Published online Sep 18, 2018. doi: 10.5312/wjo.v9.i9.173
Peer-review started: April 10, 2018
First decision: May 15, 2018
Revised: May 20, 2018
Accepted: June 27, 2018
Article in press: June 27, 2018
Published online: September 18, 2018
Processing time: 158 Days and 19.6 Hours
To perform an Internet based survey on the surgical management of bone sarcomas in the lower extremity amongst sarcoma surgeons.
All orthopedic surgical members of the Scandinavian Sarcoma Group were invited to participate in an online questionnaire. The questionnaire consisted of a clinical case involving resection of a malignant bone tumor. Several questions were asked, subdivided into categories. Among these, surgical/technical considerations, e.g., choice of implant; choice of antibiotics, dosage, and duration of treatment, choice of antithrombotic drug, initial start-up, dosage, and duration were included.
In terms of choice of implant fixation, the majority of surgeons preferred an uncemented prosthesis in younger patients until the age of 50. All participants administer intravenous prophylactic antibiotics for endoprosthetic reconstructive surgery. First choice of antibiotics was cephalosporin. Less common used was glycopeptide, penicillin, or a combination. Duration of prophylactic antibiotics ranged from less than one day to more than four days. All participants used low molecular weight heparins as antithrombotic prophylaxis and 55% of the participants answered that initial treatment was started preoperatively, 3% perioperatively and 42% postoperatively. Duration of the antithrombotic treatment ranged from five days to more than twenty-eight days.
The use of resection prosthesis in the treatment of bone sarcomas is a well-established procedure. However, therse is a significant discrepancy in the surgical treatment algorithm between the sarcoma centers. Still the treatment is mainly based on best clinical practice, due to the lack of evidence-based medicine in the surgical management of bone sarcomas.
Core tip: Today the majority of patients diagnosed with bone sarcomas located in the lower extremities are offered reconstruction with a megaprosthesis. However, no clear golden standard is available in the international surgical oncology community with regard to choice of implant, choice of antibiotics, dosage, and duration of treatment, or choice of antithrombotic drug, initial start-up, dosage, and duration. The current study reveals a clear lack of consensus.
- Citation: Baad-Hansen T, Freund SS, Bech BH, Keller J. Is there consensus regarding surgical treatment of bone sarcomas? World J Orthop 2018; 9(9): 173-179
- URL: https://www.wjgnet.com/2218-5836/full/v9/i9/173.htm
- DOI: https://dx.doi.org/10.5312/wjo.v9.i9.173
Evidence-based surgical treatment of bone sarcomas is challenged by a limited number of patients and a considerable variation in anatomical location of the bone tumors. This is in contrast to the non-surgical treatment of bone sarcomas, where standard protocols are available to test new treatment regimes because the location of the sarcoma is of less importance in this context[1,2]. Guidelines for surgical management of hip and knee replacement due to more prevalent disorders such as osteoarthritis are available from most national hip and knee societies based on randomized clinical trials and large-scale database studies[3,4]. This level of evidence is difficult to achieve dealing with endoprosthetic reconstructive surgery in osteosarcoma patients[5].
Since 2013 a randomized multicenter study, PARITY, has been ongoing with the aim to determine whether a five-day regimen of post-operative antibiotics, in comparison to a single day regimen, will change the risk of postoperative infection in patients receiving mega prosthesis[6,7]. The trial still lacks the inclusion of more than 600 patients and seven countries with a total of 35 different sarcoma centers are including patients. Such a multicenter study is time consuming, and it can be questioned if the results can be generalized to the rest of the world due to different antibiotic national policies.
The aim of the present study was to identify differences in surgical treatment strategies for patients with bone sarcomas in the lower extremities between the Scandinavian countries based on best clinical practice. The study focuses on three main areas: (1) Surgical-technical considerations; (2) use of antibiotics; and (3) antithrombotic treatment.
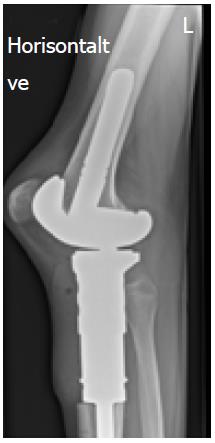
All orthopedic surgical members of the Scandinavian sarcoma group (SSG) were invited to participate in an online questionnaire. The questionnaire consisted of a clinical case involving resection of a malignant bone tumor located in the proximal tibia, reconstruction with a megaprosthesis and a gastrocnemius myocutaneous flap coverage (Figure 1). Several questions were asked, subdivided into categories, among these, surgical/technical considerations, e.g., choice of implant, choice of antibiotics, dosage, and duration of treatment, and choice of antithrombotic drug, initial start-up, dosage, and duration. A complete list of the questions can be seen in supplemental Appendix 1.
The participants could add supplementary comments to certain questions. No participants were financially compensated for completion of the survey.
A total of 42 surgeons were asked to participate. Five did not return the questionnaire; two declined to answer due to inadequate clinical experience. A total of 35 participants returned a complete survey resulting in a response rate of 83%, eight from Denmark (23%), six from Finland (17%), one from Iceland (3%), seven from Norway (20%), and thirteen from Sweden (37%). The following hospitals were represented: Aarhus University Hospital, Rigshospitalet, Copenhagen, Karolinska University Hospital, Linköping University Hospital, Sahlgrenska University Hospital, University Hospital of Umeå, Skåne University Hospital, Oslo University Hospital, Radiumhospitalet, Haukeland University Hospital, Tampere University Hospital, Helsinki University Hospital and University Hospital of Iceland.
About 50% of the participants had worked as orthopedic oncologists for more than ten years. Female: male ratio was 1:7.
With regard to preoperative planning, 47% of the participants indicated that they would always perform a preoperative templating in order to determine prosthesis size and anatomical position whereas 25% answered sometimes and 28% answered that they would never perform templating. No differences between centers or countries were observed with regards to templating.
In case a needle biopsy was done prior to the surgical procedure, 66% indicated that they would excise the biopsy tract. However, in case an open biopsy was performed there was full consensus amongst all sarcoma centers to excise the open biopsy tract if surgically feasible.
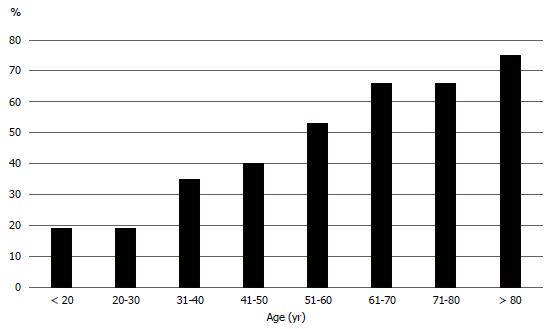
In terms of choice of implant fixation, the majority of surgeons preferred an uncemented prosthesis in the younger patients until the age of 50, whereas a cemented prosthesis was the preferred implant from age 51 and up (Figure 2).
There was an overall agreement between countries to select uncemented fixation for the younger patients, however the Danish and Swedish sarcoma centers tended to consider a cemented prosthesis also to the youngest patients below 20 years old. Several reservations were noted with regard to the implant choice. The following comments were obtained. “It depends on the individual bone stock, length of resection,” “routinely we will use uncemented prosthesis in the absence of osteoporosis and if postoperative radiotherapy is not considered,” and “We do not use uncemented prosthesis”.
In patients receiving a proximal tibia prosthesis, 72% of the participants would apply a drain. Removal of the drain was dependent on two factors: time or drain production, or a combination of the two. The majority of the participants (67%) said that they would remove the drain after 3-4 d, whereas 33% of the participants would remove the drain 1-2 d postoperatively. Looking at drain production as a parameter, 82% of the participants would remove the drain when production was less than 25-50 mL/d. With regard to drain usage, no clear pattern could be observed between countries or within individual institutions. Several comments on this topic were made: “Less than 50 mL/d or before the fifth day,” “Especially if a drain is needed in the area of the calf where the gastrocnemius muscle is rotated I will leave it for a longer time until drain production is less than 10 mL/d,” and “Max time for drain is 7 d, even if the output is > 50 mL/d”.
All participants confirmed that they administer intravenous prophylactic antibiotics for endoprosthetic reconstructive surgery. First choice of antibiotics was cephalosporin, second was penicillin followed by glycopeptide or a combination of the above mentioned. According to the answers, the antibiotic prophylaxis was initiated preoperatively by 71% of the participating surgeons and was initiated perioperatively by 29% of participants. In contrast to the sarcoma centers in Finland, Norway and Sweden, who used cephalosporin, the predominantly used prophylactic antibiotic in Denmark and Iceland was penicillin. Duration of prophylactic antibiotics ranged from less than one day to more than four days. Thirty-nine percent of the participants stated that antibiotics were used for 1-2 d. Lastly, 32% of the participants answered that they used antibiotics until drain removal. Comments were added: ”Until the surgical wound is completely dry and drain has been removed,” “Only one dose if there has been limited bleeding during surgery, and an extra dose if the bleeding has been substantial,” “3 types of antibiotics. If surgery is prolonged the regime is altered and may be given for 2-3 d,” ” If drainage is used, antibiotics will be prolonged,” and ”1-2 d sometimes longer, depending on if splitskin-graft is needed”.
The majority of the participants (58%) would use some kind of nerve block. Of these, the most common pain management strategy was an epidural catheter (45% of participants). Less commonly, a peripheral catheter was used by 22%, and a combination of the above was indicated by 33%. All answers revealed duration of epidural and/or peripheral catheters of 2-4 d.
All participants presented a united front for the use of antithrombotic treatment. All participants preferred low molecular weight heparins. Antithrombotic treatment was initiated preoperatively by 55%, perioperatively by 3%, and postoperatively by 42%. Duration of the antithrombotic treatment ranged from 5-7 d (13% of the participants) to more than 28 d (6% of the participants). Most of the participants answered 8-14 d (58%) and 23% answered 15-28 d. It was stated several times that duration of the antithrombotic therapy also depended on the degree of mobilization.
The participants were asked to indicate at which time full weight bearing was allowed in the case of insertion of an uncemented prosthesis. Exclusively looking at the fixation method, 32% answered within a week postoperatively, 10% answered one to six weeks after surgery, 48% answered seven to twelve weeks after surgery, and finally 10% answered more than twelve weeks after surgery. In contrast, 52% of the participants allowed full weight bearing of a cemented prosthesis within the first week after surgery. This pattern was observed in all sarcoma centers. Some participants raised concerns due to postoperative chemotherapy and the re-insertion of the patella tendon leading to delayed full weight bearing.
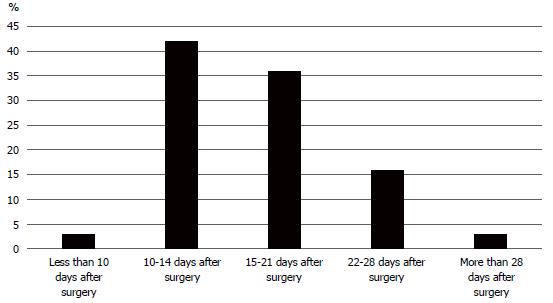
In terms of initiation of postoperative chemotherapy, a clear difference between sarcoma centers was seen. The majority of surgeons in the Swedish and Norwegian centers allowed commencement of chemotherapy 10-14 d after surgery, whereas centers in Denmark and Finland waited up to 28 d postoperatively. Figure 3 shows the results from all participants with regard to delay of postoperative chemotherapy.
The European Society for Medical Oncology (ESMO) provides Clinical Practice Guidelines for diagnosis, treatment and follow-up of bone sarcomas. However, the surgical procedures are only briefly discussed[8]. The lack of surgical recommendations is displayed in the results from the present survey revealing great heterogeneity in the surgical treatment strategy of bone sarcomas located in the proximal tibia. However, there is also some agreement between surgeons (e.g., in choice of implant fixation, use of antibiotics, and antithrombotic treatment). The individual surgeon´s preferences may be influenced by knowledge based on clinical trials almost similar to reconstruction with tumor-prostheses (e.g., primary or revision knee surgery and national recommendations for endoprosthetic joint replacement)[9,10]. Furthermore, based on the participant’s statements in the results paragraph, previous personal experiences and clinical tradition may also influence the clinical decision-making.
Some surgical procedures are widely accepted as reference standards. For decades, there has been consensus regarding removal of the biopsy tract during surgery, grounded in the theory that the biopsy tract is potentially contaminated by tumor cells[11]. According to Mankin et al[11], 15 out of 329 patients experienced what might be unnecessary amputation due to unfavorable location of the biopsy, compromising definitive reconstruction procedures. This assumption is based more on empirical knowledge than on scientific data[12]. The above-mentioned standard is in accordance with the ESMO guideline for Clinical Practice Guidelines for diagnosis, treatment and follow-up of bone sarcomas stating that the biopsy tract from an open biopsy must be considered to be contaminated with tumor cells and must be removed together with the resection specimen to avoid local recurrences. However, no randomized studies have been published to support this widely accepted procedure.
A recent systematic review by Oliveira et al[13] reports that tumor contamination along the biopsy tract in musculoskeletal cancer can cause local recurrence and lead to an unfavorable prognosis. However, it was not possible for the authors to conclude whether a needle biopsy is associated with a lower risk of contamination than an open biopsy. Nevertheless, only 66% of the asked participants in the actual survey would excise the needle biopsy tract in contrast to an open biopsy, whereas all participants would remove the open biopsy tract.
The risk of developing a deep postoperative infection in sarcoma patients undergoing large endoprosthetic reconstructions is high compared with conventional total joint replacement[14]. The reason for this is multifactorial. Immune compromised patients undergoing complex and protracted procedures increase the risk of complications[15].
The absence of a standard practice for antibiotic treatment has led to an attempt to launch an international multi-center RCT study including patients with lower extremity bone tumors undergoing endoprosthetic reconstruction, randomized to receive either cefazolin a single day post-operatively or five days post-operatively. Due to the low incidence of primary bone tumors, the study requires a sample size of approximately 600 patients, demanding inclusion of multiple sarcoma centers. Local variations in factors such as bacterial or fugal prevalence, e.g., Methicillin-resistant Staphylococcus aureus, Mycobacteria or Candida. Lack of consensus with regard to choice of implant (some manufactures provide silver or gentamycin coating) complicates the study[16]. Furthermore, cefazolin is not standard practice or available in all countries leading to a reduced number of potential participating centers. Finally, patient-related and technical concerns must be taken into account when addressing type and duration of antibiotic treatment, which also is supported by the statements of the participants of the present survey. The fact that a nearly equal percentage of orthopedic surgeons participating in our study chose the duration of prophylactic antibiotics to be from less than one day to more than four days (39%) emphasizes the need for supporting new RCTs.
According to a study by Husted et al[17], a high degree of agreement in the clinical setup of patients undergoing fast track primary knee endoprosthetic surgery in four large orthopedic institutions has been described. With regard to deep venous thrombosis prophylaxis in this patient group, the treatment was initiated 6-8 h postoperatively and continued until discharge or up to seven days postoperatively. The clinical setup in primary total knee arthroplasty (TKA) surgery, however, differs significantly from patients undergoing tumor resection and reconstruction with a megaprosthesis and a direct comparison cannot be done. Patients receiving a primary TKA are most likely to be mobilized on the same day as the index surgery or at least on the first postoperative day. In contrast, patients treated with a resection prosthesis of the proximal tibia will need soft tissue reconstruction and demands complete immobilization to achieve soft tissue healing. Furthermore, cancer patients have an increased risk of a venous thromboembolism event with a hazard ratio of 4.7 compared to the background population[18]. The current study demonstrates a clear consensus amongst the participating surgeons regarding the need for venous thrombosis prophylaxis in the treatment of bone sarcomas. In contrast, absolutely no agreement was seen with regard to time for initiating treatment or duration of venous thrombosis prophylaxis. Approximately half of the participants started initial thrombosis prophylaxis preoperatively, and duration of the treatment ranged from 5-28 d.
The current study has some limitations. National legislations may influence clinical practices (e.g., choice of antibiotics). A restrictive antibiotic policy will cause utilization of small spectrum antibiotics, leading to problematic direct comparison between countries. Furthermore, geographic variation in the prevalent bacterial/fungal species will require the surgeons to address a potential infection based on the local microbiological species. In the current study the choice of antibiotics is based on Scandinavian preferences, which potentially can lead to a reduced external validity.
In conclusion, the use of resection prosthesis in the treatment of bone sarcomas is a well-established procedure providing good functional outcomes and acceptable complication rates. However, the clinical considerations that follow management of bone sarcomas patients are predominantly based on best clinical practice and to a lower extent on evidence-based knowledge, which leads to a substantial variation in treatment protocols. In order to gain evidence-based knowledge, international multicenter studies have been initiated but are challenged by national heterogeneity with possible consequences for the generalizability of their conclusions.
Reconstructive surgery using megaprosthesis is widely applied as a treatment for bone sarcomas of the lower extremities. Even though reconstructive surgery has been used for several decades it seems that there is no clear consensus in surgical treatment strategies for this group of patients.
The low number of bone sarcomas limits the possibility to run randomized clinical trials. This is in contrast to non-surgical treatment of bone sarcomas, where standard protocols are available to test new treatment regimes since the sites of the sarcoma are of less significance. Only a few international randomized studies have been initiated to gain new evidence on the surgical treatment of bone sarcoma patients. This is due to the difficulty in achieving sufficient sample sizes due to the rarity of the disease.
The goal of this study was to identify common strategies in the surgical treatment of patients with bone sarcomas of the lower extremities in terms of surgical/technical considerations, choice of antibiotics, dosage, and duration of treatment, and choice of antithrombotic drug, initial start-up, dosage, and duration.
The study was based on an internet-based survey on the surgical management of bone sarcomas in the lower extremity amongst sarcoma surgeons in the Scandinavian countries.
This study demonstrates a large variation in the treatment of patients with bone sarcomas located in the lower extremities. With regard of implant fixation, the Danish and Swedish sarcoma centers tended to consider a cemented prosthesis not only for the older patients but also for the youngest patients below 20 years old, contrary to the rest of the Scandinavian centers. All participants would administer intravenous prophylactic antibiotics regarding endoprosthetic reconstructive surgery. First choice of antibiotics was cephalosporin. Less commonly used were glycopeptide, penicillin, or a combination. The duration of prophylactic antibiotic treatment ranged from less than one day to more than four days. All participants would administer heparins as antithrombotic prophylaxis. Fifty-five percent of the participants answered that initial treatment was started preoperatively, 3% perioperatively and 42% postoperatively. Range of antithrombotic treatment went from 5-28 d.
Today, patients diagnosed with bone sarcomas of the lower extremity are to a great extent offed treatment with a resection prosthesis. The treatment is well established, however, there is a significant inconsistency in the surgical treatment algorithm between sarcoma centers. Still the treatment is primarily based on best clinical practice, due to the absence of evidence-based medicine in the surgical management of bone sarcomas.
The current study elucidates, that the surgical sarcoma community needs to support the ongoing randomized control trials and encourage the initiation of new randomized studies to gain knowledge of the surgical treatment of bone sarcoma patients based on evidence-based medicine.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country of origin: Denmark
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Elgafy H, Li JM S- Editor: Cui LJ L- Editor: Filipodia E- Editor: Song H
| 1. | Juergens C, Weston C, Lewis I, Whelan J, Paulussen M, Oberlin O, Michon J, Zoubek A, Juergens H, Craft A. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr Blood Cancer. 2006;47:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 214] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Schuetze SM, Wathen JK, Lucas DR, Choy E, Samuels BL, Staddon AP, Ganjoo KN, von Mehren M, Chow WA, Loeb DM. SARC009: Phase 2 study of dasatinib in patients with previously treated, high-grade, advanced sarcoma. Cancer. 2016;122:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Tjørnild M, Søballe K, Hansen PM, Holm C, Stilling M. Mobile- vs. fixed-bearing total knee replacement. Acta Orthop. 2015;86:208-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Gundtoft PH, Pedersen AB, Varnum C, Overgaard S. Increased Mortality After Prosthetic Joint Infection in Primary THA. Clin Orthop Relat Res. 2017;475:2623-2631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 5. | Evaniew N, Nuttall J, Farrokhyar F, Bhandari M, Ghert M. What are the levels of evidence on which we base decisions for surgical management of lower extremity bone tumors? Clin Orthop Relat Res. 2014;472:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Ghert M, Deheshi B, Holt G, Randall RL, Ferguson P, Wunder J, Turcotte R, Werier J, Clarkson P, Damron T. Prophylactic antibiotic regimens in tumour surgery (PARITY): protocol for a multicentre randomised controlled study. BMJ Open. 2012;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Hasan K, Racano A, Deheshi B, Farrokhyar F, Wunder J, Ferguson P, Holt G, Schwartz H, Petrisor B, Bhandari M. Prophylactic antibiotic regimens in tumor surgery (PARITY) survey. BMC Musculoskelet Disord. 2012;13:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | ESMO/European Sarcoma Network Working Group. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii113-iii123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 9. | Kishida Y, Sugano N, Sakai T, Nishii T, Haraguchi K, Ohzono K, Yoshikawa H. Full weight-bearing after cementless total hip arthroplasty. Int Orthop. 2001;25:25-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Strange S, Whitehouse MR, Beswick AD, Board T, Burston A, Burston B, Carroll FE, Dieppe P, Garfield K, Gooberman-Hill R. One-stage or two-stage revision surgery for prosthetic hip joint infection--the INFORM trial: a study protocol for a randomised controlled trial. Trials. 2016;17:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am. 1982;64:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 390] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Moore TM, Meyers MH, Patzakis MJ, Terry R, Harvey JP Jr. Closed biopsy of musculoskeletal lesions. J Bone Joint Surg Am. 1979;61:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Oliveira MP, Lima PM, da Silva HJ, de Mello RJ. Neoplasm seeding in biopsy tract of the musculoskeletal system. A systematic review. Acta Ortop Bras. 2014;22:106-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Lee DK, Kim HJ, Cho IY, Lee DH. Infection and revision rates following primary total knee arthroplasty in patients with rheumatoid arthritis versus osteoarthritis: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25:3800-3807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Racano A, Pazionis T, Farrokhyar F, Deheshi B, Ghert M. High infection rate outcomes in long-bone tumor surgery with endoprosthetic reconstruction in adults: a systematic review. Clin Orthop Relat Res. 2013;471:2017-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | PARITY Investigators. Prophylactic antibiotic regimens in tumour surgery (PARITY): a pilot multicentre randomised controlled trial. Bone Joint Res. 2015;4:154-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Husted H, Solgaard S, Hansen TB, Søballe K, Kehlet H. Care principles at four fast-track arthroplasty departments in Denmark. Dan Med Bull. 2010;57:A4166. [PubMed] |
| 18. | Cronin-Fenton DP, Søndergaard F, Pedersen LA, Fryzek JP, Cetin K, Acquavella J, Baron JA, Sørensen HT. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103:947-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 285] [Article Influence: 19.0] [Reference Citation Analysis (0)] |