INTRODUCTION
Various kinds of ultraviolet (UV) blocking materials, such as sunblocks, sunglasses, films and fibers are often used to prevent skin damage from UV exposure. Although individuals all over the world use various types of sunscreens, unwanted biological influences, such as rosacea, erythema ab igne, long-term vasodilation[1,2], muscle thinning[3,4] and sagging still occur[5,6]. Most sunscreens can only block UV but not visible light and near-infrared (NIR)[5].
Both UV and visible light radiation are attenuated by melanin[7], whereas infrared (IR) can penetrate deep into human tissue where it can cause photochemical changes[8]. We previously reported that NIR penetrates the skin and is absorbed by sweat on the skin surface, water in the dermis[1,9-11], hemoglobin in dilated vessels[1,2], myoglobin in the superficial muscle[3,4], bone cortical mass and is scattered by adipose cells[12].
Appropriate NIR irradiation induces dermal heating thermally and non-thermally induces collagen and elastin stimulation, which results in skin laxity tightening. NIR irradiation also induces non-thermal DNA damage[13,14] and cell death by apoptosis[15], as well as the cell death of cancer cells and bone marrow cells[12]. In addition, NIR irradiation is used as a therapeutic option for the treatment of wound healing disorders[16-18] and malignant tumors[19-22].
However, the necessity to protect cells from NIR in order to prevent tissue damage has not been well investigated. Many studies have proven the effects of sun and UV exposure on the skin but have not investigated well the long-term effects of NIR exposure on human skin and skin cancers. Fair skin, with sparse melanin and a thin dermis, might allow NIR radiation to penetrate deeper into human tissue than dark skin, which has dense melanin and a thick dermis[5,6]. In addition to natural NIR, human skin is increasingly exposed to artificial NIR from medical devices and electrical appliances[23,24]. Thus, sunscreens should also protect against NIR[3,5,6,12,23-28] because we are exposed to tremendous amounts of NIR[5]. Our preliminary studies suggest that we should consider the biological effect of not only UV, but also NIR[3,5,6,12].
METHODS OF NIR RESEARCH
Previous in vitro research of NIR
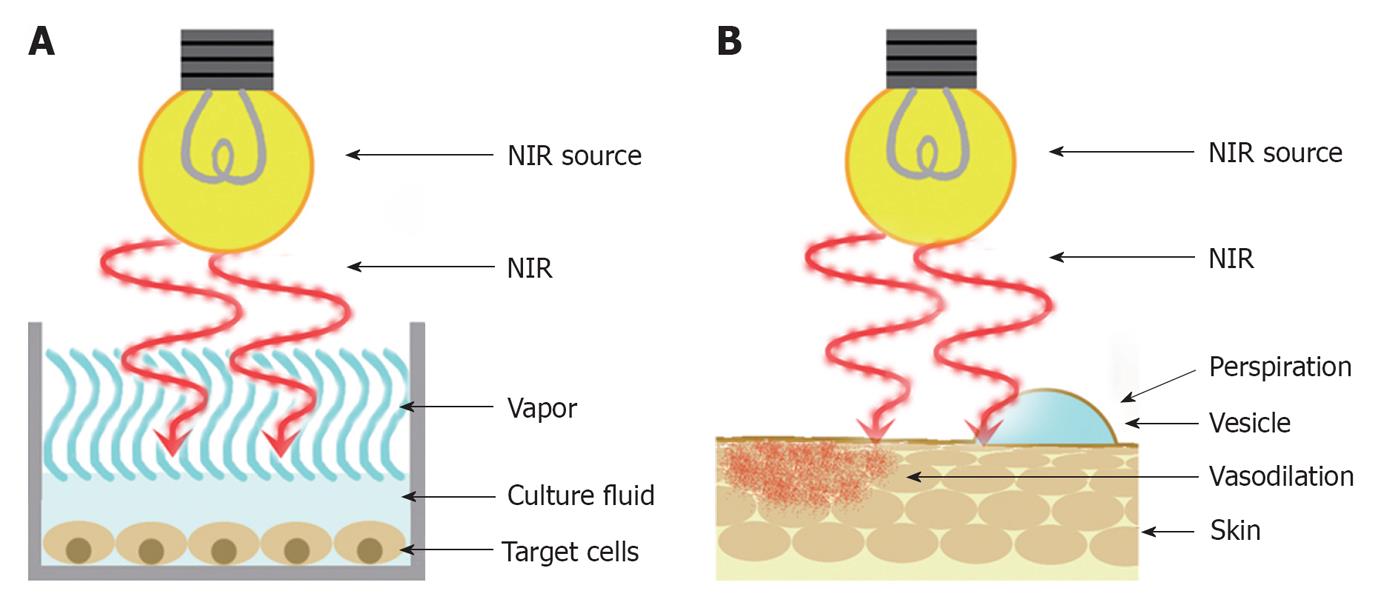
In the previous studies of the NIR, lamps emitting wide wavelengths of NIR were used as a NIR source[6,29]. The temperature of the superficial layer of the culture fluid in the laboratory dish will rise immediately by the NIR irradiation because NIR is primarily absorbed by water. Then, the energy of NIR will diminish as it penetrates deeper and will not reach the target cells in the base enough (Figure 1A). Therefore, the previous studies only described thermal effects of NIR and could not find various non-thermal biological effects of NIR[6].
Figure 1 A schematic of the previous in vitro and in vivo research.
A: In vitro research. B: In vivo research. NIR: Near-infrared.
Previous in vivo research of NIR
NIR irradiation is known to induce dermal heating, which results in skin laxity tightening[1,9,30-34]. In previous studies[16,35,36], NIR devices without a water filter or contact cooling were used to evaluate photobiological effects on the human body.
NIR increases the skin surface temperature and induces perspiration and vasodilation because NIR is primarily absorbed by water and hemoglobin. Then, a substantial amount of energy is absorbed in the superficial layers of skin and only limited NIR energy can be delivered to deeper tissues (Figure 1B). Therefore, the previous studies only described superficial and thermal effects of NIR and could not find various non-thermal biological effects of NIR[6].
My research of NIR
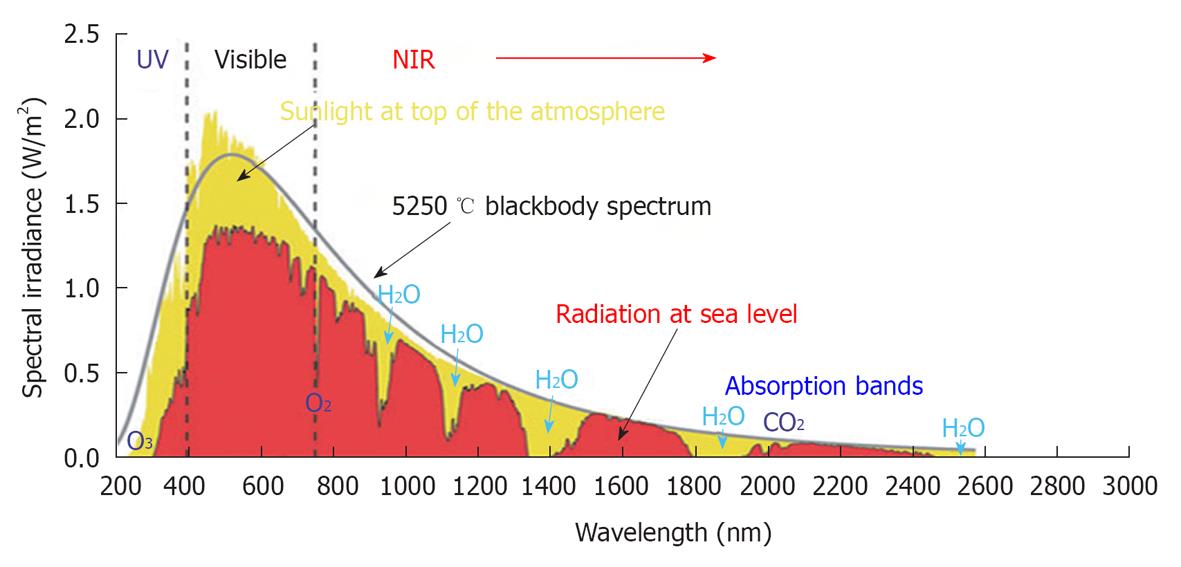
Sunlight that reaches the human skin contains solar energy composed of 6.8% UV light, 38.9% visible light and 54.3% IR radiation[37]. The IR spectral region is arbitrarily divided according to wavelength into sub-regions of NIR (760-3000 nm), middle IR (3000-30 000nm) and far IR (30 000 nm-1 mm). NIR radiation from the sun is selectively filtered by atmospheric water[7,38]; thus, most NIR radiation that reaches the Earth’s surface readily penetrates the superficial layers of the skin[3,5,6,12] (Figure 2).
Figure 2 Solar radiation.
This graph shows the radiation spectrum for direct light both at the top of the earth’s atmosphere (yellow) and at sea level (red). The sun produces light with a distribution similar to that expected from a 5250 °C blackbody (gray), which is approximately the temperature of the sun’s surface. As light passes through the atmosphere, some is absorbed by gases with specific absorption bands (blue). These curves are based on the American Society for Testing and Materials Terrestrial Reference Spectra, which are standards adopted by the photovoltaic industry to ensure consistent test conditions and are similar to the light levels expected in North America. Regions for ultraviolet, visible and near-infrared are indicated. Cited and revised from Figure 2 of reference 3.
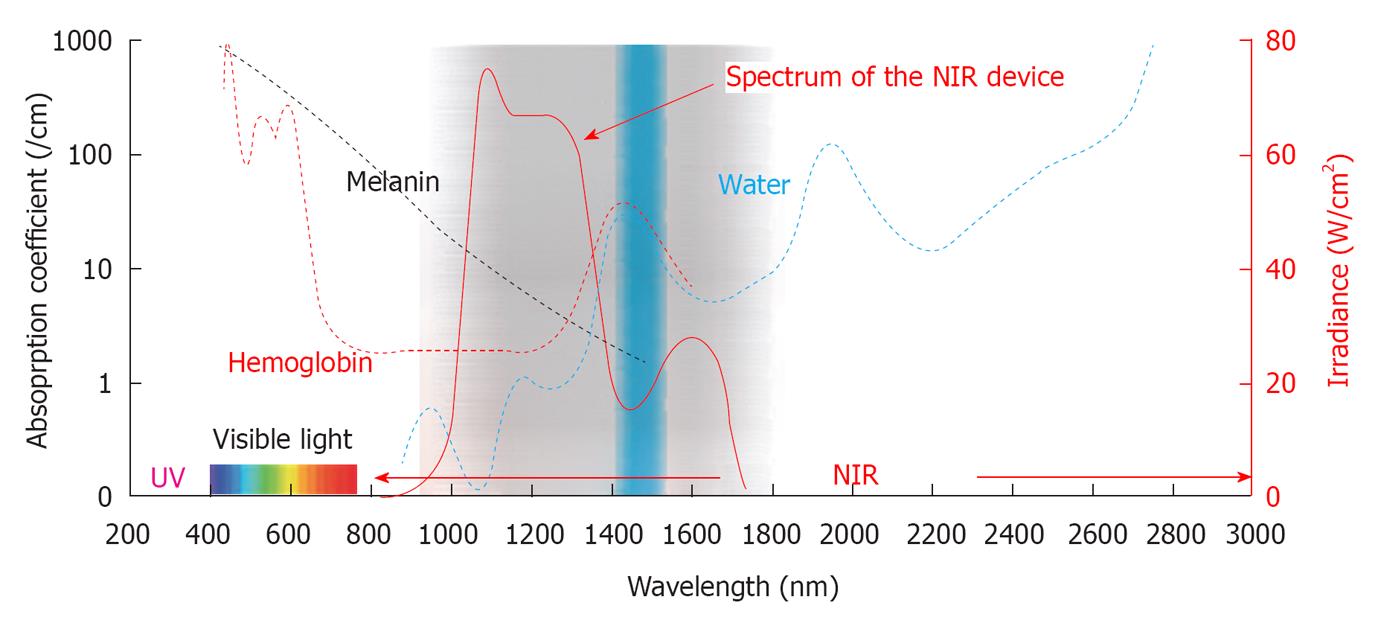
In order to simulate solar NIR that reaches the skin, a water filter is essential because solar NIR is filtered by atmospheric water. I used a NIR device that emitted a spectrum of NIR irradiation from 1100 to 1800 nm with a water-filter that excludes wavelengths between 1400 and 1500 nm, which are strongly absorbed by water and hemoglobin (Figure 3).
Figure 3 The absorption coefficients and wavelength of the near-infrared device.
This graph shows the absorption coefficients of melanin (brown), hemoglobin (red) and water (blue). The near-infrared (NIR) device used in our study emits a spectrum of NIR from 1100 to 1800 nm (bold red), with filtering of wavelengths between 1400 and 1500 nm (blue belt) that are strongly absorbed by water and hemoglobin. Cited and revised from Figure 2 of reference 3.
Wavelengths below 1100 nm are preferentially absorbed by melanin in the superficial layers of the skin. Wavelengths between 1400 and 1500 nm and those above 1850 nm are absorbed heavily by water in the superficial layers of the skin, which results in heating and can lead to painful sensations and burns[20]. Filtering out the wavelengths below 1100 nm, around 1450 nm and above 1850 nm enabled the delivery of NIR irradiation to deeper tissues[39] and also simulated solar NIR radiation that reaches the skin of humans on the earth’s surface. Therefore, a NIR device with a water-filter mimics the natural situation and allows the evaluation of solar NIR radiation that reaches the skin. However, the biological effects induced by near sub-region of IR radiation could not be evaluated with this NIR device. Further studies in near sub-region of IR are needed.
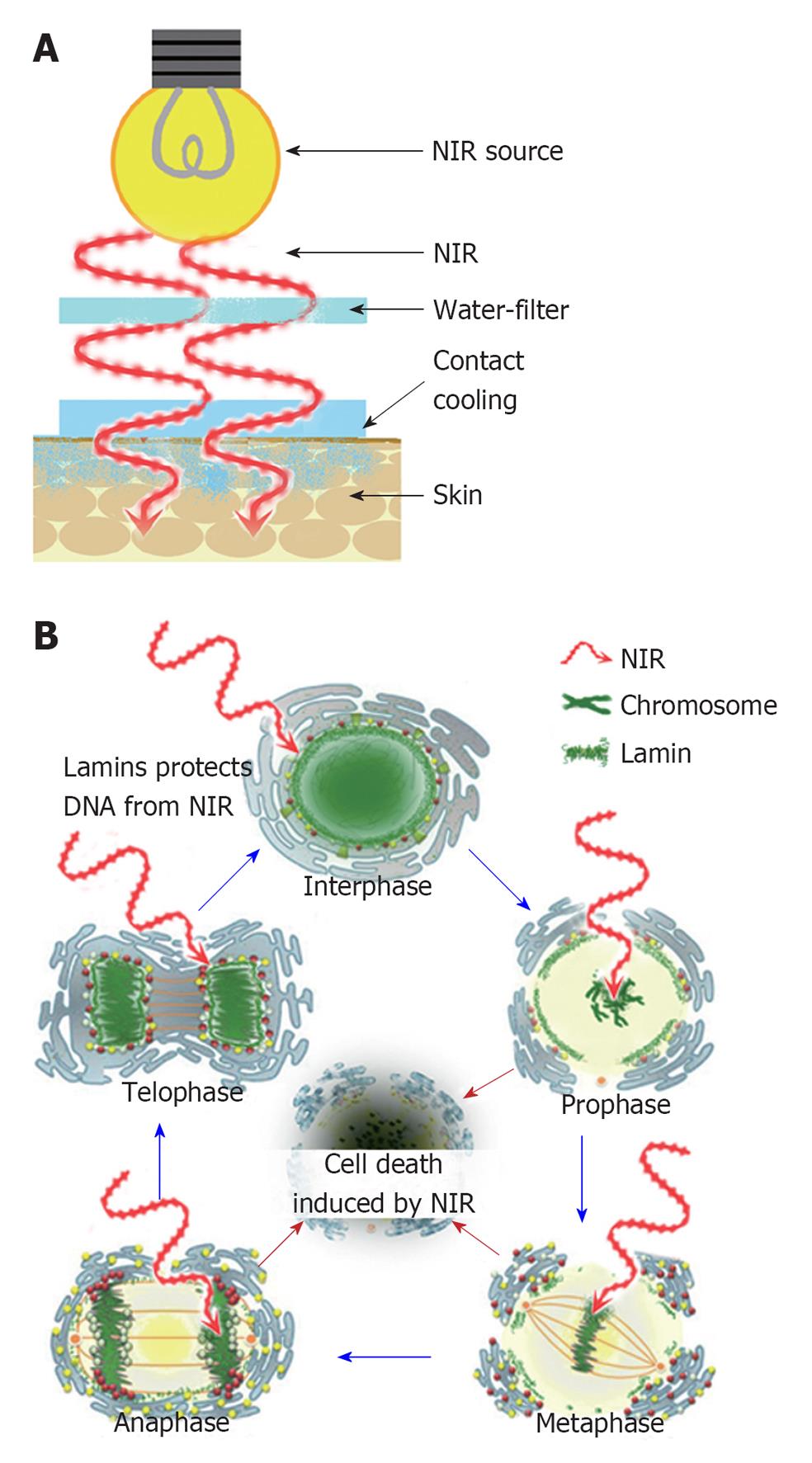
In reality, NIR increases the surface temperature and induces thermal effects so a contact cooling is needed to pursue the properties of NIR. Contact cooling through a temperature-controlled sapphire window was used to reduce the skin surface temperature and reduce perspiration and blood vessel dilation (Figure 4A).
Figure 4 A schematic of my near-infrared research and cell cycle.
A: A schematic of my near-infrared (NIR) research; B: A schematic of the cell cycle and effects of NIR. NIR cannot penetrate the nuclear envelope due to the protection of nuclear lamins in interphase and telophase. NIR may damage the chromosomes of mitotic cells in prophase, metaphase and anaphase due to the absence of nuclear lamin protection, which results in apoptotic cell death. Cited and revised from Figure 15 of reference 5.
These specific wavelengths and the cooling system enabled NIR to be delivered to the deeper tissues without pain or epidermal burns[39,40], which was evidenced by the ability to treat animals and humans without anesthesia and without contact burns or other adverse events.
Therefore, I found various non-thermal biological effects of NIR[1-6,9-14].
DISCUSSION
Properties of NIR
NIR is an electromagnetic wave that simultaneously exhibits both wave and particle properties and is strongly absorbed by water, hemoglobin and myoglobin[2]. As a consequence, NIR irradiation can penetrate the skin and affect the subcutaneous tissues, including muscles and bone marrow, with both its wave as well as its particle properties.
The penetrating 600-1300 nm wavelength region causes photochemical changes and affects a large volume and depth of tissue[7]. Actively proliferating cells show increased sensitivity to red and NIR[41,42]. NIR irradiation induces strand breaks and apoptosis[15], as well as cell death of cancer cells and bone marrow cells[12-14]. NIR irradiation is used as a therapeutic option in the treatment of wound healing disorders[16-18] and malignant tumors[19-22]. While NIR irradiation appears to damage tumor tissue, it has also been shown to reduce cellular protein damage produced by biological oxidants in normal cells[43].
We also reported that NIR irradiation was shown to thermally induce the expression of collagen[9], elastin and water-binding proteins[1,9] without scar formation[10]. Furthermore, NIR irradiation non-thermally induced long-lasting muscle thinning[3], muscle relaxation[4], bone marrow damage[12], a cytocidal effect on cancer cells[13,14], stimulation of stem cells[5,12] and DNA damage[13,14] of mitotic cells[5].
Biological effects of NIR on human skin
The biological effects of NIR have both merits and demerits. The dermis tends to increase the amount of fluid by inducing an increase in collagen, elastin and water-binding protein in order to protect subcutaneous tissues from NIR[1,9]. Pre-exposure of NIR prevents UV-induced toxicity[29,44,45] and this effect is independent of heat shock protein induction and cell division[45]. These findings suggest that NIR irradiation prepares skin to better resist the subsequent damage of UV or NIR.
In contrast, similar to UV, NIR seems to exert biological effects on human skin[23]. NIR irradiation was shown to cause skin changes similar to those observed in solar elastosis and enhanced UV-induced dermal damage[35]. NIR irradiation is able to activate mitogen-activated protein kinases and induce gene transcription and is likely to increase collagen degradation[23,24,46]. Epidemiological data and clinical reports point to the ability of NIR to cause and enhance actinic skin damage, implying that NIR is not innocuous to human skin[23,47,48].
The mean facial surface area that is covered with wrinkles is significantly smaller in African Americans than in Caucasians and characteristics of age-related periorbital changes seem to occur at a more accelerated rate in Caucasians[49]. In addition, fair skin is more sensitive to skin aging[50,51]. These findings support the observation that fair skin tends to wrinkle and sag earlier in life[52,53] because fair skin is thinner and is more susceptible to NIR damage to the underlying frontalis, orbicularis oculi and platysma muscles than dark skin[5,6]. NIR is attenuated by thick water-containing dermis. Thus, skin with sparse melanin and a thin dermis might allow NIR radiation to penetrate deeper into human tissue than skin with dense melanin and a thick dermis[5,6].
Repeated exposure to sources of heat and NIR, such as fires and stoves, results in a skin lesion described as erythema ab igne[54], which is clinically characterized by a reticular hyperpigmentation and teleangiectasia accompanied histologically by epidermal atrophy, vasodilation and dermal melanin and hemosiderin deposits. After many years, these lesions may develop thermal keratoses, such as hyperkeratosis, keratinocyte dysplasia and dermal elastosis, which are similar to the changes that occur in actinically damaged skin[55]. Similar to actinic keratoses, thermal keratoses are precancerous lesions that exhibit epidermal dysplasia, which may develop into invasive squamous cell carcinoma. There are several reports of carcinomas arising from heat induced erythema ab igne[47,56,57]. NIR radiation, similar to UV radiation, induces photoaging and potentially photocarcinogenesis[23]. In addition, skin tumors in mice appeared faster after irradiation with the full lamp spectrum containing UV, visible and NIR compared to irradiation with UV alone[58].
Biological effects of NIR on cancer cells
Wavelength of NIR anticancer therapy: Photodynamic therapy (PDT) is the most common antitumor therapy using IR for select forms of cancer[59]. PDT is based on the accumulation of a photosensitizing agent in tumors and uses wavelengths near 800 nm as a photoactivating wavelength to achieve maximum penetration depth[19,22,60]. This wavelength, however, also has high melanin absorption, which limits the ability to deliver light to highly pigmented tumors[61].
Although wavelengths near 800 nm are the standard activators for PDT, other wavelengths have also shown treatment promise. Santana-Blank et al[62] reported that NIR at 904 nm may have antitumor activity, as shown by an increase in cytomorphological changes, as well as apoptosis in neoplastic cells. Unlike wavelengths beyond 1100 nm where melanin absorption is negligible[7], absorption at 904 nm was significant. This may limit the possible uses of the 904 nm wavelength for certain body areas in races with skin that is rich in melanin.
Although many studies have shown the thermal effects of NIR irradiation on cancer cells in the field of hyperthermia, non-thermal effects of NIR irradiation were not investigated in detail. We first reported on the non-thermal effects of NIR using a specialized broad spectrum light source emitting light between 1100-1800 nm (with a filter to exclude wavelengths between 1400 and 1500 nm) on cancer cells and suggested the possibility of beneficial uses for cancer treatment[13,14]. However, further studies are needed to evaluate variations in treatment parameters and conditions, which will enable development of procedures that achieve maximum results while providing the greatest margin of safety.
Biological effects of NIR on in vivo cancer studies: The histological findings showed tumor shrinkage and dying cells in the center of the tumor mass, which supports that NIR electromagnetic properties induce these biological effects non-thermally. If the cytocidal effect of NIR was induced thermally, the histology would show a gradient cytocidal effect from the superficial layer to the center of the tumor and the thermal effect would be reduced by the contact cooling (20 °C) of the NIR device. Due to surface cooling, NIR can penetrate deeper tissue and induce a drastic non-thermal cytocidal effect in the center of the tumor mass[13].
A significant reduction in tumor volume and a high level of TUNEL-positive cells in the irradiated group indicated that NIR irradiation induces apoptosis in cancer cells. However, the mechanism of NIR-mediated tumor cell death appeared to be different than standard apoptosis because high levels of activated caspase-3 expression and ssDNA-positive cells appeared gradually after NIR irradiation, although tumor shrinkage happened rapidly.
On the other hand, NIR irradiation induced the stimulation of CD34-positive bone marrow stem cells in our previous study[12] and the frequency of Ki67-positive cells on day 45 was significantly higher than the irradiated group on day 9. These results suggest that NIR irradiation may stimulate stem cells.
The immunohistological staining results suggested that NIR may induce cell death of highly proliferative tumor cells, stimulate stem cells and then induce apoptosis of the cells which are unnecessary to promote the development of melanoma. These steps appeared to be a part of the mechanism driving the effects of NIR on cancer cells.
Biological effect of NIR on molecular structure
NIR is absorbed by water, hemoglobin and myoglobin. The NIR spectrum of biological materials is a result of the overtones and combination of O-H, C-H and N-H groups’ bond stretching vibrations[63]. Water is a polar molecule with an electrical dipole moment and possesses hydrogen bonds. A water molecule will be resonated by NIR and absorb NIR due to the O-H intramolecular hydrogen bonds and electrical dipole moment[64]. Since T2 weighted MRI enhances water as well as active proliferating cancer cells, active proliferating cells may have a rich water content, which strongly absorbs NIR[5].
Hemoglobin has four heme-binding subunits, each largely made of α helices, and myoglobin consists of eight α helices that are connected through turns with an oxygen binding site. The similarity between hemoglobin and myoglobin resides in the heme binding sites and α helices. Heme is a prosthetic group that consists of an iron atom located in the center of a large heterocyclic organic ring called porphyrin. Our results of long-lasting muscle thinning and vasodilation induced by NIR suggest that NIR might resonate and damage heme. However, our collagen, elastin and cancer studies suggest that NIR may mainly resonate helical structures, α helices and DNA. α helices are thought to be resonated by NIR and have strong amide bands in the IR spectra, which have characteristic frequencies and intensities[65]. Both hemoglobin and myoglobin are the oxygen-carrying proteins and have many α helices. It is possible that NIR induces resonance of α helices in the oxygen-carrying proteins and degenerates proteins containing α helices, which results in damage to the storage and transport of oxygen. This could be one of the mechanisms of apoptosis. In our previous study, we evaluated the effect of NIR on myoglobin; however, similar effects may also be found for hemoglobin[2].
NIR increases the amount of water retained in the dermis by inducing vasodilation and the expression of collagen and elastin[1]. Both collagen and elastin possess helical structures and hydrogen bonds. Elastin has higher absorption properties than that of water[64]. These findings suggest that we have acquired biological defense mechanisms in which induced helical structures and hydrogen bonds are resonated by NIR and absorb NIR to protect the subcutaneous tissues against NIR.
Similarly, DNA consists of two long strands in the shape of a double helix, which is stabilized by two forces: hydrogen bonds between nucleotides and base-stacking interactions among the aromatic bases. Many studies regarding DNA and cancer imaging have been performed using a NIR spectroscopy since biological molecules such as proteins, lipids and nucleic acids provide a unique absorption spectral pattern and NIR induces the vibration of DNA. IR irradiation alone appears to induce DNA strand breaks and apoptosis[15]. DNA will be also resonated and absorb NIR, which is most likely due to its helical structures and hydrogen bonds.
Biological effects of NIR on lamin
The nuclear lamina is a proteinaceous structure located underneath the inner nuclear membrane that forms a stress-resistant elastic network where it associates with the peripheral chromatin[66]. It contains lamins and lamin-associated proteins, including many integral proteins of the inner nuclear membrane, chromatin modifying proteins, transcriptional repressors and structural proteins[67-70].
Lamins are type-V intermediate filament proteins located in the nucleus, primarily in the periphery, and underlie the nuclear envelope[71]. Lamins have a conserved α helical central rod domain and variable head and tail domains[66,72,73]. α helical structures are surmised to absorb NIR and protect the nucleus and DNA from NIR.
Lamins play important roles in DNA replication, chromatin organization, adult stem cell differentiation, aging and tumorigenesis. In addition, mutations in lamin lead to laminopathic diseases[66]. Nuclei assembled in vitro in the absence of lamins are more prone to breakage than nuclei assembled in the presence of a full complement of lamins[74,75]. Disruption of the lamins results in abnormal mitosis, chromosomal segregation and cell death[76].
During mitosis, lamin molecules are transiently disassembled into monomers[77,78] through phosphorylation[79] by the protein kinase p34cdc2[80]. In addition, actively proliferating cells show increased sensitivity to NIR[41,42] and IR irradiation induces DNA strand breaks and apoptosis[15]. Therefore, these findings suggest that NIR exposure appears to damage nuclear lamins and DNA in the mitotic phase due to absence of nuclear lamins protection, which results in apoptotic cell death[5]. Thus, NIR induces non-thermal DNA damage of mitotic cells in prophase, metaphase and anaphase due to the absence of nuclear lamin protection, which may have the potential application for treating various forms of cancer[13,14] (Figure 4B).
Biological effect of NIR on stem cells
NIR irradiation abruptly induced subcutaneous adipocytes on the panniculus carnosus and CD34-positive cells around the subcutaneous adipocytes[12]. Adipose-derived stem cells express CD34 in higher percentages than bone marrow-derived mesenchymal stem cells[81]. CD34-positive human adipose-derived stem cells have a greater replicative capacity compared to CD34-negative cells[82]. These results suggest that NIR irradiation may enrich and stimulate CD34-positive adipose-derived stem cells to increase subcutaneous adipocytes on the panniculus carnosus.
Optically, fatty tissue can scatter NIR[83] and fatty acids are the major NIR absorbing materials in soft tissues[64]. The oil in the liquid phase is transparent, whereas the oil in the solid phase is highly scattering to NIR[84]. The long-lasting induction of subcutaneous adipocytes may protect the underlying tissues, including the panniculus carnosus, against NIR damage.
NIR irradiation that simulated solar radiation non-thermally affected the subcutaneous tissues, cortical bone and bone marrow[12]. The apoptotic damage to bone marrow cells might be minimized by a biological defense against NIR irradiation by means of an increase in subcutaneous and bone marrow adipocytes, as well as cortical bone mass through the enrichment of CD34-positive stem cells at the inner surface of the bone cortex.
Lamin A and pre-lamin A regulate stem cell maintenance and differentiation by influencing key signaling pathways in stem cells[66]. Lamin A/C expression seems to be reduced or absent in undifferentiated or proliferative cells but is observed in differentiated or non-proliferative cells, such as quiescent adult stem cells[85]. Lamin A regulates stem cell maintenance through a range of regenerative signaling pathways, which suggests that the regulation of adult stem cell aging may occur at a number of different pathway steps that intersect with lamin A, including adult stem cells, their progenitors and/or stem cell niches[85]. These results suggest that NIR radiation may stimulate stem cells, including cancer stem cells[5].
CONCLUSION
In order to simulate solar NIR that reaches the skin and to pursue the properties of NIR, a water filter and contact cooling are essential for a NIR source.
Appropriate NIR irradiation induces dermal heating thermally and non-thermally induces collagen and elastin stimulation, which results in skin laxity tightening. NIR also induces non-thermal DNA damages of mitotic cells in prophase, metaphase and anaphase due to the absence of nuclear lamin protection. NIR irradiation might have a potential application for treating various forms of cancer, including highly proliferative cells, since the schedule reduces discomfort and side effects, reaches the deep subcutaneous tissues and facilitates repeated irradiations.
In contrast, solar NIR radiation may also cause unexpected muscle thinning and stimulation of stem cells, including cancer stem cells, in areas of the body that are exposed to the sun. Although various kinds of sunscreen materials are often used to prevent skin damage from UV exposure, these materials cannot block visible light or NIR.
Therefore, exposed skin should be protected with sunscreens that block not only UV, but also NIR radiation, in order to prevent overlying skin ptosis, photoaging and oncogenicity. Additional non-thermal studies are required to decipher the biological effects of NIR in humans.