Peer-review started: September 28, 2014
First decision: November 19, 2014
Revised: December 1, 2014
Accepted: December 18, 2014
Article in press: December 19, 2014
Published online: February 2, 2015
Processing time: 115 Days and 9.3 Hours
Frontal fibrosing alopecia (FFA) is a recently described form of primary cicatricial alopecia, characterized by progressive recession of the frontotemporal hairline and eyebrow loss, occurring predominantly in postmenopausal women. The incidence of FFA has increased significantly during the last decade and we may be facing an epidemic of the disease. Because this condition causes permanent hair loss, prompt diagnosis and treatment are essential for obtaining optimal outcome. This article reviews existing knowledge on epidemiology, etiopathogenesis, clinico-histological features, diagnosis, and treatment modalities of FFA.
Core tip: Frontal fibrosing alopecia (FFA) is a recently described form of primary cicatricial alopecia, characterized by progressive recession of the frontotemporal hairline and eyebrow loss, occurring predominantly in postmenopausal women. The incidence of FFA has increased significantly during the last decade and we may be facing an epidemic of the disease. Because this condition causes permanent hair loss, prompt diagnosis and treatment are essential for obtaining optimal outcome. This article reviews existing knowledge on epidemiology, etiopathogenesis, clinico-histological features, diagnosis, and treatment modalities of FFA.
- Citation: Lyakhovitsky A, Barzilai A, Amichai B. Frontal fibrosing alopecia update. World J Dermatol 2015; 4(1): 33-43
- URL: https://www.wjgnet.com/2218-6190/full/v4/i1/33.htm
- DOI: https://dx.doi.org/10.5314/wjd.v4.i1.33
Frontal fibrosing alopecia (FFA) is a relatively recently recognized form of primary cicatricial alopecia (PCA), characterized by progressive recession of the frontotemporal hairline and eyebrow loss, occurring predominantly in postmenopausal women. It was described by Kossard[1] in 1994. The number of patients with this condition has markedly increased over last decade and there are dermatologists who believe that we are facing a possible epidemic of this challenging disease[2-7]. Due to the clinical and histologic similarities with lichen planopilaris (LPP), many dermatologists consider it to be a clinical variant of LPP with marginal distribution. Both entities show perifollicular erythema and follicular hyperkeratosis, and lichenoid lymphocytic infiltrate along with perifollicular fibrosis leading to hair follicle destruction[1,4,5,7-11]. The cause of “marginal march” in FFA is unknown and therefore whether it is an LPP variant or a distinct entity with shared clinical features, remains to be determined[4,12,13]. The pathogenesis of FFA is poorly understood, although an autoimmune reaction and hormonal androgen-driven factors seem to play a role[4,9,13]. Several familial cases have been reported that raise the possibility of a genetic inheritance factor[3,14,15]. Some researchers have also suggested that there may be an environmental trigger for the disease[16,17]. The natural history of FFA is variable, although slow progression with spontaneous remission is the most frequently reported outcome[2,8,13,18]. The general uncertainties with this entity begin with an unknown origin and pathogenesis and continue with the difficulty of finding effective treatment. A range of topical as well as systemic treatments has been disappointing. Several researchers have reported stabilization with topical and intralesional corticosteroids, antibiotics, hydroxychloroquine, immunomodulators, and 5-alpha-reductase inhibitors[2-5,7,11,13,18-21].
In 1994 Kossard[1] described 6 postmenopausal women with distinctive progressive scarring alopecia affecting the frontal hairline and frequently extending to the temporal and parietal regions. This contrasted with the usual multifocal appearance of LPP, yet had similar histopathological features. Kossard[1] named this entity “postmenopausal frontal fibrosing alopecia” and further characterized it clinically and histologically in subsequent studies[1,8]. Since 1994, numerous case reports and studies on FFA have been published. Several reports have included men and premenopausal women, and it has been proposed that “postmenopausal frontal fibrosing alopecia” is better termed “frontal fibrosing alopecia”. The incidence of FFA is unknown, although most dermatologists agree that there has been an increase in the number of patients with this condition in recent years[2-7]. A ten-fold increase in the number of cases seen annually over the last decade was described by MacDonald et al[5]. According to reports published to date, over 85% of the patients reported were white postmenopausal women[2-5,17,18,20]. However, this condition has also been reported in Black, Asian, and Hispanic male and female patients[3,4,20]. Several studies showed a high incidence of early menopause, up to 17%, compared with an incidence of 6% in the general population[2,3]. In addition, several reports described that in a considerable number of women the menopause had been surgically precipitated, post-hysterectomy[2,3]. The course of the disease does not seem to be affected by the onset of hormone replacement therapy[4,21]. Age of onset of the disease ranged from 18 to 87 years with the highest incidence in the sixth decade[2-5,7,20]. One study showed an earlier age of onset of FFA in black patients, with 74% of those cases starting prior to menopause[22], yet another study described the age of onset to be similar to that in a white population[23]. There have been several reports of familial cases of FFA mentioned as well[3,14,15]. A family history of FFA in 8% of patients was recently described in a multicenter review consisting of 355 patients[3]. Most researchers found no association between previous medical background and administered medications, except for the increased incidence of autoimmune diseases[3,5-7,9,13]. One recent study described dyslipidemia, hypothyroidism, hypertension, and osteoporosis as the most frequent comorbidities[3]. Another study described an association between beta-blockers and nonsteroidal anti-inflammatory drugs, with a possible protective effect of angiotensin-converting enzyme inhibitors[5]. Concurrent female pattern hair loss or senescent alopecia has been described in 0%-68% of patients[3-5,7,13]. Thyroid abnormalities are probably the main association described, with an incidence between 9% and 23%[3,4]. Previous or concurrent lichen planus (LP) was reported in FFA patients, with a frequency ranging from 2% to 17%. Compared to LPP, in which LP is found in 28%-50% of cases, the association between cutaneous and mucosal lichen planus and FFA seems to be less common[4,5,20]. Other autoimmune diseases reported to be associated with FFA include vitiligo, alopecia areata, atopy, psoriasis, rheumatoid arthritis, lupus erythematosus, and polymyositis[3-5,20]. FFA has also been described in patients following hair transplantation and face-lift surgery[24]. One study assessed the socioeconomic and smoking status of patients with FFA and showed an association with the higher affluent group and significant preponderance of nonsmokers within the cohort of Scottish patients comparing with national data[5]. Another study from Spain showed 87% were nonsmoking patients, but it was found to be similar to the percentage of Spanish women matched by age, so their results did not support the protective effect of tobacco against FFA[3]. Laboratory work-up that included hormonal profile evaluation was unremarkable in several studies[3,12,13,19].
The etiopathogenesis of FFA remains uncertain. A key element seems to be destruction of the epithelial hair follicle (HF) stem cells located in the bulge region of the HF leading to permanent hair loss.
Most authors consider FFA as a clinical variant of LPP in a patterned distribution that primarily affects postmenopausal women. In fact, the North American Hair Research classification system currently classifies FFA as a lymphocytic primary cicatricial alopecia within the spectrum of LPP. This hypothesis is based on similar histopathologic features. Both entities show a lichenoid lymphocytic inflammatory infiltrate involving the upper and midportion of the hair follicle, with perifollicular fibrosis, and hair follicle destruction. Several authors reported coexistent mucosal or cutaneous lesions of LP in patients with FFA and postulated the phenotypical relation of these two conditions[1,4,5,7,8,10]. Previous or concurrent LP was reported in FFA patients, with a frequency ranging from 2% to 17%, in comparison to LPP, in which LP is found in 28%-50% of cases[4,5,20]. This indicates that the association between mucosal and cutaneous LP and FFA seems to be less common than in LPP. Poblet et al[12] examined the clinicopathological features of FFA as well as the similarities and differences between FFA and LPP. No clear-cut histological differences between these two entities were reported; however, their study demonstrated that, in general, FFA tends to show more apoptosis and less lichenoid tissue reaction and damage to basal cells, along with spared interfollicular epidermis. In their opinion, whether FFA can be considered a variant of LPP just because they share a common inflammatory pattern, or it is a distinct entity, is still questionable[12]. Several studies have shown patchy fibrinogen deposition and globular deposits of IgM or IgA along the epidermal or infundibular basement membrane zones (BMZ) in direct immunofluorescence (DIF) in LPP patients, but these findings were not observed in FFA[6,12,19]. In addition to these histological findings, it appears that there are differences in treatment response, indicating that FFA and LPP should be considered two separate entities. High and moderate potency topical corticosteroid preparations and systemic corticosteroid preparations and hydroxychloroquine were reported as effective in LPP patients, whereas in the cases of FFA the response to these treatments was less impressive if at all. On the other hand, there are several reports of the effectiveness of anti-androgenic drugs such as dutasteride and finasteride in FFA patients[3-5,7,9-11,13,20,21].
Next to an inflammatory process caused by infiltrating lymphocytes, hormonal influence has been also suggested as a potential factor in the pathogenesis of FFA. It is argued that the FFA is a scarring variant of pattern hair loss[13]. The role of androgens was suggested due to involvement of the androgen-dependent HF at the frontal line and onset of the disease after menopause. It was further supported by reported clinical improvement with anti-androgen therapy, such as 5-alpha-reductase inhibitors (finasteride and dutasteride)[3,7,13,18]. However, several studies have examined the hormonal profile of patients with FFA and did not show increased levels of androgens or any other hormonal abnormalities[3,13,19]. According to existing reports, hormone replacement therapy did not cause an improvement in patients with FFA[7,8,13]. A lack of any correlation to peripheral sex hormone levels or the use of hormone supplementation may suggest that regional factors of the frontotemporal scalp are involved. In addition, FFA has also been reported in men and premenopausal women[3-5,7,9,20]. The majority of patients with FFA have experienced progressive hair loss from the frontal hairline that was not preceded by progressive miniaturization of HF. In addition, FFA targets the follicles not linked to the areas of patterned hair loss. For example, involvement of the eyebrows typical of FFA does not occur in cases of androgenetic alopecia. In summary, according to existing data there is not enough evidence to implicate a hormonal basis as the cause of FFA.
The occurrence of familial cases of FFA points to a possible genetic contribution[3,14,15,17]. It is also well documented that inherited genetic traits codetermine susceptibility to autoimmune diseases as well as the influence of different environmental factors. On the other hand, one could argue that the accumulation of a number of cases in the same family simply indicates exposure to a common environmental trigger. The development of FFA in transplanted HF also raises the issue of whether the influence of local factors in the frontal area determines follicular destruction rather than the inherent qualities of the follicles at this site[24]. Further accumulation of familial cases may allow assessment of the role of genetic factors and identification of gene mutations predisposing to FFA.
Several studies showed that in FFA the lymphocytic infiltrate and fibrosis selectively affect the HF of the frontal margin and eyebrows. The disease concomitantly involves hair follicles of different types: terminal, intermediate, and vellus hairs, and different stages of the cycling (from anagen to telogen)[13,25]. Preferential involvement of vellus and intermediate HF is supported by several authors[13,26]. The reason for this selection is still unknown. A T-cell-mediated autoimmune reaction against follicular keratinocytes appears to play a major role in this process. Important mechanisms implicated in the irreversible follicular destruction include collapse of the HF immune privilege, cytotoxic cell-mediated follicular damage along with increased proinflammatory response, and increased apoptosis[12,17,27]. The inflammatory cells attack and destroy keratinocytes expressing particular antigens. These target follicular antigens have as yet not been defined. The inflammation is mostly located around the bulge area, where the stem cells are present, thus causing permanent hair loss. The fact that several autoimmune diseases have been associated with FFA also suggests an autoimmune pathogenesis[3,5-7,9,13].
Epithelial-mesenchymal transition (EMT) may contribute to fibrosis in FFA. Previous studies suggested a possible contribution of EMT in renal, liver, and pulmonary fibrosis. Recently, Nakamura et al[28] demonstrated that an EMT marker, snail 1, was expressed in the fibrotic dermis of FFA patients. This observation suggests a possible role of EMT conversion of HF epithelial cells to fibroblasts regulated by transforming growth factor-β in the pathogenesis of the FFA[17,28].
Peroxisome proliferator-activated receptor gamma (PPARγ) is a member of the nuclear receptor super-gene family that regulates the expression of genes involved in lipid homeostasis and inflammatory responses and has a crucial role in maintaining the pilosebaceous unit. Recent gene expression studies identified a deficiency in PPARγ-mediated signaling in LPP patients and suggested that the loss of this function may trigger the pathogenesis of LPP. Several authors reported the efficacy of PPARγ-agonists in treatment of LPP. The deficiency of PPARγ has yet to be studied in FFA[16,29].
The epidemiology of FFA strongly suggests a role of environmental factors in its development. The occurrence of the disease in several members of the same family may indicate exposure to common environmental triggers. A contact sensitizer, environmental toxin, dietary factor, or infectious agent could be responsible for triggering the process in predisposed subjects. For example, photoallergic reaction to pyridoxine hydrochloride found in a wide variety of hair care products, needs to be evaluated as potential trigger for scarring alopecia. Up to date a role of application cosmetic products and hair-care practices in the pathogenesis of FFA were not documented and their role remains unclear. The possible role of microbial antigens or super-antigens in this context remains to be elucidated. Recently the possible link between the aryl hydrocarbon receptor (AHR) and its overexpression in cicatricial alopecia was suggested. Since the ligand for the AHR is activated by dioxin, the possibility that dioxin-like substances can trigger the disease via the AHR was discussed recently, but remains unproven[16,17,29]. Dioxin-like chemicals are environmental pollutants that are ingested mostly with food of animal origin: meat, dairy products, or fish predominate, depending on the country. These toxins persist in the environment and are very slowly eliminated from the human body. Chronic low-dose exposure may lead to the accumulation of dioxins in lipid-rich regions such as sebaceous glands, causing loss of PPARγ expression and thereby scarring alopecia in susceptible individuals.
In conclusion, future investigation is needed to clarify the pathogenesis of FFA and to define the role of genetic, autoimmune, hormonal, and environmental factors and lipid metabolism in this challenging condition.
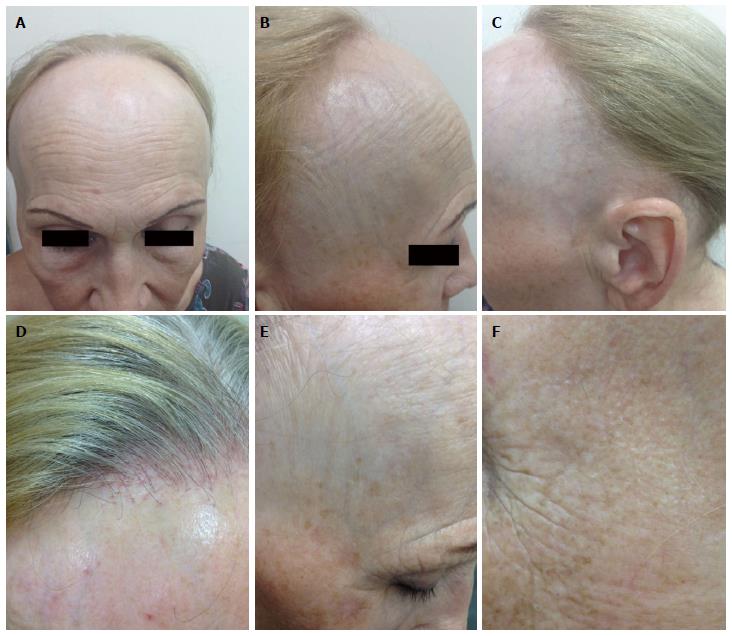
FFA has a distinct clinical presentation characterized by recession of the anterior hairline with loss of follicular orifices in the area of hair loss. The band-like zone of alopetic skin appears pale, shiny, and smooth contrasting to the mottled, photo-aged skin of the forehead. Although the frontal area is most commonly affected, and despite the name “frontal” fibrosing, FFA may appear on other sites, such as the temporo-parietal as well as the retroauricular and occipital areas. Hairline recession usually occurs symmetrically and bilaterally, giving rise to a band of alopecia between 0.5 and 10 cm from its original site (Figure 1A-C). Although the progression is relatively slow, distribution can be widespread, involving the entire hairline and leaving only a band of hair on the top of the scalp, which is described as “clown alopecia”. Increased venous vasculature on the temples is also a common observation (Figure 1B). Perifollicular erythema and papules along the new hairline indistinguishable from that seen in LPP are common findings as well (Figure 1D). Degree of erythema and scaling may be variable, being marked in some, but minimal in others[1-11,13,18-20]. The striking feature is the unnatural appearance of the hairline caused by loss of both terminal and vellus hair (Figure 1D)[13,30]. The presence of “lonely hair“ described by Tosti et al[31] is also a useful diagnostic clue (Figure 1E). This sign describes the presence of isolated terminal hairs in the middle of a bald band marking the original hairline prior to hair loss. This is a common but non-specific sign of FFA. While some patients can present with associated itching, pain, and burning sensations, it is not unusual to see patients who are completely asymptomatic without frank inflammation. Most patients with FFA exhibit some degree of eyebrow diminution, recognized as a characteristic feature of this disorder. It can occur either before or after the onset of frontotemporal recession. Hair loss from the lateral third of the eyebrows is typical, but in some cases, there may be diffuse thinning or total loss of eyebrows (Figure 1E). Eyelash involvement was also reported, albeit less frequently (Figure 1E)[1-11,13,18-20]. Recently, non-inflammatory asymptomatic facial papules, mainly confined to the temporal area of the face, were reported as possible sign of facial vellus hair involvement (Figure 1F)[32]. Several authors described decrease or absence of facial vellus hair as well as loss of hair from peripheral body sites and generalized hair loss[2,6,8,13,18]. It should be noted that despite frequent reports of axillary and pubic hair loss, it cannot be clearly attributed to the disease process considering that this is also a common symptom in postmenopausal and older women.
Findings on trichoscopy include the absence of follicular openings, absence of vellus hairs, white dots (corresponding to the follicular fibrosis seen in dark-skinned individuals), and brown halos (expressing the inflammation). Reduced hair density, follicular plugging, perifollicular scale (also called peripilar casts), and perifollicular erythema are frequent around the existing hairs at the anterior hairline[30,33]. Follicular red dots on the forehead corresponding to another sign indicating vellus hair involvement were described recently[34].
A 4-mm punch biopsy from an active inflammatory lesion within the hair-bearing margin of the alopetic patch is preferred for pathological study. Horizontal sections seem more informative than vertical orientation. Typical histopathological findings in FFA show lymphocytic infiltrate mainly localized to the isthmus and infundibular regions with the lower portion of HF being spared. The inflammatory infiltrate shows lichenoid characteristics of variable severity, with a follicular interface dermatitis pattern, whereas the overlying interfollicular epidermis is spared. The number of HFs is reduced, perifollicular lamellar fibrosis is evident, and HFs are often replaced by fibrous tracts. The sebaceous glands are absent or only focally present, and the external root sheaths may show vacuolar degeneration of the basal layer and apoptosis of follicular keratinocytes. Perivascular and periadnexal inflammation is absent. Histopathologic findings are variable according to disease progression. In its active stage the inflammation is much more prominent, while in the “burned-out” stage the specimen may show only a follicular scar. Most authors consider FFA as a clinically distinct variant of LPP with patterned distribution, based on their similar histopathological findings[1,6,8]. However, recently a number of subtle histopathological differences between these two entities were described[12]. In general, the lichenoid tissue reaction was shown to be milder in FFA than in LPP. While LPP cases may show intense damage of the basal cell layer, this feature is not observed in FFA. The involvement of interfollicular epidermis was not found in FFA, but it is common in LPP. FFA cases tend to show much more apoptosis than LPP. The foreign body reaction to the follicular destruction of the external root sheath and the hair shafts trapped in the dermis were found to be more frequent and more prominent in FFA[12]. In addition, immunofluorescence tests are negative in FFA, while they can show patchy fibrinogen deposition and globular deposits of IgM or IgA along the epidermal or infundibular basement membrane zones in LPP[6,12,19]. It was also reported that there may be a predilection for the involvement of intermediate and vellus follicles in FFA[13]. Recently, the sign of “follicular triad” was suggested as a possible diagnostic clue to the diagnosis of FFA[26]. This sign describes the simultaneous involvement of HF of different types: terminal, intermediate (0.03-0.06 mm), and vellus (< 0.03 mm) and in different stages of cycling (anage, catagen, and telogen) with inflammatory infiltrate and perifollicular fibrosis. Although clinically noninflammatory, several studies have shown that histologically eyebrow, facial, and peripheral hair loss demonstrate inflammation and fibrosis, suggesting that the process of permanent hair loss is generalized rather than localized only to the frontal scalp[2,6,13].
In most cases the diagnosis of FFA can be made on clinical grounds and supported by trichoscopy. When the clinical diagnosis is not conclusive, for example, in the early stages when the follicular density is almost normal, in partially treated cases, or in advanced cases when clinical signs of inflammation are subtle, a dermoscopy-guided biopsy from a site of disease activity is indicated to provide for histopathological confirmation[2,5,10,13].
Key features of FFA that help to make clinical diagnosis are as follows: (1) largely affects postmenopausal women; (2) symmetric and progressive band-like recession of the fronto-temporal region with loss of follicular ostia; (3) pale and atrophic skin devoid of follicular orifices in the zone of alopecia, contrasted with the hyperpigmented sun-damaged skin of the forehead; (4) perifollicular erythema and follicular hyperkeratosis around the existing hairs at the margin of alopecia; (5) presence of isolated terminal hairs in the bald band on the forehead (“lonely hair” sign); and (6) marked decrease or complete loss of the eyebrows.
On trichoscopy: (1) absence of follicular openings; (2) reduced hair density, absence of vellus hair at hairline margins; (3) follicular plugging, perifollicular scale, and erythema around the existing hairs at the margin of alopecia; and (4) peripilar white dots and brown halos (incontinentia pigmenti).
Histopathological features include: (1) lichenoid lymphocytic infiltrate mainly localized to the isthmus and infundibulum; (2) follicular interface dermatitis pattern, with sparing of interfollicular epidermis; (3) reduced number of HFs, perifollicular lamellar fibrosis, replacement of HFs by fibrous tracts; (4) the sebaceous glands are absent or only focally present; (5) vacuolar degeneration of the basal layer and apoptosis of follicular keratinocytes in the external root sheaths; (6) absence of perivascular and periadnexal inflammation; and (7) follicular triad sign: simultaneous involvement of different types of hairs (terminal, intermediate, and vellus) and in different stages of cycling (anage, catagen, and telogen).
FFA should be differentiated from several conditions that cause marginal scalp hair loss. In some cases it is difficult to distinguish between FFA and alopecia areata involving hairline, for example in sisaipho or ophiatic pattern alopecia areata. Presence of follicular orifices, exclamation mark hairs, yellow dots, black dots, and dystrophic hairs on dermoscopy can help establish the diagnosis of alopecia areata. On the other hand, lack of follicular ostia, perifollicular inflammation, and symmetric eyebrow involvement appear in FFA. When it is difficult to differentiate between the diseases clinically, skin biopsy is recommended.
Frontotemporal form of female pattern hair loss (FPHL) is another condition that may be confused with FFA. Unlike FFA, female pattern hair loss is not a cicatricial process. In FPHL, the vellus hair is present, and there is no perifollicular inflammation or scale.
Another condition that can cause hair loss in the periphery of the scalp is traction alopecia. Differentiation from traction alopecia can usually be made from the history of traction or chemical straightening, and supported by the finding of broken hairs of various lengths in the affected area. There is no eyebrow and peripheral hair loss or perifollicular inflammation, and vellus hair is preserved. The lichenoid follicular inflammation and fibrosis are absent and sebaceous glands are preserved in histologic sections.
Cicatricial marginal alopecia was recently described by Goldberg[35] in women who presented with hair loss limited to the periphery of the scalp. There was no history of traction. The clinical picture revealed hair thinning or almost complete alopecia of the scalp margin and eyebrows were not involved. Dermoscopic features were characterized by low hair density with loss of follicular ostia, reduced diameter of remaining hairs, and absence of perifollicular erythema or hyperkeratosis. Histological sections revealed a decrease in HF density with normal sebaceous glands and no signs of inflammation.
There are cases where the possibility of genetic family high hairline should be considered. In this condition, the front line of hair can be located relatively high, but it has a natural appearance, and contains all types of hair (terminal, intermediate, and vellus). There are no clinical signs of inflammation or eyebrow involvement.
The differential diagnosis with other diseases from the PCA group can be challenging. The distribution pattern of alopecia (single plaque, multifocal, hairline recession), the involvement of other hairy sites (eyebrows, axillae, body hair), and the presence of other associated cutaneous manifestations may allow clinical distinction in a large number of cases.
While FFA patients are mostly asymptomatic and only rarely have mild itching, intense pruritus, pain, burning, and scalp tenderness are common in LPP patients. The distribution of the hair loss also differs. There is a multifocal disease with a predilection to central scalp in LPP. The involvement of hair in the peripheral portion of the scalp typical for FFA is uncommon. Histologic sections of LPP show less apoptosis and more inflammation. DIF is not uncommonly negative, with some cases showing patchy deposition of fibrinogen and IgM, or less commonly IgA and C3, along the follicular BMZ. Elastin staining reveals a superficial, wedged-shaped scar associated with loss of the upper follicular elastic sheath.
Piccardi-Lasseueur-Graham-Little Syndrome is classically considered a type of LPP; however, several researchers suggest that it is a distinct entity. It is characterized by a triad of scarring patchy scalp alopecia, nonscarring alopecia of the axillae and the pubic area, and grouped or disseminated follicular papules with spinous scale on the trunk and extremities. Face and eyebrows can be also affected. Pathology shows features of both LPP and keratosis pilaris atrophicans.
Fibrosing alopecia in a pattern distribution was described by Zinkernagel et al[36]. This entity shares clinical and histologic features similar to LPP, but is limited to an area of androgenic hair loss. The centrovertical scalp is usually diffusely affected, but some patients also have frontotemporal loss similar to that seen in FFA. Biopsy specimens demonstrate the HF miniaturization and a lichenoid inflammatory infiltrate targeting the upper follicle region.
Discoid lupus erythematosus (DLE) is usually multifocal and does not have a predilection for the marginal scalp. In contrast to FFA, the center of lesion, rather than the hair-bearing periphery, is affected in the active disease stage. A search for DLE elsewhere on the body or for coexisting signs of systemic lupus erythematosus is essential. The histologic presentation of DLE is also different. Superficial and deep perivascular and perieccrine inflammation and dermal mucin deposition are typical features of DLE and are not found in FFA. In addition, the interface change is vacuolar and not lichenoid, often with a thickened basement membrane. The interfollicular epidermis is generally not spared. The dermal sclerosis is usually less severe than in FFA and telangiectases are present. The DIF of DLE is usually specific with deposition of immunoglobulin (Ig) G or IgM and C3 in a granular or homogenous band-like pattern at the dermal interface with the follicular epithelium and epidermis. Elastin staining reveals a diffuse dermal uptake that spares the fibrous tracts of extinct follicles.
Pseudopelade of Brocq is another type of PCA that predominantly affects the vertex and parietal areas of scalp with irregular multifocal areas of scarring alopecia. The lesions are asymptomatic, limited to the scalp, with no visible perifollicular inflammation or follicular hyperkeratosis. Multifocal coin-sized plaques and larger polycyclic or irregular plaques of alopecia coalesce and create a “footprints in the snow” pattern. There are no pathognomonic features on histology. In early lesions, massive lymphocyte-mediated apoptosis of the follicular sheath has been observed. Lymphocytic perifollicular infiltrate around the infundibulum without interface changes, and prominent concentric lamellar fibroplasia are described as established disease features. Findings on DIF are usually negative, but scanty IgM deposition along the follicular infundibular basal membrane zone can be seen. Elastin stains reveal hyalinized dermis with markedly thickened elastic fibers throughout and broad follicular fibrous tracts with intact elastic sheaths, unlike the pattern seen in LPP and DLE.
Central centrifugal scarring alopecia predominantly occurs in adult black women and presents with asymp-tomatic, noninflammatory alopecia that begins in the midline central scalp, with gradual centrifugal spread over the years. The histologic features are not specific in this type of PCA and resemble Pseudopelade of Brocq.
Alopecia mucinosa has a polymorphous presentation and can be nonscarring or scarring. Histology is diagnostic showing mucinous degeneration of the follicular and sebaceous epithelium associated with perifollicular and perivascular lymphocytic infiltrate.
Keratosis Follicularis Spinulosa Decalvans (KFSD) may also share features similar to FFA. Unlike FFA, it usually begins in infancy and may improve during puberty. There are sporadic and familial cases. Men are more likely to present with a more severe disease. Triad of KFSD includes widespread keratotic papules succeeded by atrophy, cicatricial alopecia, and photophobia. Alopetic plaques have multifocal distribution with patchy involvement of the scalp, eyebrows, and eyelashes, with perifollicular hyperkeratosis and erythema at the edges. It can be associated with focal palmoplantar keratoderma. In some cases, follicular pustules appear at the hair-bearing margins, resembling folliculitis decalvans. The histopathology shows a mixed inflammatory infiltrate with follicular plugging, hypergranulosis, and neutrophilic spongiosis of the infundibulum and adjacent epidermis in acute disease and perifollicular lymphocytic infiltrate thereafter.
Neutrophilic type cicatricial alopecia can also simulate FFA, especially in advanced stages of the disease, when pustules and crusts are absent, and neutrophilic infiltrate of folliculitis decalvans disappears. Multifocal distribution without predilection to marginal scalp and presence of tufted HFs are characteristic of folliculitis decalvans.
Because the disease has been described relatively recently, little is known about the natural history of FFA. The rate and extent of frontal recession is highly variable. According to several studies an average rate of recession is 0.9-1.05 mm per month[2,3,5]. It has been described that FFA may advance slowly and stabilize over time regardless of treatment continuation, whereas in some patients the recession progresses despite therapeutic intervention and eventually involves the entire scalp[2,8,13,18]. One recent study demonstrated that eyelash loss, presence of facial papules, and body hair involvement may be associated with poor prognosis, while eyebrow loss as the initial clinical presentation was associated with mild forms of FFA[3]. It has also been described that visible erythema does not always correlate with alopecia progression[5]. Because the alopecia is cicatricial, hair regrowth is not possible, except when therapy has been started at the very beginning of the disease; therefore, early diagnosis is crucial. The treatment goals are to abort the disease progression, prevent further alopecia, and reduce symptoms. The fact that FFA progression is slow, with spontaneous cessation of the disease years after onset, makes both treating the disease and assessing the effectiveness of the administered treatment difficult. Given the tendency of FFA for spontaneous stabilization, some apparent responses may simply have been part of the natural course of the disease. The available evidence on the treatment of FFA comes from retrospective observation studies. There are no controlled clinical trials to evaluate the available modalities. It should be noted also that there is currently no standardized outcome measure for the evaluation of treatment efficacy in FFA. Several researchers have used LPP activity index in order to assess treatment efficacy in FFA patients[20,37]. In view of the differences that exist between FFA and LPP, such as a lower weight of subjective symptoms and clinical signs of inflammation in FFA, the relevance of this index in FFA patients is questionable. Recently, a clinical severity scale of FFA was suggested, basing on measuring the area of hairline recession. It includes 5 grades of severity: I (< 1 cm), II (1-2.99 cm), III (3-4.99 cm), IV (5-6.99 cm), and V (≥ 7 cm, also called clown alopecia), which are grouped as mild FFA (grades I and II) and severe FFA (grades III, IV, and V)[3]. We believe that the main measure to be considered in evaluating the treatment efficacy in FFA is progression of hairline recession. Changes in erythema or subjective symptoms should be evaluated separately. Treatment of FFA has been disappointing. It is unclear whether treatments alter the natural history of the disease. Several topical and systemic therapeutic options have been reported to have some efficacy in halting the disease progression in retrospective studies.
According to previous reports, almost all patients had been treated with moderate to high potency topical corticosteroids. Although in some cases this treatment resulted in reduced inflammation, it did not halt progression of alopecia in most cases[7,11,21].
Intralesional corticosteroid injections have been considered as first-line treatment by most researchers. Triamcinolone acetonide at a concentration of 5-10 mg/mL (to a maximum of 2 mL) for the scalp and 2 mg/mL for the eyebrows (location is more susceptible to atrophy) every 4-8 wk produced a response rate of up to 60%[21]. Higher concentrations of triamcinolone acetonide were reported as well, and in these cases the time intervals between injections were longer. For example, triamcinolone acetonide injections at a concentration of 20 mg/mL were made every 3 mo[13,18]. However, most researchers prefer to employ a lower concentration in order to reduce the risk of cutaneous atrophy. Intralesional corticosteroids are more effective in the early stages of the disease, especially in patients with prominent clinical and histologic inflammation. Once alopecia has advanced to the fibrotic phase, it provides no benefit and may even worsen the fibrosis and atrophy that characterize the advanced stages of FFA[18,19]. It should be noted that in most reported cases this treatment was given in combination with other treatment modalities, which makes it difficult to judge its own efficacy. Several researchers have emphasized that the results of this treatment are less impressive compared with those in the cases of LPP[2-5,29].
Administration of oral prednisone may be useful in rapidly progressive diseases, especially when the signs of inflammation are prominent. However, after the discontinuation of treatment a relapse is common. There are different protocols in the literature. Usually oral prednisone is administered in the dose of 0.5-1 mg/kg per day for a period of 1 to 18 mo and tapered to discontinuation over 2-4 mo[1,8,32,38]. There are researchers who preferred intramuscular triamcinolone pulses of 0.5-1 mg/kg every 3-4 wk for 3-4 mo[13,29]. The combined treatment of systemic corticosteroids with other topical or systemic treatments was also described. Existing literature indicates that, like with other anti-inflammatory treatments, corticosteroids in FFA are less effective than in LPP.
Because there is an opinion that FFA is a clinical variant of LPP, antimalarial medications known to be effective in LPP were one of the frequently used systemic medications in FFA patients. According to existing reports, results are inconclusive. There are studies that show the disease stabilization in more than 50% of cases[3,7,20,29,37]. Other studies have noted less impressive results or simply a lack of efficacy[2,6,11,39]. Protocols described include administration of hydroxychloroquine in a dose of 200-400 mg per day, or chloroquine diphosphate 250 mg per day, for at least 6 mo[3,7,32].
Several reports describe treatment with tetracycline 500 mg twice daily or minocycline 100 mg twice daily in FFA patients. Like other anti-inflammatory treatments, tetracyclines caused a decrease in inflammation in some cases, but their efficacy in controlling the alopecia was uncertain[4,20].
Topical tacrolimus and pimecrolimus were used with disappointing results in most patients[2,5,7,11].
Although the hormonal basis of FFA has not been proven, several retrospective studies have shown efficacy of anti-androgen therapy with 5-alpha-reductase inhibitors, finasteride and dutasteride. A number of studies comparing the efficacy of various treatments in patients with FFA suggest that this treatment is more effective than others in halting the disease progression. Due to its teratogenic potential (pregnancy category X) this treatment needs to be combined with oral contraceptive therapy in premenopausal women. Several open label studies demonstrated the efficacy of finasteride at a dose of 2.5 mg daily given for at least 6 mo[3,4,7,13,18]. Treatment with dutasteride was also reported to be effective, producing even more impressive results than with finasteride, probably due to its ability to achieve greater suppression of dihydrotestosterone[7,25]. Therapeutic protocols of treatment with dutasteride reported in the literature include treatment at a dose of 0.5 mg per day for at least six months[40] or loading dose of 0.5 mg per day for 2 wk and reducing to maintenance dose of 0.5 mg per week thereafter[2-4,7,25]. Some authors question the effectiveness of 5-alpha-reductase inhibitors in treatment of FFA. They argue that in all cases reported, the treatment with 5-alpha-reductase inhibitors was combined with other medications, such as topical or intralesional corticosteroids[40], calcineurin inhibitors[40], or minoxidil[13,18,40], thus making it difficult to judge which of medications given can be associated with the outcome. Some authors also suggest that the improvement obtained may be related to a positive effect of medications on accompanying female pattern hair loss by preventing miniaturization and stimulating of remaining hairs[5,11]. Therefore, although several preliminary reports are promising, further evaluation of the therapeutic role of dutasteride with randomized controlled trials is warranted.
Limited reports on the use of topical minoxidil solution did not slow the progression of FFA in most patients[3,9,19,21]. It should be noted that in several reports the treatment with minoxidil was combined with topical steroids and 5-alpha-reductase inhibitors. These studies showed positive results. It is unclear whether the results are related to the effect of minoxidil or to the additional medication that had been taken, and if the improvement is due to the influence on FFA or on an accompanying female pattern hair loss.
Limited reports on the use of PPPAR-γ agonists, methotrexate, cyclosporin[29], mycophenolate mofetil[20], griseofulvin[9], isotretinoin, acitretin, and UVB[3,21] on FFA preclude any conclusion regarding efficacy.
There is some concern about hair transplantation in FFA patients because of reported cases of FFA that began after hair transplantation in patients with androgenetic alopecia[24,37]. There is also a description of the development of FFA following face-lift surgery[37,41]. However, several reports have shown promising results[21,42]. It was suggested that hair transplants might be proposed when there is no progression of the disease for at least 1 year.
Different methods of camouflage including wigs, hairpieces, scalp micropigmentation, and eyebrow tattooing may be recommended to cover hair loss in severe refractory and end-stage cases of FFA[2,5].
FFA is form of PCA, characterized by progressive reces-sion of the frontotemporal hairline and eyebrow loss, occurring predominantly in postmenopausal women. The pathogenesis of FFA is poorly understood, although an autoimmune reaction and hormonal androgen-driven factors seem to play a role. Typical histopathological findings of FFA show lichenoid lymphocytic inflammatory infiltrate localized to the upper portion of hair follicle, perifollicular fibrosis, and HF destruction. Therapeutic options are limited. Long-term prospective cohort studies are needed to further elucidate the etiopathogenesis, to evaluate the effectiveness of existing treatments, and to find new treatment options. Until then, management of this fascinating disorder remains unsatisfactory.
P- Reviewer: Crisostomo MR, Zhang X S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Kossard S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. [PubMed] |
| 2. | Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, Arias-Santiago S, Rodrigues-Barata AR, Garnacho-Saucedo G, Martorell-Calatayud A, Fernández-Crehuet P, Grimalt R, Aranegui B. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 307] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 4. | Banka N, Mubki T, Bunagan MJ, McElwee K, Shapiro J. Frontal fibrosing alopecia: a retrospective clinical review of 62 patients with treatment outcome and long-term follow-up. Int J Dermatol. 2014;53:1324-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 6. | Chew AL, Bashir SJ, Wain EM, Fenton DA, Stefanato CM. Expanding the spectrum of frontal fibrosing alopecia: a unifying concept. J Am Acad Dermatol. 2010;63:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Ladizinski B, Bazakas A, Selim MA, Olsen EA. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66. [PubMed] |
| 9. | Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37; quiz 38-40. [PubMed] |
| 10. | Olsen EA, Bergfeld WF, Cotsarelis G, Price VH, Shapiro J, Sinclair R, Solomon A, Sperling L, Stenn K, Whiting DA. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110. [PubMed] |
| 11. | Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Poblet E, Jiménez F, Pascual A, Piqué E. Frontal fibrosing alopecia versus lichen planopilaris: a clinicopathological study. Int J Dermatol. 2006;45:375-380. [PubMed] |
| 13. | Tosti A, Piraccini BM, Iorizzo M, Misciali C. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60. [PubMed] |
| 14. | Dlova N, Goh CL, Tosti A. Familial frontal fibrosing alopecia. Br J Dermatol. 2013;168:220-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Junqueira Ribeiro Pereira AF, Vincenzi C, Tosti A. Frontal fibrosing alopecia in two sisters. Br J Dermatol. 2010;162:1154-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Karnik P, Tekeste Z, McCormick TS, Gilliam AC, Price VH, Cooper KD, Mirmirani P. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 17. | Ohyama M. Primary cicatricial alopecia: recent advances in understanding and management. J Dermatol. 2012;39:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Moreno-Ramírez D, Camacho Martínez F. Frontal fibrosing alopecia: a survey in 16 phaoatients. J Eur Acad Dermatol Venereol. 2005;19:700-705. [PubMed] |
| 19. | Moreno-Ramírez D, Ferrándiz L, Camacho FM. [Diagnostic and therapeutic assessment of frontal fibrosing alopecia]. Actas Dermosifiliogr. 2007;98:594-602. [PubMed] |
| 20. | Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Rácz E, Gho C, Moorman PW, Noordhoek Hegt V, Neumann HA. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Dlova NC, Jordaan HF, Skenjane A, Khoza N, Tosti A. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Miteva M, Whiting D, Harries M, Bernardes A, Tosti A. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Kossard S, Shiell RC. Frontal fibrosing alopecia developing after hair transplantation for androgenetic alopecia. Int J Dermatol. 2005;44:321-323. [PubMed] |
| 25. | Georgala S, Katoulis AC, Befon A, Danopoulou I, Georgala C. Treatment of postmenopausal frontal fibrosing alopecia with oral dutasteride. J Am Acad Dermatol. 2009;61:157-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Miteva M, Tosti A. The follicular triad: a pathological clue to the diagnosis of early frontal fibrosing alopecia. Br J Dermatol. 2012;166:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Harries MJ, Paus R. The pathogenesis of primary cicatricial alopecias. Am J Pathol. 2010;177:2152-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Nakamura M, Tokura Y. Expression of Snail1 in the fibrotic dermis of postmenopausal frontal fibrosing alopecia: possible involvement of an epithelial-mesenchymal transition and a review of the Japanese patients. Br J Dermatol. 2010;162:1152-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Miteva M, Tosti A. Treatment options for alopecia: an update, looking to the future. Expert Opin Pharmacother. 2012;13:1271-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Lacarrubba F, Micali G, Tosti A. Absence of vellus hair in the hairline: a videodermatoscopic feature of frontal fibrosing alopecia. Br J Dermatol. 2013;169:473-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Tosti A, Miteva M, Torres F. Lonely hair: a clue to the diagnosis of frontal fibrosing alopecia. Arch Dermatol. 2011;147:1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Donati A, Molina L, Doche I, Valente NS, Romiti R. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 33. | Inui S, Nakajima T, Shono F, Itami S. Dermoscopic findings in frontal fibrosing alopecia: report of four cases. Int J Dermatol. 2008;47:796-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Pirmez R, Donati A, Valente NS, Sodré CT, Tosti A. Glabellar red dots in frontal fibrosing alopecia: a further clinical sign of vellus follicle involvement. Br J Dermatol. 2014;170:745-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Goldberg LJ. Cicatricial marginal alopecia: is it all traction? Br J Dermatol. 2009;160:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Zinkernagel MS, Trüeb RM. Fibrosing alopecia in a pattern distribution: patterned lichen planopilaris or androgenetic alopecia with a lichenoid tissue reaction pattern? Arch Dermatol. 2000;136:205-211. [PubMed] |
| 37. | Chiang C, Sah D, Cho BK, Ochoa BE, Price VH. Hydroxychloroquine and lichen planopilaris: efficacy and introduction of Lichen Planopilaris Activity Index scoring system. J Am Acad Dermatol. 2010;62:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 38. | Naz E, Vidaurrázaga C, Hernández-Cano N, Herranz P, Mayor M, Hervella M, Casado M. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:25-27. [PubMed] |
| 39. | Vaisse V, Matard B, Assouly P, Jouannique C, Reygagne P. Postmenopausal frontal fibrosing alopecia: 20 cases. Ann Dermatol Venereol. 2003;130:607-610. [PubMed] |
| 40. | Katoulis A, Georgala E, Papadavid E, Kalogeromitros D, Stavrianeas N. Frontal fibrosing alopecia: treatment with oral dutasteride and topical pimecrolimus. J Eur Acad Dermatol Venereol. 2009;23:580-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Chiang YZ, Tosti A, Chaudhry IH, Lyne L, Farjo B, Farjo N, Cadore de Farias D, Griffiths CE, Paus R, Harries MJ. Lichen planopilaris following hair transplantation and face-lift surgery. Br J Dermatol. 2012;166:666-370. [PubMed] |
| 42. | Harries MJ, Sinclair RD, Macdonald-Hull S, Whiting DA, Griffiths CE, Paus R. Management of primary cicatricial alopecias: options for treatment. Br J Dermatol. 2008;159:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |