Published online Jun 18, 2017. doi: 10.5312/wjo.v8.i6.441
Peer-review started: February 3, 2017
First decision: March 8, 2017
Revised: March 20, 2017
Accepted: April 6, 2017
Article in press: April 10, 2017
Published online: June 18, 2017
Processing time: 142 Days and 5.1 Hours
Total knee replacement (TKR) is one of the most common surgeries over the last decade. Patients undergoing TKR are at high risk for postoperative anemia and furthermore for allogeneic blood transfusions (ABT). Complications associated with ABT including chills, rigor, fever, dyspnea, light-headedness should be early recognized in order to lead to a better prognosis. Therefore, perioperative blood management program should be adopted with main aim to reduce the risk of blood transfusion while maximizing hemoglobin simultaneously. Many blood conservation strategies have been attempted including preoperative autologous blood donation, acute normovolemic haemodilution, autologous blood transfusion, intraoperative cell saver, drain clamping, pneumatic tourniquet application, and the use of tranexamic acid. For practical and clinical reasons we will try to classify these strategies in three main stages/pillars: Pre-operative optimization, intra-operative and post-operative protocols. The aim of this work is review the strategies currently in use and reports our experience regarding the perioperative blood management strategies in TKR.
Core tip: Total knee replacement is one of the most common elective surgeries in orthopaedics. Blood loss during surgery is putting the patient at risk for a blood transfusion. A number of reviews and meta-analyses have tried to analyze the best blood conservation strategy. Our objective is to review any blood saving method/strategy into the preoperative, intraoperative and postoperative period and analyze their possible combination. A zero allogenic blood transfusion rate with safe and cost-effective methods should be the aim and an achievable goal.
- Citation: Themistoklis T, Theodosia V, Konstantinos K, Georgios DI. Perioperative blood management strategies for patients undergoing total knee replacement: Where do we stand now? World J Orthop 2017; 8(6): 441-454
- URL: https://www.wjgnet.com/2218-5836/full/v8/i6/441.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i6.441
Total knee arthroplasty (TKA) is currently the most cost-effective and efficacious way for treating patients with end-stage knee osteoarthritis who suffer from severe pain, activity limitation and for whom conservative treatment is unsuccessful. Based on National registries, TKA is considered to be the most common major orthopaedic surgery performed worldwide[1]. It’s really important to mention that the number of TKA surgeries performed each year increases and is projected to have a five to six-fold increase by 2030[2].
Blood loss during TKA is putting the patient at risk for a blood transfusion. It’s reported that patients undergoing TKA may result in blood loss between 1000 mL and 1500 mL which necessitates subsequent allogeneic blood transfusion (ABT) in 10%-38% of them[3-7]. Thus, it becomes prudent to minimize the ABTs while trying to maintain hemoglobin (Hb) in a safe and efficient level to help patient’s rehabilitation. Many strategies have been used in order to minimize blood loss including preoperative autologous blood donation (PAD), acute normovolemic haemodilution (ANH), autologous blood transfusion (ABT), intraoperative cell saver, drain clamping, pneumatic tourniquet application, and the use of tranexamic acid (TXA)[8-10].
Although many strategies and algorithms have been proposed for ABTs reduction there is not a consensus about the most efficient/successful combination[8,11]. This article will try to review the latest strategies, analyze the results and our experience regarding the use of TXA. Summarizing, these strategies can be divided in three stages: Pre-operative, intra-operative and post-operative (Table 1).
| Pre-operative | Intra-operative | Post-operative |
| Detection of anaemia and iron deficiency treatment | MIS and navigated MIS TKA | Compression and cryotherapy |
| Erythropoietin | Tourniquet | Limb position |
| Perioperative management of antiplatelet agents | Hypotensive epidural anesthesia | Post-operative cell saving |
| Transfusion protocol agreement | Acute normovolemic haemodilution | Drainage clamping |
| Pre-operative autologous blood donation | Antifibrinolytic agents | |
| Topical fibrin sealants | ||
| Intra-operative cell salvage | ||
| Peri/intra-articular (bupivacaine and epinephrine) injections | ||
| Bipolar vs monopolar sealant | ||
| Platelet-rich plasma | ||
| Bone wax | ||
| Sealing femoral tunnel |
The main aim of blood management is to eliminate ABTs and prevent anaemia simultaneously. In order to avoid anaemia’s clinical symptoms we need to preserve post-operative Hb values as higher as possible. Therefore, we highlight the significant effect of high pre-operative Hb on the requirement of ABT in TKA.
Anaemia has been defined by the World Health Organization as an Hb concentration < 130 g/L for men, < 120 g/L for non-pregnant women[12]. Regarding patients undergoing TKA it’s been reported that 8% to 21% of them were anaemic before the procedure[13,14].
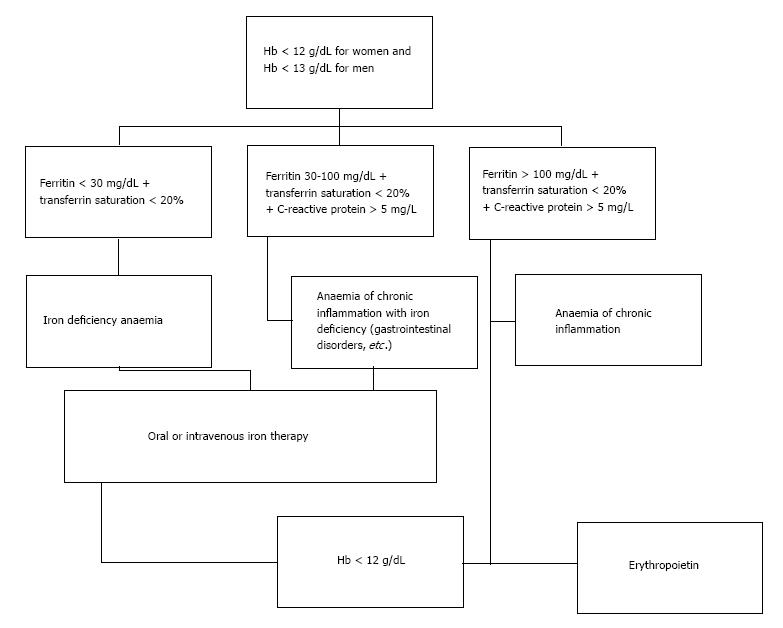
Pre-operative assessment of patients should be performed at least 30 d (some reviews suggest at least 60 d) before the procedure in order to have enough time to investigate the cause and/or plan the required treatment[15-17]. In case of low Hb additional lab tests should be carried out including at least full blood count, serum ferritin, transferrin saturation index (TSAT), vitamin B12, folic acid, a marker of inflammation (e.g., serum CRP) and a marker of renal function (e.g., serum Creatinine) (Figure 1)[18]. Any other low Hb cause apart from iron deficiency anaemia (IDA) should be carefully investigated.
IDA is the main cause of low Hb. It’s been reported that IDA counts up to 50% of the patients with Hb lower than 12 g/dL[19,20]. It’s been suggested that patients undergoing TKA should meet WHO’s criteria regarding the minimum pre-operative Hb. Otherwise, surgery should be postponed[15]. Furthermore, a recent, retrospective study demonstrated that preoperative anaemia (haematocrit < 25%) and ABTs are the two “evils” that increased the post-operative morbidity and mortality[21].
Adult patients with IDA who are candidates for TKA should be treated before the surgery. Either intravenous or oral iron therapy has been found to be effective in the treatment of pre-operative anaemia, meanwhile reducing the rehabilitation’s duration[14,22]. Moreover, the superiority of intravenous iron therapy with respect to oral iron therapy has been reported[23]. A 3-wk duration, administration of intravenous iron, just before surgery seems to be the most efficient and safe treatment[24]. Additionally, oral iron may not be efficacious in patients with malabsorption such as coeliac disease[25].
Erythropoietin (EPO) is a great tool in correcting anaemia as it is an essential hormone for red blood cell production. Without it, definitive erythropoiesis does not take place. Under hypoxic conditions, the kidney will produce and secrete erythropoietin to increase the production of red blood cells[26,27]. Its role in blood loss management has been thoroughly studied, showing a 60% reduction of ABTs in patients who received EPO compared to control group[28-30]. Three or four weekly subcutaneous injections (600 IU/kg) seems to be the most frequently used protocol with the best results[31-35]. Weber et al[36] reports a mean rise in pre-operative Hb of 1.9 g/dL in patients that received EPO. A big disadvantage of EPO is the really big cost which is being estimated to 1500 dollars per patient (4 weekly injections)[37]. For this reason, EPO use is being suggested when the patient has anemia and meets the criteria for blood transfusion, but declines a blood transfusion because of religious beliefs (e.g., Jehovah’s Witness), or the appropriate blood type is not available because of the patient’s red cell antibodies[38]. Adverse events have been reported in 5% of patients that have been treated with EPO. These complications include deep venous thrombosis (DVT), pulmonary embolism (PE), fever, hypokalemia, urinary tract infection, nausea, hypoxia, and vomiting[39-41]. Briefly, EPO can reduce the need for ABTs in high-risk patients undergoing TKA; however, it was not found to be cost-effective compared to other blood conservation methods[42].
Cardiovascular disease is common in patients planning to undergo to TKA. Antiplatelet agents, used as monotherapy or in combination, have a key role in preventing cardiac and vascular events[43]. Many of these patients have already undergone previous percutaneous coronary intervention (PCI) with stent implantation. American Heart Association’s/American College of Cardiology Foundation’s guidelines suggest dual antiplatelet therapy with aspirin and an adenosine diphosphate (ADP) inhibitor (e.g., clopidogrel) for at least 1 mo after bare-metal stent implantation and for 1 year after drug-eluting stent implantation in order to avoid late thrombosis[44]. There is a distinct proof that elective surgeries like TKA should be avoided (if it’s possible) within the first year of stent implantation, as it’s been reported a 5- to 10-fold increase in acute stent thrombosis[45]. Of course, after the first year most of these patients continue with single antiplatelet therapy[46].
Our main concern about antiplatelet agents is the perioperative bleeding that can occur during the procedure. Recent review reports bleeding increase up to 50% in patients with dual antiplatelet therapy. Regarding the monotherapy, the same review found that blood loss increased 2.5%-20%[47]. From an anaesthesiologist’s perspective, the incidence of spinal haematomas associated with epidural or spinal anaesthesia is the main reason for antiplatelet’s discontinuation. Regarding the literature, 61 cases of spinal haematomas associated with epidural or spinal anaesthesia are reported between 1906 and 1994[48].
The two most prescribed antiplatelet drugs (with different mechanism of action) are aspirin and clopidogrel. Regarding the aspirin, guidelines suggest its discontinuation 7-10 d before surgery without major consequences. Postoperatively, aspirin should be resumed preferably within 24 h (when bleeding risk is low). Conversely, patients who are in high cardiovascular risk should not stop aspirin therapy in the perioperative period[49]. Clopidogrel acts by inhibiting the ADP receptor on platelet cell membranes. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions (ACCF/AHA/SCAI) suggest discontinuation of clopidogrel 5 d prior to surgery and if additional DVT prophylaxis is needed a low molecular weight heparin (LMWH) should be used.
The key point is that both the continuation and the discontinuation of antiplatelet therapy can be associated with major risks. Therefore, (especially in dual antiplatelet therapy) the management of these medications in the perioperative setting should be discussed between the cardiologist, orthopaedic surgeon, and anaesthesiologist. This “team” should weigh the patient’s risk of thrombosis with the risk of surgical bleeding to determine the right choice for him and if/when dual antiplatelet therapy can safely be discontinued.
ABTs are responsible for many complications like human immunodeficiency virus (HIV)’s, hepatitis’B and C transmission (despite donor screening), whereas allergic reactions may cause minor reactions (e.g., fever) to fatal ABO blood group incompatibility[50,51]. Therefore, it’s really crucial to analyse and update the transfusion protocols that are being used in hospitals and especially in orthopaedic departments. We’d like to notice that although transfusion is a post-operative process, we include it in pre-operative measures as an agreement/protocol about the “transfusion trigger” should be achieved before the surgery.
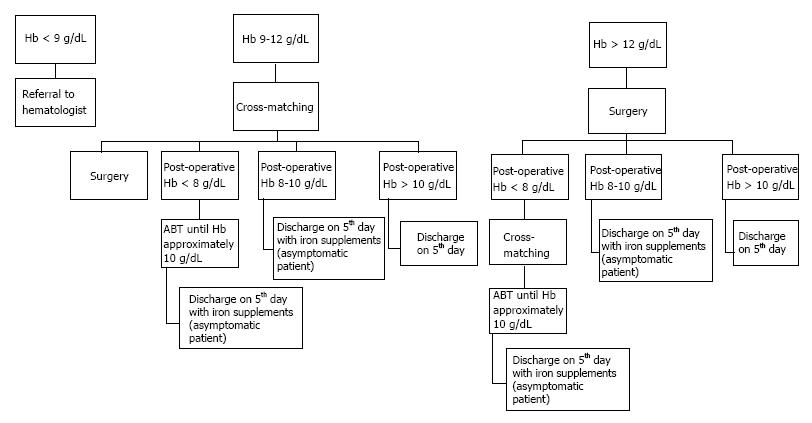
The main factor that should be investigated is the so called “transfusion trigger”. It’s the Hb threshold at which the physician decides to transfuse the patient. Many protocols/rules like 10/30 have been used in the past; but it’s not the case any longer[52]. Low transfusion trigger point seems to be effective in reducing ABTs[53,54]. Reviews suggest transfusion triggers (Hb levels) between 8 g/dL and 9 g/dL (excluding severe cardiovascular disease, renal failure, and hematologic disorders)[55,56]. Unquestionably, symptomatic anaemia resulting in tachycardia, change in mental status, cardiac ischemia or shortness of breath should always been treated followed by ABT. Based on literature, in our department we use a mini transfusion algorithm/protocol (Figure 2). This protocol has already documented significant reductions in the rates of red cell transfusion and worthwhile blood conservation. Noticeably, this strategy seems to be really cost-effective.
Briefly, a blood management protocol with restrictive typing and screening, cross-matching, and transfusion should be adopted by national health systems in order to reduce the wastage of unused blood units and the rate of ABTs without increasing patients’ morbidity or mortality.
In 1980, the recognition that ABTs were associated with potential risks like viral transmission (e.g., HIV) and bacterial infection prompted the development of PAD programs[57,58]. In 1992, PAD accounted for nearly 8.5% of all blood collected in United States. Nevertheless, pre-donation decreased to 3.5% of the blood units collected by 1997[59].
PAD’s main target is providing a resource of safe blood for patients that are candidates for scheduled surgery (like TKA). Meanwhile, this process increases the patient’s total red blood cell (RBC) mass due to the PAD-induced stimulation of erythropoiesis before elective surgery.
Many studies and meta-analyses concluded that PAD strategy managed to reduce the use of ABTs by 40%-52%, increase the overall transfusions (allogeneic and autologous) by 30%. On the contrary, it’s really important to mention that patients’ Hb concentration decreased by more than 1 g/dL from before starting PAD to immediately prior to surgery[60-62]. PAD’s poorly cost-effectiveness (about 300$ per unit), combined with new blood saving strategies and new drugs has led to a decline in its use[63,64]. In our days, the use of PAD has therefore lost its acceptance and is no longer being used in TKA patients.
Plentiful methods, strategies, technologies and drugs have contributed in blood loss minimization and ABTs’ reduction. Some of them have gained ground during the last decades and others didn’t manage to prove their effectiveness. Intra-operative blood saving seems to play the most important role between the strategies and techniques indicated in the three pillars of patient blood management.
Many of the patients that have decided to have a TKA might consider a minimally invasive procedure with or without navigation. This type of surgery uses smaller incisions and less cutting of the tissue surrounding the knee. The advantage of such a surgery except for the smaller incision is the promising recovery, a shorter hospital stay and less blood loss.
A meta-analysis revealed the superiority of minimal invasive (MIS) to the standard parapatellar approach in visual analog score (VAS) and range of motion (ROM) in the short term (postoperative 2 wk)[65]. No differences were noticed in straight leg raise, hospital stay, postoperative complications and blood loss. Comparable results pointed out between MIS TKA and MIS navigated TKA[66]. In conclusion, MIS TKA has proved the ability to couple the benefits of less invasive surgical approach without compromising the long-term established success of conventional TKA, especially in blood loss.
A tourniquet is a compressing device, used to control venous and arterial circulation to an extremity (lower extremity in TKA) for a period of time. Although the majority of orthopaedic surgeons still use it widely, its role is controversial. Tourniquet’s use was believed to be effective in decreasing intraoperative blood loss. However, reactive blood flow after tourniquet’s release seems to balance out the total blood loss compared to the non-tourniquet TKA method[67]. A meta-analysis of thirteen randomized controlled trials (RCTs) demonstrated that non tourniquet use in TKA has better clinical outcomes, less complications and better ROM in early postoperative period. The most important finding of this meta-analysis is that the true blood loss in TKA was not reduced using a tourniquet[68]. Therefore, it can be explicitly deduced that TKA with a tourniquet reduces the intra-operative blood loss but postoperatively increases the hidden blood loss[68]. To sum up, tourniquet’s effectiveness and safety in TKA should be carefully considered when surgeon decides to use it.
In April of 1989 Sharrock et al[69] published the first description of hypotensive epidural anesthesia. To date, HEA is not a popular method in elective orthopaedic surgery like TKA. HEA was developed to combine the advantages of epidural anesthesia (airway problem, reduced rate of DVT) with the benefits of induced hypotension.
Its mechanism of action is well-described. A sympathetic blockade (including cardiac sympathetic fibers), using local anesthetic at an upper lumber interspace (T12-L1/L1-L2), causes a reduction in arterial pressure. Mean arterial pressure (MAP) is maintained at 50-55 mmHg with end result the reduction of blood loss. It’s really important to mention that concurrently, a low dose of epinephrine is being infused (till MAP reaches 75-80 mmHg) achieving circulation’s stabilisation[70,71].
Although HEA’s use seems to be really advantageous, without complications, it’s not a “first line” method regarding blood loss in TKA. A few studies have proved its safety and efficacy in total hip arthroplasty (THA), but further studies are needed to assess its use in TKA[70,72,73].
Acute normovolemic haemodilution (ANH) is a technique in which whole blood is removed from a patient, while circulating volume is maintained with crystalloid fluid. It is performed shortly before or shortly after induction of anaesthesia. A close monitoring of the patient is necessary and when Hb level drops down to 8-9 g/dL ANH is being halted[74]. Postoperatively, sufficient blood is administered to maintain patient’s Hb over 8-9 g/dL.
Many studies suggest ANH’s use in elective orthopaedic surgeries as it contributes in ABT’s reduction[75-77]. In contrast, there are studies that noted no significant difference between control and ANH group[78-81]. Undoubtedly, more studies would be needed to prove/rebut its efficacy in blood loss management.
The most famous blood saving management of the last decade is the use of antifibrinolytic agents. TXA, ε-aminocaproic acid (EACA) and aprotinin are the most commonly used antifibrinolytic agents[82-84].
TXA and EACA are lysine analog antifibrinolytics that reversibly bind both plasmin and plasminogen. TXA is a current trend in TKA and THA. Many studies have proved its efficacy without an increased risk of complications (DVT, PE, and wound infection). Latest studies and meta-analyses focused on the best route of administration combined with multiple dose regimens[85-88]. Regarding the route of administration and plasma concentration, maximum plasma concentration of TXA is reached within 5-15 min after intravenous (IV) injection, 30 min after intramuscular (IM) injection and 2 h after oral tablets[89]. IV TXA seems to be more effective compared to topical administration. However, the topical administration seems to outcompete IV in patients with high risk of thromboembolic events[90]. On the contrary, a recent meta-analysis showed no statistically significant difference in total blood loss, drain output, transfusion requirements and thromboembolic complications between topical TXA and IV-TXA in TKA[91].
The most efficacious regimen is still under debate, but multiple IV boluses regimens (pre/intra/post-operatively) prove to have a better result compared to a single IV dose[92]. Nevertheless, two RCTs concluded that intra-articular regimen of TXA is as effective as three doses IV regimen in preventing blood loss without any difference in thromboembolic complications[93,94]. In addition to all these studies some authors have noticed that the combination of IV and intra-articular TXA is more effective than either regimen used alone[95,96]. All these conflicting results suggest that more well-conducted randomised controlled trials are needed to produce strong evidences about it. In our orthopaedic department two RCTs have already been completed, showing the high effectiveness of TXA’s both in TKA with tourniquet and TKA without tourniquet and one more is currently running[87,88]. The aim of the current study is to determine whether or not repeated dosing of IV TXA reduces (additionally) the post-operative reduction in hemoglobin, hematocrit, number of transfusions, and post-operative blood loss following primary TKA.
Studies comparing EACA to TXA on the reduction of perioperative bleeding and on the number of transfusions needed showed no significant differences between the two antifibrinolytic agents. The only advantage of TXA compared to EACA is its lower price[97].
Aprotinin, a nonlysine antifibrinolytic agent, was more effective at decreasing blood loss but was associated with increased cardiovascular complications (increased risk for myocardial infarction) and was therefore removed from the market in 2008[98-100].
Fibrin sealant is comprised mostly of fibrinogen and human thrombin which form a stable fibrin clot and can mimic the last phase of physiological blood coagulation cascade. Many studies have proved their efficacy without increasing the risk of DVT, PE, hematoma, wound infection or other complications for patients undergoing TKA[101,102]. However, their main disadvantage is the high cost compared to other blood management methods (like TXA)[103,104]. Moreover, newer studies appear to confute the initial hypothesis of fibrin sealants’ haemostatic role. All these studies report no effect of fibrin sealant in terms of blood or transfusion savings after TKA[105-107].
Intraoperative blood salvage, also known as cell salvage, is a medical procedure involving recovering blood lost during surgery and re-infusing it into the patient[108]. Many devices and processes have been developed to assist in salvaging the patient’s own whole blood since the 1970s, when it was popularized in major thoracic or abdominal procedures[109]. Unwashed blood revealed poor results as it may contain hemolyzed RBC, clotting factors and cytokines[110,111]. Therefore, cell separation and washing showed better results with an autologous red cell concentrate with normal function and no complications[112].
Literature’s evidence strength is really limited regarding the safety and effectiveness of this method. Current studies have low level of evidence which means that they are incompetent to compare the post-operative infection rates with and without cell salvage use. A general outcome of these studies is that intra-operative cell salvage reduce ABTs but more studies needed to clarify the importance and the risk of this method[113-115].
Epinephrine is the agent of choice for topical haemostatic vasoconstriction[116]. Anderson et al[117] injected bupivacaine and epinephrine just before wound closure (one-third pericapsular, two-thirds peri-incisional). They managed to prove a 32% less drain output in study group. However, no statistically significant differences were noticed in the transfusion rate between the two groups. Moreover, a new study by Yang et al[118] reports controversial results, as the initial hypothesis regarding the haemostatic role of intra-articular epinephrine after TKA is not being supported by the various bleeding parameters.
Monopolar electrocautery is a device that delivers electrical current to patient’s tissue through a pen-like stylus. Intra-operative temperatures can be higher than 300 °C, resulting in smoke and eschar formation[119]. Opposed to monopolar electrocautery, bipolar sealing delivers radiofrequency energy combined with continuous-flow saline in order to prevent temperatures higher than 100 °C. Although bipolar sealant is being used for decades in oncology, thoracic, spine and brain surgery it seems to be a novel approach in TKA[120-123]. However, latest studies (including RCTs) and the results of the comparison between bipolar and monopolar sealers used in TKA report no significant difference in postoperative drain output, postoperative Hb level and transfusion requirement[119,124,125].
Platelet-rich plasma (PRP) has been used in surgeries to promote cell regeneration since 1987[126]. Today, PRP injections is being safely used in many fields like cosmetics, sports medicine, orthopaedics, and fasciomaxillary[127,128].
PRP is defined as plasma with a platelet level above peripheral blood concentration. There are two methods to obtain it: (1) ready PRP kits (higher cost); and (2) a wide variation of reported protocols for standardization and preparation of PRP (most of them use two-step centrifugation protocol)[129,130]. The final volume contains platelets and factors (e.g., platelet-derived growth factor and transforming growth factor-β) whose haemostatic and wound-healing effects have been well-described[131-134]. Gardner et al[135] in their retrospective study report less blood loss during the post-operative period. Despite that a consensus about the high concentration of growth factors and its efficacy in wound healing has been reached, its haemostatic role is still debatable[136,137].
As a final point, we’d like to note that understanding of basic principles of centrifugation is of vital importance in preparation of PRP. Many protocols have been described with different consistency of PRP yield. Thus, it is advisable to standardize individual, cost-effective preparation protocols, which are easy to adapt in clinical practice[130].
Bone wax is a waxy substance used to help mechanically control bleeding from bone surfaces during surgical procedures. It consists of a mixture of beeswax, paraffin and isopropyl palmitate[138]. Although its use in elective orthopaedic surgery hasn’t been well-demonstrated, Moo et al[139] suggest bone wax’s application in TKA for reducing total blood loss and maintaining higher hemoglobin levels.
It’s remarkable to mention that complications like allergic reaction, inflammation and foreign bodies formation need extra attention by the physicians[140]. Undoubtedly, further studies are needed to confirm its safety and efficacy in TKA.
In recent decades most of the orthopaedic surgeons use an intramedullary alignment system regarding the placement of the femoral component in TKA[141]. The intramedullary (IM) femoral rod that is being used damages the cancellous bone and its vascularization resulting in high blood loss. Nowadays, many surgeons seal this tunnel with autologous bone in order to minimize the bleeding. Although autologous bone grafting is a safe and non-time consuming process, its efficacy regarding the reduction in blood loss is still debateable[142,143]. Additionally, studies report that the use of an extramedullary (EM) femoral alignment guide system resulted in reduction of the drained blood and consequently in lower transfusion rates[144,145]. Our only concern is the influence of IM and EM femoral cutting guides on survivorship of the TKA, as IM seems to demonstrate superiority over the EM[146].
Last but not least, post-operatively blood saving methods are integrated in order to reduce blood loss and blood transfusion, and promote the rehabilitation of patients. Post-operative strategies include compression, cryotherapy, use (or not) of drainage systems, cell saving systems and post-operative leg position.
Knee swelling after TKA is common and most of the time impairs early rehabilitation. Use of an inelastic compression bandage after TKA seems not to reduce total blood loss. However, it offers a slight but non-significant improvement regarding the postoperative pain and early functional outcomes[147,148]. On the other hand many studies report no difference in compression method[149-151].
Recently Desteli et al[152] and Kullenberg et al[153] reported that cryotherapy was beneficial in minimizing blood loss after TKA. Many cryotherapy devices have been used in the past (gel packs, circulating ice water) in order to help patients’ rehabilitation[154,155]. However, Adie et al[156] in their systematic review and meta-analysis does not support the routine use of cryotherapy after TKA.
Another option in order to reduce blood loss after TKA is the limb position. Different knee flexion positions (e.g., hip elevation by 60° combined with 60° knee flexion) have been reported to have promising results with respect to reducing perioperative blood loss[157-159]. Based on these studies, we conclude that post-operative knee flexion is an easy, inexpensive and effective method in blood loss reduction.
It’s been calculated that 50% of the total blood loss in a TKA occurs post-operatively[6]. Therefore, post-operative cell saving and return of unwashed, filtered blood from drains represents an alternative to ABTs method[160]. This system consists of a collection bag and an autologous transfusion bag (filtered blood collected). Re-transfusion can take place in the first 6 h after the end of surgery in order to avoid bacterial infection[161-163]. After this period it can be used as a vacuum drain. Its cost-effectiveness and efficacy seems to be maximized in patients with pre-operative Hb between 12 g/dL and 15 g/dL, whereas in patients with Hb < 12 g/dL post-operative cell saving system should be combined with other blood-saving techniques in order to increase its efficacy[164].
Although it is commonly believed that a suction drain, placed intra-articularly reduces the formation of a haemarthrosis and enhances rehabilitation, many studies have yielded controversial results regarding its use[165-169]. Senthil Kumar et al[170] in report that most of the post-operative blood loss occurs in the first few hours and especially in the first four hours. As a result, drainage’s clamping should help in minimizing blood loss acting like a tamponade. Although drainage’s use is still debatable, many different drainage’s clamp intervals have been described[168,171-173]. In a prospective study, Yamada et al[174] noted that extended drainage’s clamping increased complications significantly. There is no consensus about the best protocol but it’s noticeable that drainage’s clamping combined with TXA can reduce blood loss after TKA[175]. Surprisingly and in contrast with the above literature, 2010 Tai et al[176] found no advantage of using the “clamping” method compared with non-drainage at all.
It’s more than clear that TKA is a surgery with a blood loss reaching up to 1500 mL. Undoubtedly, the consequent ABTs and/or anaemia occurring post-operatively are causes of increased morbidity, cardiovascular risks, length of stay, decreased vigor and slow rehabilitation. Over recent decades, many blood saving strategies and methods have been described. Nevertheless, there are no concise guidelines, as few/limited studies have compared the relative efficacy of these techniques.
The common target of all blood saving methods is the cost-effective decrease of ABTs. The aim of this review was to evaluate current evidence regarding the efficacy, the safety and the cost-effectiveness on the various pre/intra/post-operative management strategies for patients undergoing TKA. As we described above there is a plethora of methods that can be used in the different periods of the surgery. Many studies have successfully/unsuccessfully described the advantages/disadvantages of each method with/without their limitations. We faced many controversial results in the majority of these strategies. For that reason larger prospective randomized studies comparing not only the individual strategies, but also their combination, are needed.
Scrutinizing the recent literature, we conclude that there is no “consensus success story” about a common efficient/safe blood management strategy in TKA. And if we hazard a guess, we’d say that this consensus cannot be achieved. The current trend is the patient-specific strategy (PSS). This idea is based on the notion that each patient has a different impact on the risk of requiring a transfusion. For example the PSS in a healthy man with Hb > 13 g/dL who undergoes TKA could be a “do nothing” (except Hb reaches transfusion trigger). Conversely, a Jehovah’s Witness patient and/or a patient with significant cardiopulmonary compromise should be monitored carefully and more blood management strategies should be considered in order to avoid ABTs. In other words, the above methods that have been analyzed, the advantages and the disadvantages of each method, are just the different parameters that every surgeon should take on board in order to achieve the best result in a specific patient.
The take home message after our in-depth search is that the first important step in blood management is the thorough pre-operative evaluation of each patient. Consideration should be given to the existing physiologic/pathologic variables of the patient and the concomitant actions that should be taken in order to allow prompt optimization of the patient’s physiologic status. The 2nd principal arm of effective blood management is the restriction of ABTs’ to patients meeting well-established transfusion criteria. Nowadays, this trigger has been decreased to 8 g/dL. The old common belief that all patients with Hb below 10 g/dL should be transfused, has been surpassed. However, when clearly the blood is indicated (clinical signs and symptoms of anemia), administration should not be delayed. Additionally, the use of TXA perioperatively (with different routes of administration) is a widely accepted, effective and safe method in reducing perioperative blood transfusion. These three steps are the “baseline” in our daily practice regarding the perioperative care of the surgical patient.
In our daily practice, it’s been proven to be really challenging and unfeasible to apply the same practices in all patients. In simple terms, no single method achieved to provide significantly superior results over another in ABTs’ reduction. Primarily, every orthopaedic surgeon should be able to plow through and understand each method separately. Consequently, he must tailor these methods to result in an individualistic blood saving model.
In conclusion, an appropriate combination of the above blood management strategies could further result in ABT’s reduction. Additionally, we should highlight the importance of a team approach (e.g., orthopaedic surgeon, anesthesiologist, hematologist) in order to optimize the patients perioperatively and succeeding in the best result.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hasegawa M, Malik H, Robertson GA S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Kurtz SM, Ong KL, Lau E, Widmer M, Maravic M, Gómez-Barrena E, de Pina Mde F, Manno V, Torre M, Walter WL. International survey of primary and revision total knee replacement. Int Orthop. 2011;35:1783-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 2. | Jasper LL, Jones CA, Mollins J, Pohar SL, Beaupre LA. Risk factors for revision of total knee arthroplasty: a scoping review. BMC Musculoskelet Disord. 2016;17:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Park JH, Rasouli MR, Mortazavi SM, Tokarski AT, Maltenfort MG, Parvizi J. Predictors of perioperative blood loss in total joint arthroplasty. J Bone Joint Surg Am. 2013;95:1777-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Wong J, Abrishami A, El Beheiry H, Mahomed NN, Roderick Davey J, Gandhi R, Syed KA, Muhammad Ovais Hasan S, De Silva Y, Chung F. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92:2503-2513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 384] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 5. | Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19:281-287. [PubMed] |
| 6. | Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86:561-565. [PubMed] |
| 7. | Kalairajah Y, Simpson D, Cossey AJ, Verrall GM, Spriggins AJ. Blood loss after total knee replacement: effects of computer-assisted surgery. J Bone Joint Surg Br. 2005;87:1480-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Loftus TJ, Spratling L, Stone BA, Xiao L, Jacofsky DJ. A Patient Blood Management Program in Prosthetic Joint Arthroplasty Decreases Blood Use and Improves Outcomes. J Arthroplasty. 2016;31:11-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Moonen AF, Neal TD, Pilot P. Peri-operative blood management in elective orthopaedic surgery. A critical review of the literature. Injury. 2006;37 Suppl 5:S11-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016;98-B:98-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Helm AT, Karski MT, Parsons SJ, Sampath JS, Bale RS. A strategy for reducing blood-transfusion requirements in elective orthopaedic surgery. Audit of an algorithm for arthroplasty of the lower limb. J Bone Joint Surg Br. 2003;85:484-489. [PubMed] |
| 12. | Organization WH. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. [published 2011 June]. Available from: http://www.who.int/vmnis/indicators/haemoglobin/en/. |
| 13. | Goodnough LT, Vizmeg K, Sobecks R, Schwarz A, Soegiarso W. Prevalence and classification of anemia in elective orthopedic surgery patients: implications for blood conservation programs. Vox Sang. 1992;63:90-95. [PubMed] |
| 14. | Andrews CM, Lane DW, Bradley JG. Iron pre-load for major joint replacement. Transfus Med. 1997;7:281-286. [PubMed] |
| 15. | Goodnough LT, Maniatis A, Earnshaw P, Benoni G, Beris P, Bisbe E, Fergusson DA, Gombotz H, Habler O, Monk TG. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 384] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 16. | Liumbruno GM, Bennardello F, Lattanzio A, Piccoli P, Rossetti G; Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party. Recommendations for the transfusion management of patients in the peri-operative period. I. The pre-operative period. Blood Transfus. 2011;9:19-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 17. | Rogers BA, Cowie A, Alcock C, Rosson JW. Identification and treatment of anaemia in patients awaiting hip replacement. Ann R Coll Surg Engl. 2008;90:504-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Shander A, Knight K, Thurer R, Adamson J, Spence R. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:58S-69S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Guyatt GH, Patterson C, Ali M, Singer J, Levine M, Turpie I, Meyer R. Diagnosis of iron-deficiency anemia in the elderly. Am J Med. 1990;88:205-209. [PubMed] |
| 20. | Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 911] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 21. | Loor G, Rajeswaran J, Li L, Sabik JF 3rd, Blackstone EH, McCrae KR, Koch CG. The least of 3 evils: exposure to red blood cell transfusion, anemia, or both? J Thorac Cardiovasc Surg. 2013;146:1480-1487.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Cuenca J, García-Erce JA, Martínez F, Cardona R, Pérez-Serrano L, Muñoz M. Preoperative haematinics and transfusion protocol reduce the need for transfusion after total knee replacement. Int J Surg. 2007;5:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Onken JE, Bregman DB, Harrington RA, Morris D, Acs P, Akright B, Barish C, Bhaskar BS, Smith-Nguyen GN, Butcher A. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. 2014;54:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Theusinger OM, Leyvraz PF, Schanz U, Seifert B, Spahn DR. Treatment of iron deficiency anemia in orthopedic surgery with intravenous iron: efficacy and limits: a prospective study. Anesthesiology. 2007;107:923-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Halfdanarson TR, Litzow MR, Murray JA. Hematologic manifestations of celiac disease. Blood. 2007;109:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 26. | Lacombe C, Mayeux P. The molecular biology of erythropoietin. Nephrol Dial Transplant. 1999;14 Suppl 2:22-28. [PubMed] |
| 27. | Lacombe C. Erythropoietin: from molecular biology to clinical use. Eur Cytokine Netw. 1997;8:308-310. [PubMed] |
| 28. | Perkins HA, Busch MP. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress. Transfusion. 2010;50:2080-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81:2-10. [PubMed] |
| 30. | Kopolovic I, Ostro J, Tsubota H, Lin Y, Cserti-Gazdewich CM, Messner HA, Keir AK, DenHollander N, Dzik WS, Callum J. A systematic review of transfusion-associated graft-versus-host disease. Blood. 2015;126:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 31. | So-Osman C, Nelissen RG, Koopman-van Gemert AW, Kluyver E, Pöll RG, Onstenk R, Van Hilten JA, Jansen-Werkhoven TM, van den Hout WB, Brand R. Patient blood management in elective total hip- and knee-replacement surgery (Part 1): a randomized controlled trial on erythropoietin and blood salvage as transfusion alternatives using a restrictive transfusion policy in erythropoietin-eligible patients. Anesthesiology. 2014;120:839-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Feagan BG, Wong CJ, Kirkley A, Johnston DW, Smith FC, Whitsitt P, Wheeler SL, Lau CY. Erythropoietin with iron supplementation to prevent allogeneic blood transfusion in total hip joint arthroplasty. A randomized, controlled trial. Ann Intern Med. 2000;133:845-854. [PubMed] |
| 33. | Gombotz H, Gries M, Sipurzynski S, Fruhwald S, Rehak P. Preoperative treatment with recombinant human erythropoietin or predeposit of autologous blood in women undergoing primary hip replacement. Acta Anaesthesiol Scand. 2000;44:737-742. [PubMed] |
| 34. | Bezwada HP, Nazarian DG, Henry DH, Booth RE. Preoperative use of recombinant human erythropoietin before total joint arthroplasty. J Bone Joint Surg Am. 2003;85-A:1795-1800. [PubMed] |
| 35. | Moonen AF, Thomassen BJ, Knoors NT, van Os JJ, Verburg AD, Pilot P. Pre-operative injections of epoetin-alpha versus post-operative retransfusion of autologous shed blood in total hip and knee replacement: a prospective randomised clinical trial. J Bone Joint Surg Br. 2008;90:1079-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Weber EW, Slappendel R, Hémon Y, Mähler S, Dalén T, Rouwet E, van Os J, Vosmaer A, van der Ark P. Effects of epoetin alfa on blood transfusions and postoperative recovery in orthopaedic surgery: the European Epoetin Alfa Surgery Trial (EEST). Eur J Anaesthesiol. 2005;22:249-257. [PubMed] |
| 37. | Etchason J, Petz L, Keeler E, Calhoun L, Kleinman S, Snider C, Fink A, Brook R. The cost effectiveness of preoperative autologous blood donations. N Engl J Med. 1995;332:719-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 332] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 38. | (UK) NIfHaCE. Blood Transfusion. London: National Institute for Health and Care Excellence (UK). In: NICE, ed. Vol (NICE Guideline, No. 24.). Available from: https://www.ncbi.nlm.nih.gov/books/NBK327570/. |
| 39. | Deutsch A, Spaulding J, Marcus RE. Preoperative epoetin alfa vs autologous blood donation in primary total knee arthroplasty. J Arthroplasty. 2006;21:628-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Keating EM, Callaghan JJ, Ranawat AS, Bhirangi K, Ranawat CS. A randomized, parallel-group, open-label trial of recombinant human erythropoietin vs preoperative autologous donation in primary total joint arthroplasty: effect on postoperative vigor and handgrip strength. J Arthroplasty. 2007;22:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Rosencher N, Poisson D, Albi A, Aperce M, Barré J, Samama CM. Two injections of erythropoietin correct moderate anemia in most patients awaiting orthopedic surgery. Can J Anaesth. 2005;52:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Bedair H, Yang J, Dwyer MK, McCarthy JC. Preoperative erythropoietin alpha reduces postoperative transfusions in THA and TKA but may not be cost-effective. Clin Orthop Relat Res. 2015;473:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | Tendera M, Wojakowski W. Role of antiplatelet drugs in the prevention of cardiovascular events. Thromb Res. 2003;110:355-359. [PubMed] |
| 44. | Savonitto S, Caracciolo M, Cattaneo M, DE Servi S. Management of patients with recently implanted coronary stents on dual antiplatelet therapy who need to undergo major surgery. J Thromb Haemost. 2011;9:2133-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Di Minno MN, Prisco D, Ruocco AL, Mastronardi P, Massa S, Di Minno G. Perioperative handling of patients on antiplatelet therapy with need for surgery. Intern Emerg Med. 2009;4:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Lee HL, Chiu KY, Yiu KH, Ng FY, Yan CH, Chan PK. Perioperative antithrombotic management in joint replacement surgeries. Hong Kong Med J. 2013;19:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 47. | Chassot PG, Delabays A, Spahn DR. Perioperative antiplatelet therapy: the case for continuing therapy in patients at risk of myocardial infarction. Br J Anaesth. 2007;99:316-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 48. | Vandermeulen EP, Van Aken H, Vermylen J. Anticoagulants and spinal-epidural anesthesia. Anesth Analg. 1994;79:1165-1177. [PubMed] |
| 49. | Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, Dunn AS, Kunz R. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S-e350S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1080] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 50. | Dwyre DM, Fernando LP, Holland PV. Hepatitis B, hepatitis C and HIV transfusion-transmitted infections in the 21st century. Vox Sang. 2011;100:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 51. | Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406-3417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 417] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 52. | Nelson CL, Fontenot HJ, Flahiff C, Stewart J. An algorithm to optimize perioperative blood management in surgery. Clin Orthop Relat Res. 1998;36-42. [PubMed] |
| 53. | Ballantyne A, Walmsley P, Brenkel I. Reduction of blood transfusion rates in unilateral total knee arthroplasty by the introduction of a simple blood transfusion protocol. Knee. 2003;10:379-384. [PubMed] |
| 54. | Carson JL, Hill S, Carless P, Hébert P, Henry D. Transfusion triggers: a systematic review of the literature. Transfus Med Rev. 2002;16:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 55. | Practice Guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology. 1996;84:732-747. [PubMed] |
| 56. | Laupacis A, Fergusson D. Drugs to minimize perioperative blood loss in cardiac surgery: meta-analyses using perioperative blood transfusion as the outcome. The International Study of Peri-operative Transfusion (ISPOT) Investigators. Anesth Analg. 1997;85:1258-1267. [PubMed] |
| 57. | Giordano GF, Dockery J, Wallace BA, Donohoe KM, Rivers SL, Bass LJ, Fretwell RL, Huestis DW, Sandler SG. An autologous blood program coordinated by a regional blood center: a 5-year experience. Transfusion. 1991;31:509-512. [PubMed] |
| 58. | Kruskall MS, Glazer EE, Leonard SS, Willson SC, Pacini DG, Donovan LM, Ransil BJ. Utilization and effectiveness of a hospital autologous preoperative blood donor program. Transfusion. 1986;26:335-340. [PubMed] |
| 59. | Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion medicine. First of two parts--blood transfusion. N Engl J Med. 1999;340:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 596] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 60. | Forgie MA, Wells PS, Laupacis A, Fergusson D. Preoperative autologous donation decreases allogeneic transfusion but increases exposure to all red blood cell transfusion: results of a meta-analysis. International Study of Perioperative Transfusion (ISPOT) Investigators. Arch Intern Med. 1998;158:610-616. [PubMed] |
| 61. | Carless P, Moxey A, O’Connell D, Henry D. Autologous transfusion techniques: a systematic review of their efficacy. Transfus Med. 2004;14:123-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Biesma DH, Marx JJ, Kraaijenhagen RJ, Franke W, Messinger D, van de Wiel A. Lower homologous blood requirement in autologous blood donors after treatment with recombinant human erythropoietin. Lancet. 1994;344:367-370. [PubMed] |
| 63. | Birkmeyer JD, Goodnough LT, AuBuchon JP, Noordsij PG, Littenberg B. The cost-effectiveness of preoperative autologous blood donation for total hip and knee replacement. Transfusion. 1993;33:544-551. [PubMed] |
| 64. | Tretiak R, Laupacis A, Rivière M, McKerracher K, Souêtre E. Cost of allogeneic and autologous blood transfusion in Canada. Canadian Cost of Transfusion Study Group. CMAJ. 1996;154:1501-1508. [PubMed] |
| 65. | Xu SZ, Lin XJ, Tong X, Wang XW. Minimally invasive midvastus versus standard parapatellar approach in total knee arthroplasty: a meta-analysis of randomized controlled trials. PLoS One. 2014;9:e95311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Chang CW, Wu PT, Yang CY. Blood loss after minimally invasive total knee arthroplasty: effects of imageless navigation. Kaohsiung J Med Sci. 2010;26:237-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Ejaz A, Laursen AC, Kappel A, Laursen MB, Jakobsen T, Rasmussen S, Nielsen PT. Faster recovery without the use of a tourniquet in total knee arthroplasty. Acta Orthop. 2014;85:422-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 68. | Tai TW, Lin CJ, Jou IM, Chang CW, Lai KA, Yang CY. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2011;19:1121-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 69. | Sharrock NEMB, Mineo CB, Robert MS, Urquhart CRNA, Barbara RN. Hemodynamic Effects of Low Dose Epinephrine and Sodium Nitroprusside during Epidural Hypotensive Anesthesia. Regional Anesthesia. 1989;14:12. |
| 70. | Kiss H, Raffl M, Neumann D, Hutter J, Dorn U. Epinephrine-augmented hypotensive epidural anesthesia replaces tourniquet use in total knee replacement. Clin Orthop Relat Res. 2005;184-189. [PubMed] |
| 71. | Sharrock NE, Salvati EA. Hypotensive epidural anesthesia for total hip arthroplasty: a review. Acta Orthop Scand. 1996;67:91-107. [PubMed] |
| 72. | Danninger T, Stundner O, Ma Y, Bae JJ, Memtsoudis SG. The Impact of Hypotensive Epidural Anesthesia on Distal and Proximal Tissue Perfusion in Patients Undergoing Total Hip Arthroplasty. J Anesth Clin Res. 2013;4:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 73. | Juelsgaard P, Larsen UT, Sørensen JV, Madsen F, Søballe K. Hypotensive epidural anesthesia in total knee replacement without tourniquet: reduced blood loss and transfusion. Reg Anesth Pain Med. 2001;26:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 74. | Schmied H, Schiferer A, Sessler DI, Meznik C. The effects of red-cell scavenging, hemodilution, and active warming on allogenic blood requirements in patients undergoing hip or knee arthroplasty. Anesth Analg. 1998;86:387-391. [PubMed] |
| 75. | Karakaya D, Ustün E, Tür A, Bariş S, Sarihasan B, Sahinoğlu H, Güldoğuş F. Acute normovolemic hemodilution and nitroglycerin-induced hypotension: comparative effects on tissue oxygenation and allogeneic blood transfusion requirement in total hip arthroplasty. J Clin Anesth. 1999;11:368-374. [PubMed] |
| 76. | Oishi CS, D’Lima DD, Morris BA, Hardwick ME, Berkowitz SD, Colwell CW. Hemodilution with other blood reinfusion techniques in total hip arthroplasty. Clin Orthop Relat Res. 1997;132-139. [PubMed] |
| 77. | Olsfanger D, Fredman B, Goldstein B, Shapiro A, Jedeikin R. Acute normovolaemic haemodilution decreases postoperative allogeneic blood transfusion after total knee replacement. Br J Anaesth. 1997;79:317-321. [PubMed] |
| 78. | Goodnough LT, Despotis GJ, Merkel K, Monk TG. A randomized trial comparing acute normovolemic hemodilution and preoperative autologous blood donation in total hip arthroplasty. Transfusion. 2000;40:1054-1057. [PubMed] |
| 79. | Mielke LL, Entholzner EK, Kling M, Breinbauer BE, Burgkart R, Hargasser SR, Hipp RF. Preoperative acute hypervolemic hemodilution with hydroxyethylstarch: an alternative to acute normovolemic hemodilution? Anesth Analg. 1997;84:26-30. [PubMed] |
| 80. | Entholzner E, Mielke L, Plötz W, Malek A, Kling M, Burgkart R, Hargasser S, Hipp R. [Hypervolemic hemodilution as a means of preventing homologous blood transfusion. A simple alternative to acute normovolemic hemodilution]. Fortschr Med. 1994;112:410-414. [PubMed] |
| 81. | Bennett SR. Perioperative autologous blood transfusion in elective total hip prosthesis operations. Ann R Coll Surg Engl. 1994;76:95-98. [PubMed] |
| 82. | Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18:132-138. [PubMed] |
| 83. | Lerman DM, Rapp TB. Minimizing Blood Loss in Orthopaedic Surgery The Role of Antifibrinolytics. Bull Hosp Jt Dis (2013). 2015;73:83-89. [PubMed] |
| 84. | Meeran H. Should antifibrinolytics be used in orthopaedic surgery? Hosp Med. 2003;64:190. [PubMed] |
| 85. | Hsu CH, Lin PC, Kuo FC, Wang JW. A regime of two intravenous injections of tranexamic acid reduces blood loss in minimally invasive total hip arthroplasty: a prospective randomised double-blind study. Bone Joint J. 2015;97-B:905-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 86. | Zhang P, Liang Y, Chen P, Fang Y, He J, Wang J. Intravenous versus topical tranexamic acid in primary total hip replacement: A meta-analysis. Medicine (Baltimore). 2016;95:e5573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 87. | Drosos GI, Ververidis A, Valkanis C, Tripsianis G, Stavroulakis E, Vogiatzaki T, Kazakos K. A randomized comparative study of topical versus intravenous tranexamic acid administration in enhanced recovery after surgery (ERAS) total knee replacement. J Orthop. 2016;13:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Tzatzairis TK, Drosos GI, Kotsios SE, Ververidis AN, Vogiatzaki TD, Kazakos KI. Intravenous vs Topical Tranexamic Acid in Total Knee Arthroplasty Without Tourniquet Application: A Randomized Controlled Study. J Arthroplasty. 2016;31:2465-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 89. | Benoni G, Björkman S, Fredin H. Application of Pharmacokinetic Data from Healthy Volunteers for the Prediction of Plasma Concentrations of Tranexamic Acid in Surgical Patients. Clinical Drug Investigation. 1995;10:280. [RCA] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Sun X, Dong Q, Zhang YG. Intravenous versus topical tranexamic acid in primary total hip replacement: A systemic review and meta-analysis. Int J Surg. 2016;32:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | Meena S, Benazzo F, Dwivedi S, Ghiara M. Topical versus intravenous tranexamic acid in total knee arthroplasty. J Orthop Surg. 2017;25:230. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Xie J, Ma J, Yao H, Yue C, Pei F. Multiple Boluses of Intravenous Tranexamic Acid to Reduce Hidden Blood Loss After Primary Total Knee Arthroplasty Without Tourniquet: A Randomized Clinical Trial. J Arthroplasty. 2016;31:2458-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 93. | Maniar RN, Kumar G, Singhi T, Nayak RM, Maniar PR. Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res. 2012;470:2605-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 94. | Soni A, Saini R, Gulati A, Paul R, Bhatty S, Rajoli SR. Comparison between intravenous and intra-articular regimens of tranexamic acid in reducing blood loss during total knee arthroplasty. J Arthroplasty. 2014;29:1525-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 95. | Lin SY, Chen CH, Fu YC, Huang PJ, Chang JK, Huang HT. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty. 2015;30:776-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 96. | Shang J, Wang H, Zheng B, Rui M, Wang Y. Combined intravenous and topical tranexamic acid versus intravenous use alone in primary total knee and hip arthroplasty: A meta-analysis of randomized controlled trials. Int J Surg. 2016;36:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 97. | Sepah YJ, Umer M, Ahmad T, Nasim F, Chaudhry MU, Umar M. Use of tranexamic acid is a cost effective method in preventing blood loss during and after total knee replacement. J Orthop Surg Res. 2011;6:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Martin K, Wiesner G, Breuer T, Lange R, Tassani P. The risks of aprotinin and tranexamic acid in cardiac surgery: a one-year follow-up of 1188 consecutive patients. Anesth Analg. 2008;107:1783-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 99. | Mangano DT, Tudor IC, Dietzel C; Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 707] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 100. | Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, Teoh K, Duke PC, Arellano R, Blajchman MA. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 724] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 101. | Wang H, Shan L, Zeng H, Sun M, Hua Y, Cai Z. Is fibrin sealant effective and safe in total knee arthroplasty? A meta-analysis of randomized trials. J Orthop Surg Res. 2014;9:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 102. | Liu J, Cao JG, Wang L, Ma XL. Effect of fibrin sealant on blood loss following total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2014;12:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 103. | Molloy DO, Archbold HA, Ogonda L, McConway J, Wilson RK, Beverland DE. Comparison of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomised controlled trial. J Bone Joint Surg Br. 2007;89:306-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 104. | McConnell JS, Shewale S, Munro NA, Shah K, Deakin AH, Kinninmonth AW. Reducing blood loss in primary knee arthroplasty: a prospective randomised controlled trial of tranexamic acid and fibrin spray. Knee. 2012;19:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Choufani C, Barbier O, Bajard X, Ollat D, Versier G. [Medical and economic impact of a haemostatic sealant on the rate of transfusion after total knee arthroplasty]. Transfus Clin Biol. 2015;22:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 106. | Randelli F, D’Anchise R, Ragone V, Serrao L, Cabitza P, Randelli P. Is the newest fibrin sealant an effective strategy to reduce blood loss after total knee arthroplasty? A randomized controlled study. J Arthroplasty. 2014;29:1516-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Aguilera X, Martinez-Zapata MJ, Bosch A, Urrútia G, González JC, Jordan M, Gich I, Maymó RM, Martínez N, Monllau JC. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95:2001-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 108. | Clark CR, Spratt KF, Blondin M, Craig S, Fink L. Perioperative autotransfusion in total hip and knee arthroplasty. J Arthroplasty. 2006;21:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 109. | Bridgens JP, Evans CR, Dobson PM, Hamer AJ. Intraoperative red blood-cell salvage in revision hip surgery. A case-matched study. J Bone Joint Surg Am. 2007;89:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 110. | Muñoz M, García-Vallejo JJ, Ruiz MD, Romero R, Olalla E, Sebastián C. Transfusion of post-operative shed blood: laboratory characteristics and clinical utility. Eur Spine J. 2004;13 Suppl 1:S107-S113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 111. | Ramírez G, Romero A, García-Vallejo JJ, Muñoz M. Detection and removal of fat particles from postoperative salvaged blood in orthopedic surgery. Transfusion. 2002;42:66-75. [PubMed] |
| 112. | Dusik CJ, Hutchison C, Langelier D. The merits of cell salvage in arthroplasty surgery: an overview. Can J Surg. 2014;57:61-66. [PubMed] |
| 113. | Thomas D, Wareham K, Cohen D, Hutchings H. Autologous blood transfusion in total knee replacement surgery. Br J Anaesth. 2001;86:669-673. [PubMed] |
| 114. | Blatsoukas KS, Drosos GI, Kazakos K, Papaioakim M, Gioka T, Chloropoulou P, Verettas DA. Prospective comparative study of two different autotransfusion methods versus control group in total knee replacement. Arch Orthop Trauma Surg. 2010;130:733-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 115. | Sinclair KC, Clarke HD, Noble BN. Blood management in total knee arthroplasty: a comparison of techniques. Orthopedics. 2009;32:19. [PubMed] |
| 116. | Groenewold MD, Gribnau AJ, Ubbink DT. Topical haemostatic agents for skin wounds: a systematic review. BMC Surg. 2011;11:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 117. | Anderson LA, Engel GM, Bruckner JD, Stoddard GJ, Peters CL. Reduced blood loss after total knee arthroplasty with local injection of bupivacaine and epinephrine. J Knee Surg. 2009;22:130-136. [PubMed] |
| 118. | Yang CY, Chang CW, Chen YN, Chang CH. Intra-articular injection of bupivacaine and epinephrine does not save blood loss after total knee arthroplasty. BJJ. 2016;98:68. |
| 119. | Huang Z, Ma J, Shen B, Yang J, Zhou Z, Kang P, Pei F. Use of a Bipolar Blood-Sealing System During Total Joint Arthroplasty. Orthopedics. 2015;38:757-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 120. | Yim AP, Rendina EA, Hazelrigg SR, Chow LT, Lee TW, Wan S, Arifi AA. A new technological approach to nonanatomical pulmonary resection: saline enhanced thermal sealing. Ann Thorac Surg. 2002;74:1671-1676. [PubMed] |
| 121. | Samdani AF, Torre-Healy A, Asghar J, Herlich AM, Betz RR. Strategies to reduce blood loss during posterior spinal fusion for neuromuscular scoliosis: a review of current techniques and experience with a unique bipolar electrocautery device. Surg Technol Int. 2008;17:243-248. [PubMed] |
| 122. | Marulanda GA, Krebs VE, Bierbaum BE, Goldberg VM, Ries M, Ulrich SD, Seyler TM, Mont MA. Hemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop (Belle Mead NJ). 2009;38:E179-E183. [PubMed] |
| 123. | Kamath AF, Austin DC, Derman PB, Clement RC, Garino JP, Lee GC. Saline-coupled bipolar sealing in simultaneous bilateral total knee arthroplasty. Clin Orthop Surg. 2014;6:298-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 124. | Rosenthal BD, Haughom BD, Levine BR. A Retrospective Analysis of Hemostatic Techniques in Primary Total Knee Arthroplasty: Traditional Electrocautery, Bipolar Sealer, and Argon Beam Coagulation. Am J Orthop (Belle Mead NJ). 2016;45:E187-E191. [PubMed] |
| 125. | Nielsen CS, Gromov K, Jans Ø, Troelsen A, Husted H. No Effect of a Bipolar Sealer on Total Blood Loss or Blood Transfusion in Nonseptic Revision Knee Arthroplasty-A Prospective Study With Matched Retrospective Controls. J Arthroplasty. 2017;32:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 126. | Ferrari M, Zia S, Valbonesi M, Henriquet F, Venere G, Spagnolo S, Grasso MA, Panzani I. A new technique for hemodilution, preparation of autologous platelet-rich plasma and intraoperative blood salvage in cardiac surgery. Int J Artif Organs. 1987;10:47-50. [PubMed] |
| 127. | Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1:165-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 324] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 128. | Leo MS, Kumar AS, Kirit R, Konathan R, Sivamani RK. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J Cosmet Dermatol. 2015;14:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 129. | Akhundov K, Pietramaggiori G, Waselle L, Darwiche S, Guerid S, Scaletta C, Hirt-Burri N, Applegate LA, Raffoul WV. Development of a cost-effective method for platelet-rich plasma (PRP) preparation for topical wound healing. Ann Burns Fire Disasters. 2012;25:207-213. [PubMed] |
| 130. | Dhurat R, Sukesh M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J Cutan Aesthet Surg. 2014;7:189-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 382] [Cited by in RCA: 552] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 131. | Celotti F, Colciago A, Negri-Cesi P, Pravettoni A, Zaninetti R, Sacchi MC. Effect of platelet-rich plasma on migration and proliferation of SaOS-2 osteoblasts: role of platelet-derived growth factor and transforming growth factor-beta. Wound Repair Regen. 2006;14:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 132. | Hosgood G. Wound healing. The role of platelet-derived growth factor and transforming growth factor beta. Vet Surg. 1993;22:490-495. [PubMed] |
| 133. | Knighton DR, Hunt TK, Thakral KK, Goodson WH. Role of platelets and fibrin in the healing sequence: an in vivo study of angiogenesis and collagen synthesis. Ann Surg. 1982;196:379-388. [PubMed] |
| 134. | Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18:93-103. [PubMed] |
| 135. | Gardner MJ, Demetrakopoulos D, Klepchick PR, Mooar PA. The efficacy of autologous platelet gel in pain control and blood loss in total knee arthroplasty. An analysis of the haemoglobin, narcotic requirement and range of motion. Int Orthop. 2007;31:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 136. | Guerreiro JP, Danieli MV, Queiroz AO, Deffune E, Ferreira RR. Platelet-rich plasma (PRP) applied during total knee arthroplasty. Rev Bras Ortop. 2015;50:186-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 137. | Tingstad EM, Bratt SN, Hildenbrand KJ, O’Malley BA, Mitchell ER, Gaddis CE, Jacobson CA. Platelet-rich plasma does not decrease blood loss in total knee arthroplasty. Orthopedics. 2015;38:e434-e436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 138. | Schonauer C, Tessitore E, Barbagallo G, Albanese V, Moraci A. The use of local agents: bone wax, gelatin, collagen, oxidized cellulose. Eur Spine J. 2004;13 Suppl 1:S89-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 139. | Moo IH, Chen JY, Pagkaliwaga EH, Tan SW, Poon KB. Bone Wax Is Effective in Reducing Blood Loss After Total Knee Arthroplasty. J Arthroplasty. 2017;32:1483-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 140. | Solomon LB, Guevara C, Büchler L, Howie DW, Byard RW, Beck M. Does bone wax induce a chronic inflammatory articular reaction? Clin Orthop Relat Res. 2012;470:3207-3212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 141. | Teter KE, Bregman D, Colwell CW. The efficacy of intramedullary femoral alignment in total knee replacement. Clin Orthop Relat Res. 1995;117-121. [PubMed] |
| 142. | Batmaz AG, Kayaalp ME, Oto O, Bulbul AM. [Sealing of Femoral Tunnel with Autologous Bone Graft Decreases Blood Loss]. Acta Chir Orthop Traumatol Cech. 2016;83:348-350. [PubMed] |
| 143. | Ko PS, Tio MK, Tang YK, Tsang WL, Lam JJ. Sealing the intramedullary femoral canal with autologous bone plug in total knee arthroplasty. J Arthroplasty. 2003;18:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 144. | Jeon SH, Kim JH, Lee JM, Seo ES. Efficacy of extramedullary femoral component alignment guide system for blood saving after total knee arthroplasty. Knee Surg Relat Res. 2012;24:99-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 145. | Kandel L, Vasili C, Kirsh G. Extramedullary femoral alignment instrumentation reduces blood loss after uncemented total knee arthroplasty. J Knee Surg. 2006;19:256-258. [PubMed] |
| 146. | Meding JB, Berend ME, Ritter MA, Galley MR, Malinzak RA. Intramedullary vs extramedullary femoral alignment guides: a 15-year follow-up of survivorship. J Arthroplasty. 2011;26:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 147. | Andersen LØ, Husted H, Otte KS, Kristensen BB, Kehlet H. A compression bandage improves local infiltration analgesia in total knee arthroplasty. Acta Orthop. 2008;79:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 148. | Brock TM, Sprowson AP, Muller S, Reed MR. Short-stretch inelastic compression bandage in knee swelling following total knee arthroplasty study (STICKS): study protocol for a randomised controlled feasibility study. Trials. 2015;16:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 149. | Munk S, Jensen NJ, Andersen I, Kehlet H, Hansen TB. Effect of compression therapy on knee swelling and pain after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 150. | Pinsornsak P, Chumchuen S. Can a modified Robert Jones bandage after knee arthroplasty reduce blood loss? A prospective randomized controlled trial. Clin Orthop Relat Res. 2013;471:1677-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 151. | Cheung A, Lykostratis H, Holloway I. Compression bandaging improves mobility following total knee replacement in an enhanced recovery setting. J Perioper Pract. 2014;24:84-86. [PubMed] |
| 152. | Desteli EE, Imren Y, Aydın N. Effect of both preoperative andpostoperative cryoceutical treatment on hemostasis and postoperative pain following total knee arthroplasty. Int J Clin Exp Med. 2015;8:19150-19155. [PubMed] |
| 153. | Kullenberg B, Ylipää S, Söderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21:1175-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 154. | Song M, Sun X, Tian X, Zhang X, Shi T, Sun R, Dai W. Compressive cryotherapy versus cryotherapy alone in patients undergoing knee surgery: a meta-analysis. Springerplus. 2016;5:1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 155. | Schinsky MF, McCune C, Bonomi J. Multifaceted Comparison of Two Cryotherapy Devices Used After Total Knee Arthroplasty: Cryotherapy Device Comparison. Orthop Nurs. 2016;35:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 156. | Adie S, Naylor JM, Harris IA. Cryotherapy after total knee arthroplasty a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2010;25:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 157. | Yang Y, Yong-Ming L, Pei-jian D, Jia L, Ying-ze Z. Leg position influences early blood loss and functional recovery following total knee arthroplasty: A randomized study. Int J Surg. 2015;23:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 158. | Faldini C, Traina F, De Fine M, Pedrini M, Sambri A. Post-operative limb position can influence blood loss and range of motion after total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;23:852-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 159. | Wu Y, Yang T, Zeng Y, Si H, Li C, Shen B. Effect of different postoperative limb positions on blood loss and range of motion in total knee arthroplasty: An updated meta-analysis of randomized controlled trials. Int J Surg. 2017;37:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 160. | Muñoz M, Slappendel R, Thomas D. Laboratory characteristics and clinical utility of post-operative cell salvage: washed or unwashed blood transfusion? Blood Transfus. 2011;9:248-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 161. | Moonen AF, Knoors NT, van Os JJ, Verburg AD, Pilot P. Retransfusion of filtered shed blood in primary total hip and knee arthroplasty: a prospective randomized clinical trial. Transfusion. 2007;47:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 162. | Strümper D, Weber EW, Gielen-Wijffels S, Van Drumpt R, Bulstra S, Slappendel R, Durieux ME, Marcus MA. Clinical efficacy of postoperative autologous transfusion of filtered shed blood in hip and knee arthroplasty. Transfusion. 2004;44:1567-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 163. | Han CD, Shin DE. Postoperative blood salvage and reinfusion after total joint arthroplasty. J Arthroplasty. 1997;12:511-516. [PubMed] |
| 164. | Muñoz M, Ariza D, Campos A, Martín-Montañez E, Pavía J. The cost of post-operative shed blood salvage after total knee arthroplasty: an analysis of 1,093 consecutive procedures. Blood Transfus. 2013;11:260-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 165. | Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10:185-189. [PubMed] |
| 166. | Martin A, Prenn M, Spiegel T, Sukopp C, von Strempel A. [Relevance of wound drainage in total knee arthroplasty--a prospective comparative study]. Z Orthop Ihre Grenzgeb. 2004;142:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 167. | Li C, Nijat A, Askar M. No clear advantage to use of wound drains after unilateral total knee arthroplasty: a prospective randomized, controlled trial. J Arthroplasty. 2011;26:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 168. | Esler CN, Blakeway C, Fiddian NJ. The use of a closed-suction drain in total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 2003;85:215-217. [PubMed] |
| 169. | Hong KH, Pan JK, Yang WY, Luo MH, Xu SC, Liu J. Comparison between autologous blood transfusion drainage and closed-suction drainage/no drainage in total knee arthroplasty: a meta-analysis. BMC Musculoskelet Disord. 2016;17:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 170. | Senthil Kumar G, Von Arx OA, Pozo JL. Rate of blood loss over 48 hours following total knee replacement. Knee. 2005;12:307-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 171. | Stucinskas J, Tarasevicius S, Cebatorius A, Robertsson O, Smailys A, Wingstrand H. Conventional drainage versus four hour clamping drainage after total knee arthroplasty in severe osteoarthritis: a prospective, randomised trial. Int Orthop. 2009;33:1275-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 172. | Raleigh E, Hing CB, Hanusiewicz AS, Fletcher SA, Price R. Drain clamping in knee arthroplasty, a randomized controlled trial. ANZ J Surg. 2007;77:333-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 173. | Kim YH, Cho SH, Kim RS. Drainage versus nondrainage in simultaneous bilateral total knee arthroplasties. Clin Orthop Relat Res. 1998;188-193. [PubMed] |
| 174. | Yamada K, Imaizumi T, Uemura M, Takada N, Kim Y. Comparison between 1-hour and 24-hour drain clamping using diluted epinephrine solution after total knee arthroplasty. J Arthroplasty. 2001;16:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 175. | Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 176. | Tai TW, Jou IM, Chang CW, Lai KA, Lin CJ, Yang CY. Non-drainage is better than 4-hour clamping drainage in total knee arthroplasty. Orthopedics. 2010;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |