Published online Feb 18, 2017. doi: 10.5312/wjo.v8.i2.130
Peer-review started: June 19, 2016
First decision: July 27, 2016
Revised: September 23, 2016
Accepted: November 16, 2016
Article in press: November 18, 2016
Published online: February 18, 2017
Processing time: 243 Days and 3.7 Hours
To investigate the efficacy of a chitosan/polyethylene glycol blended paste as a local antibiotic delivery device, particularly in musculoskeletal wounds.
Acidic (A) chitosan sponges and neutralized (N) chitosan/polyethylene glycol (PEG) blended sponges were combined in ratios of 3A:2N, 1A:1N, and 2A:3N; then hydrated with phosphate buffered saline to form a chitosan/PEG paste (CPP). Both in vitro and in vivo studies were conducted to determine the potential CPP has as a local antibiotic delivery device. In vitro biocompatibility was assessed by the cytotoxic response of fibroblast cells exposed to the experimental groups. Degradation rate was measured as the change in dry mass due to lysozyme based degradation over a 10-d period. The antibiotic elution profiles and eluate activity of CPP were evaluated over a 72-h period. To assess the in vivo antimicrobial efficacy of the CPP, antibiotic-loaded paste samples were exposed to subcutaneously implanted murine catheters inoculated with Staphylococcus aureus. Material properties of the experimental paste groups were evaluated by testing the ejection force from a syringe, as well as the adhesion to representative musculoskeletal tissue samples.
The highly acidic CPP group, 3A:2N, displayed significantly lower cell viability than the control sponge group. The equally distributed group, 1A:1N, and the highly neutral group, 2A:3N, displayed similar cell viability to the control sponge group and are deemed biocompatible. The degradation studies revealed CPP is more readily degradable than the chitosan sponge control group. The antibiotic activity studies indicated the CPP groups released antibiotics at a constant rate and remained above the minimum inhibitory concentrations of the respective test bacteria for a longer time period than the control chitosan sponges, as well as displaying a minimized burst release. The in vivo functional model resulted in complete bacterial infection prevention in all catheters treated with the antibiotic loaded CPP samples. All experimental paste groups exhibited injectability and adhesive qualities that could be advantageous material properties for drug delivery to musculoskeletal injuries.
CPP is an injectable, bioadhesive, biodegradable, and biocompatible material with potential to allow variable antibiotic loading and active, local antibiotic release to prevent bacterial contamination.
Core tip: The study investigates the efficacy of a chitosan-polyethylene glycol paste as a local antibiotic delivery device to prevent bacterial infection, particularly in high risk, severe musculoskeletal wounds complex in shape and experiencing decreased vascularity. Research focusing on three different paste formulations categorized by the ratio of acidic to neutral components involved in vitro evaluation of the paste cytotoxicity, degradation, antibiotic elution, as well as an in vivo functional infection model evaluating the antimicrobial efficacy of the paste. Preliminary study outcomes demonstrate the potential of a chitosan-polyethylene glycol paste as a local antibiotic delivery device capable of infection prevention.
- Citation: Rhodes CS, Alexander CM, Berretta JM, Courtney HS, Beenken KE, Smeltzer MS, Bumgardner JD, Haggard WO, Jennings JA. Evaluation of a chitosan-polyethylene glycol paste as a local antibiotic delivery device. World J Orthop 2017; 8(2): 130-141
- URL: https://www.wjgnet.com/2218-5836/full/v8/i2/130.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i2.130
Musculoskeletal wounds are among the most prevalent types of injuries in the United States, accounting for over 60% of unintentional injuries per year[1], and are among the leading causes of death in all age groups[2]. Systemic antibiotic therapy is standard prophylactic treatment[3], but compromised vasculature in some complex musculoskeletal wounds reduces systemic distribution allowing for proliferation of contaminating bacteria and establishment of infection[4,5]. A complex musculoskeletal wound has an estimated 20% chance of becoming infected in a civilian[1,5,6] and 65% chance for soldiers suffering an open fracture in battlefield conditions[6,7]. Musculoskeletal infections severely impair wound healing[1] and can be complicated even further when antibiotic resistant and/or biofilm-forming bacterial strains are present, such as Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa (P. aeruginosa)[6,8,9], resulting in the need for higher concentrations of systemic antibiotics[10]. Increased systemic antibiotics can help clear infection, but may lead to adverse side effects[4]. Even with systemic antibiotics delivered in a clinical setting, patients still develop infections, reported by Mlynek et al[11]. Antibiotics present at levels below the minimum inhibitory concentration (MIC) in a S. aureus infection can lead to the development of more biofilm and a resistance to the antibiotic. One could rationally associate the peaks and troughs of antibiotic bioavailability seen in systemic delivery with the development of antibiotic resistant bacterial strains and biofilm. A local antibiotic delivery system could increase antibiotic levels at the musculoskeletal wound without increasing risk to the patient[10].
Vancomycin and amikacin are both optimal for local delivery to musculoskeletal trauma, and were used for all studies involving antibiotics. Vancomycin is effective against S. aureus which can evolve into methicillin resistant S. aureus, a difficult to treat strain of bacteria attracted to open and avascular musculoskeletal wounds. Amikacin has a broad spectrum of efficacy including against Gram negative bacteria, such as the biofilm forming P. aeruginosa. Both antibiotics are considered reliable because they’re capable of sustained activity over an extended elution time, storage time, or variable environmental conditions such as a low pH.
Biomaterials currently used as local antibiotic delivery devices, including polymethylmethacrylate and calcium sulfate, increase the local antibiotic levels within the tissue surrounding a wound; which could enhance treatment outcome for contaminated wounds. However, the current options present limitations such as surgical removal after a period of time[12,13], rapid degradation[14], or a limited choice of antibiotics utilized at the time of application[15]. These limitations, among others, drive the need for the development of novel local drug delivery devices in order to provide enhanced treatment over current devices; particularly in complex trauma wounds or patients with infection risk factors (i.e., diabetes, positive skin cultures, history of infection)[16-20]. The study objective is to develop a biocompatible, local drug delivery device capable of being loaded with physician-selected antimicrobials at the time of surgical intervention, presenting extended drug release profiles, and degrading in vivo.
Chitosan has been shown to be effective as a drug delivery system in several forms (i.e., films, sponges), capable of releasing antibiotics at a predictable rate with in vivo degradation[16,21-24]. Additionally, when blended with polyethylene glycol (PEG), chitosan sponges have demonstrated improved biocompatibility, biodegradability, and antibiotic release profiles compared to chitosan alone[17,18]. Chitosan devices have been approved by the Food and Drug Administration to be used clinically as a hemostatic wound dressing. Starting in 2003, HemCon wound dressings were used widespread by the United States military in combat operations in Iraq and Afghanistan to effectively control hemorrhaging injuries[25]. Results of previous studies investigating the antimicrobial drug delivery characteristics of sponges consisting of a chitosan/PEG blend as well as sponges made from chitosan alone support their use as local antibiotic delivery device[16-20]. However, chitosan sponges have shortcomings, including incomplete wound coverage and some implant migration[19,26]. A chitosan paste developed from sponges and modified with PEG was evaluated to address the shortcomings of sponges[17,18]. For this body of work, acidic chitosan and neutral PEG blended chitosan sponges were fabricated and combined in various ratios to form a chitosan/PEG paste (CPP).
The aim of the current study is to determine the feasibility of the CPP as a local drug delivery device for the prevention of bacterial wound infections by evaluating the following aspects: Biocompatibility, degradation, antibiotic elution, efficacy in preventing a biofilm-based bacterial infection, the ease of injection, and adhesion to musculoskeletal tissue.
All materials purchased from Fisher Scientific (Pittsburgh, PA) unless otherwise noted. Chitosan and PEG-blended chitosan products were prepared as previously reported using chitosan powder (Chitinor AS, Tromsø, Norway) and 6000g/mol PEG (Sigma Aldrich, St. Louis, MO)[17,18]. Chitosan and PEG were dissolved in a 1% acetic acid solution (v/v) containing 0.5% chitosan and 0.5% PEG (w/v), the PEG must first be dissolved then the chitosan added after. Control chitosan only solution was also made using a 1% acetic acid solution, but instead containing 1% chitosan (w/v). Chitosan/PEG and chitosan solutions (333 mL) were cast, frozen overnight (-80 °C), and lyophilized in a LabConco FreeZone 4.5 Liter Benchtop Freeze Dry System (Kansas City, MO) to create slightly acidic, dehydrated sponges. After lyophilization, the control chitosan sponges and the chitosan/PEG sponges were neutralized via submersion in NaOH solution. The chitosan/PEG sponges were submerged in 0.25 mol/L NaOH for 15 min and the control chitosan sponges were in 0.6 mol/L NaOH for 20 min, followed by rinsing cycles with distilled water until a neutral pH was reached. Finally, neutralized sponges were again frozen and lyophilized. Neutral chitosan sponges were used as the control for all experiments, excluding paste injectability evaluations. Acidic and neutral chitosan/PEG sponges were ground separately into a powder, with flake sizes ≤ 0.5 mm in diameter, using a blade grinder. Three different combinations of CPP were made by varying the mass ratios of acidic (A) chitosan to neutral (N) chitosan/PEG powder: 3A:2N, 1A:1N, and 2A:3N. Phosphate buffered saline (PBS) solution was used to hydrate the chitosan/PEG powder with a ratio of PBS volume to dry paste mass of 7.5. Dry CPP components used for biological testing and the infection prevention model were sterilized with ethylene oxide gas (EtO) prior to hydration, and PBS was sterile filtered.
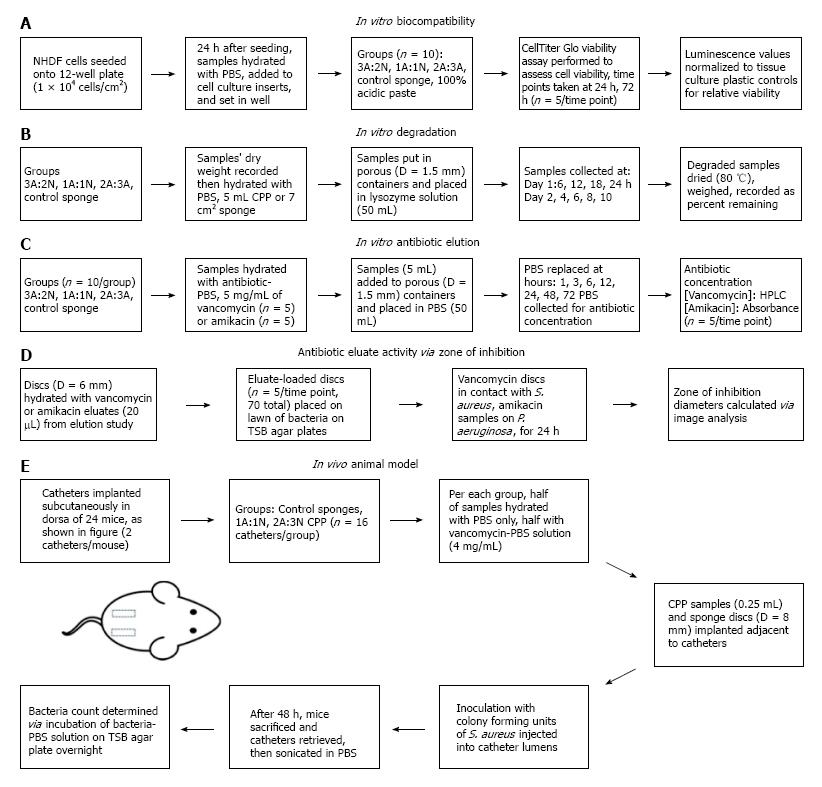
In vitro cytocompatibility was assessed using a modified protocol[18] (Figure 1A). Normal human dermal fibroblast (NHDF) cells (Lonza, Walkersville, MD) were seeded at 1.0 × 104 cells/cm2 in 12-well tissue culture plates in 1.5 mL of Dulbecco’s Modified Eagle’s Medium solution supplemented with 10% fetal bovine serum and 1 × antibiotic-antimycotic solution (100 units/mL Penicillin G, 100 μg/mL streptomycin sulfate, 0.25 μg/mL amphotericin B, Corning Inc., Manassas, VA). Twenty-four hours after cell seeding, hydrated paste samples (0.5 mL) and control sponge samples (8 mm diameter) were added to cell culture inserts (8 μm pore membrane, Corning Inc., Manassas, VA) (n = 5/group at each time point), and placed into wells containing NHDF cells and media. To asses NHDF viability, at 24 and 72 h post-exposure a Cell Titer-Glo® assay (Promega, Madison, WI) was used to measure cell viability using a BioTek Synergy H1 plate reader (Winooski, VT). Tissue culture plastic controls from each time point were used to calibrate luminescence values to a cell viability relative to the tissue culture plastic.
In vitro degradation was assessed by weight reduction over time based on a previous method[18] (Figure 1B). Sample weights were recorded before hydration (n = 5), and 5 mL paste samples or 22 mm × 23 mm sponges were placed in metal, hemispherical containers (Norpro, Everett, WA) with 1.5 mm diameter holes. Container openings were covered with para-film with appropriate lower holes occluded, in order to prevent leakage of CPP and still allow the transfer of media across the sample surface. The porous vessels containing the samples were placed inside plastic containers, para-film side down, in 50 mL of lysozyme solution (1 mg/mL Lysozyme Type VI, MP Biomedicals, Santa Ana, CA), and placed in an incubator on a shaker. Lysozyme is a naturally occurring enzyme found in human macrophages, among many other tissues and bodily fluid; used for its natural degradation of chitosan, as described by Varum et al[27]. Samples were gently shaken at 37 °C for the duration of the study. Time points were taken every 6 h through day 1, 24 h later at day 2, and subsequently every 48 h through day 10. Lysozyme solution was completely replaced every 6 h over the entire 10 d degradation period. Degraded samples were dried (80 °C), weighed, and percent CPP remaining was calculated.
The in vitro concentration release profile of vancomycin and amikacin was determined over 72 h by high performance liquid chromatography (HPLC) (Figure 1C). Seventy-two hours was selected as the average period of time between the debridement and irrigation of a complex musculoskeletal wound in a clinical setting[28]. Contrary to the degradation study, samples were hydrated with an antibiotic loaded PBS (5 mg/mL of amikacin or vancomycin, n = 5, separate groups per antibiotic type), and the porous hemispheres were placed in 50 mL of PBS, instead of a lysozyme solution. Samples were gently shaken at 37 °C for the duration of the study.
Samples used for the HPLC and antibiotic activity from the elution solution, were collected at 1, 3, 6, 12, 24, 48 and 72 h with complete elution solution replacement at each time point with 50 mL of PBS; thereby implementing infinite sink conditions. Using a modified HPLC protocol[29,30], vancomycin concentrations were measured utilizing a reversed-phase C18 column with mobile phase containing 35% acetonitrile and 65% phosphate buffer at 0.1 mol/L and 3 pH. Vancomycin had a 2.5 min retention time (1.0 mL/min flow rate, 250 nm UV detection). Vancomycin absorbance readings were normalized to corresponding concentration values via a standard curve made form serial dilutions of vancomycin.
Concentration of amikacin in eluates was determined via a spectrofluorometric method[31]. Amikacin eluate samples were reacted with acetylacetone and formaldehyde in a buffer solution containing a combination of boric, acetic, and phosphoric acid at a pH of 2.7. Closed sample vials were heated in an oven at 100 °C for 20 min. Absorbance of the reacted product was measured at 450 nm with a BioTek Synergy H1 plate reader (Winooski, VT). Absorbance values were normalized to concentrations via a standard curve of known amikacin concentrations.
Antibiotic activity of vancomycin and amikacin eluted from samples obtained in the elution study was determined using zone of inhibition (ZOI) as described by[32] (Figure 1D). On trypticase soy broth (TSB) agar plates, blank discs (6 mm diameter) hydrated with vancomycin or amikacin eluates (20 μL) were placed on lawns of S. aureus (ATCC 12598) or P. aeruginosa (ATCC 27317), respectively. S. aureus and P. aeruginosa were chosen as the representative bacterial strains because they are found in infected musculoskeletal wounds and are known to be capable of forming biofilm[33]. The sample size for each test group was n = 5. TSB agar plates were incubated (37 °C) and removed after 24 h for photography and measurement of ZOI diameters, excluding discs.
Animal care and use statement: Study protocols were approved by the University of Arkansas for Medical Sciences IACUC and all appropriate measures were taken to minimize pain and discomfort.
Following an established mouse model protocol[17,34,35] (Figure 1E) approved by the University of Arkansas for Medical Sciences IACUC (protocol #3608), 24 mice were used to assess in vivo prevention of biofilm-forming S. aureus (ATCC 49230) growth. The functional evaluation was used to determine the in vivo infection prevention efficacy of the CPPs. The more acidic CPP group (3A:2N) was not tested due to the results of the cytocompatibility study indicating its cytotoxic qualities.
Incisions 0.3 cm in length were made on the left and right dorsal surface of the hip and two polytetrafluoroethylene catheters, 1 cm length, were implanted subcutaneously into each mouse. Simultaneously, control 1% chitosan sponges, 1A:1N CPP, and 2A:3N CPP samples were hydrated with either a PBS-vancomycin solution, containing 4 mg/mL vancomycin, or PBS alone. There were six test groups with four animals (8 catheters) in each group. Paste samples (0.25 mL) were injected adjacent to each catheter using a U-100 insulin syringe (BD, Franklin Lakes, NJ) with needle removed. Control chitosan sponge discs (D = 8 mm) were also placed adjacent to each catheter via tweezers. Incisions were closed with surgical glue. S. aureus (UAMS-1) was injected into the catheter lumens at a concentration of 1 × 105 colony forming units (CFUs) in 2 μL of solution. Mice were sacrificed after 48 h and catheters were surgically removed and placed in a sterile saline solution and sonicated to remove biofilm. The resultant bacterial PBS solutions were serially diluted, plated on TSB agar, and incubated at 37 °C overnight in order to quantify the CFUs of S. aureus attached to each catheter.
Injectability was assessed by ejecting paste from a standard 25 mL repeater pipette syringe (Eppendorf; Hamburg, Germany) with 3.25 mm diameter tips (n = 3). Syringes were loaded with 6 mL of paste and fixed in an Instron 33R Universal Testing Machine model 4465 (Instron, Norwood, MA) with 5kN load cell, automated by Instron’s Bluehill 2 (v2.13) software, compressing the plunger 1 mm/s to fully eject paste. Injectability was also visually assessed by ejecting 0.5 mL of paste from modified repeater pipette syringes (n = 5).
To determine the quality of adhesion of CPP to a representative musculoskeletal tissue, porcine cervical vertebrae were used (Kroger, Memphis, TN) coated in fetal bovine serum simulating blood-like components. Adhesion was visually assessed by adhering 5 mL of paste or 22 mm × 23 mm sponge to FBS-coated tissue and timing adherence for a minimum of 1 min (n = 3). Samples were then doused with 10 mL of PBS, to partially simulate wound fluid exudate from the representative tissues, to determine if samples would dislodge.
The statistical methods for this study were reviewed by Dr. Amber Jennings, professor of Biostatistics in the department of Biomedical Engineering at the University of Memphis. For all applicable data, normality was determined via the Shapiro-Wilk test. Data from the in vitro degradation, biocompatibility, antibiotic elution, and antibiotic activity studies was analyzed using a two-way Analysis of Variance (ANOVA), and further analyzed via Holm-Sidak post hoc tests. Injectability and remaining CFUs from the in vivo model were analyzed using Kruskal-Wallis one-way ANOVA on ranks followed by Tukey post hoc tests. An a priori power analysis was performed using results from previous studies[35] by assuming standard deviation of 0.9 log-CFUs to determine eight catheters per group were required for 87% power to detect a mean difference of 1.85 log-CFUs between control and experimental groups at significance level P < 0.05. All results, excluding percent clearance of bacteria, are presented as average ± standard deviation (SD). SigmaPlot 12.5 (Systat Software Inc, San Jose, CA) was used for analysis. Statistical significance level was set at α = 5%.
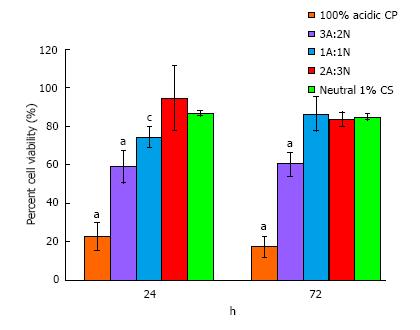
Lower cell viability at both time points (approximately 60%) was observed for 3A:2N CPP compared with all other samples (P≤ 0.006), excluding 100% acidic paste (P < 0.001; negative control) (Figure 2). 1A:1N CPP exhibited lower viability than 2A:3N CPP and neutral sponge (positive control) after 24 h (P≤ 0.025). However, after 72 h the groups displayed similar viability (P≥ 0.800). From microscopic observations, no evidence was found of cellular malformation, sloughing, or lysis for any samples except the 100% acidic paste.
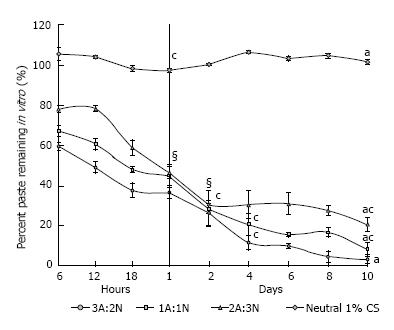
After undergoing lysozyme mass-based degradation, the pastes displayed a greater degradation rate for the first 24 to 48 h than the control sponge group. After approximately 48 h, steady degradation was shown for the 3A:2N and 1A:1N CPP groups at a higher rate than the 2A:3N group (Figure 3). The control group of 1% chitosan sponges remained at a constant mass through day 10. 3A:2N, 1A:1N, and 2A:3N CPP variations displayed significantly greater percentage loss of mass compared to control sponges (P≤ 0.001), 98%, 93% and 81%, respectively.
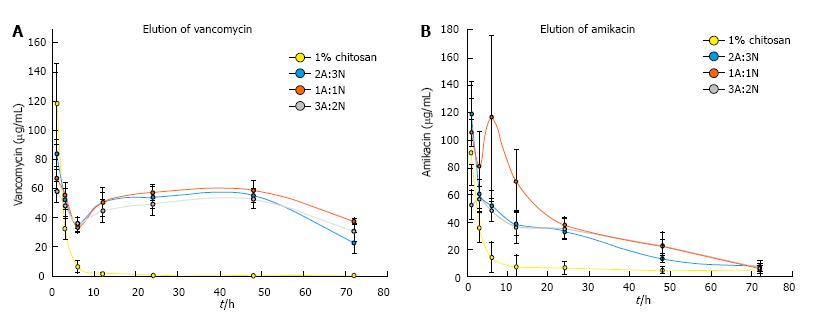
All paste variations steadily released vancomycin over 72 h. The control chitosan sponges released an initial, high burst with minimal to no release after 6 h. Vancomycin released from the CPP groups remained above the MIC for S. aureus of 2 μg/mL[36] through 72 h while sponges released vancomycin concentrations above the MIC through 6 h (Figure 4A). Percent vancomycin released from CPP was lower after 6 h and the initial burst release effect (P≤ 0.001), but the vancomycin concentrations increased, in part due to the degradation of the paste, through 48 h and was again significantly lower at 72 h (P≤ 0.001). Similarly to the vancomycin elution profile, all CPP groups released amikacin at a concentration above the MIC for P. aeruginosa of 4-25 μg/mL[16,37] for 48 h. The control chitosan sponges initially released amikacin at a very high concentration for 3 h then minimal elution after (Figure 4B).
Vancomycin eluates from the paste samples remained active against S. aureus through 72 h. The eluates from sponges only remained active through 6 h. Amikacin eluates from the CPP groups were active against P. aeruginosa through 24 h, while eluates from the control sponge group were active for 3 h (Table 1). Vancomycin eluates from sponges exhibited similar ZOI diameters to 2A:3N CPP at 1 h (P = 0.252) but significantly smaller than CPP at all other time points (P≤ 0.001). Although vancomycin eluates from the CPP groups exhibited significantly lower ZOI diameters at 72 h (P≤ 0.001), activity of S. aureus was still inhibited. Amikacin eluates from sponges also displayed similar ZOI diameters to the CPP groups at 1 h (P≥ 0.288), but the diameters quickly decreased to minimal levels by 6 h. All CPP sample eluates exhibited a significantly lower diameter at 48 and 72 h (P≤ 0.001), with only 3A:2N remaining active against P. aeruginosa through 48 h.
| Group | Antibiotic eluate time points (h) | ||||||
| 1 | 3 | 6 | 12 | 24 | 48 | 72 | |
| Staphylococcus aureus zone of inhibition diameter (mm, n = 5) of vancomycin eluates | |||||||
| 3A:2N CPP | 13 ± 1.1 | 11 ± 0.5a | 10 ± 0.4a | 12 ± 0.4 | 12 ± 0.4 | 12 ± 0.5 | 10 ± 0.8 |
| 1A:1N CPP | 13 ± 0.5 | 13 ± 1.1 | 11 ± 0.0a | 12 ± 0.4 | 11 ± 0.4a | 13 ± 0.4 | 10 ± 0.8 |
| 2A:3N CPP | 12 ± 0.4 | 11 ± 1.1 | 11 ± 0.5 | 11 ± 0.5 | 11 ± 0.5 | 12 ± 0.0 | 8 ± 0.9 |
| Neut 1% CS | 12 ± 0.7c | 7 ± 0.5 | 1 ± 3.1 | 0 | 0 | 0 | 0 |
| Pseudomonas aeruginosa zone of inhibition diameter (mm, n = 5) of amikacin eluates | |||||||
| 3A:2N CPP | 10 ± 0.9 | 9 ± 2.3 | 10 ± 2.2 | 8 ± 1.5 | 8 ± 1.8 | 2 ± 3.8 | 0 |
| 1A:1N CPP | 13 ± 2.2 | 10 ± 2.1 | 9 ± 1.1a | 5 ± 3.2a | 4 ± 3.8a | 0 | 0 |
| 2A:3N CPP | 11 ± 1.6 | 9 ± 1.3 | 8 ± 0.7a | 9 ± 1.6 | 5 ± 3.2a | 0 | 0 |
| Neut 1% CS | 10 ± 1.9c | 5 ± 3.2 | 0 | 0 | 0 | 0 | 0 |
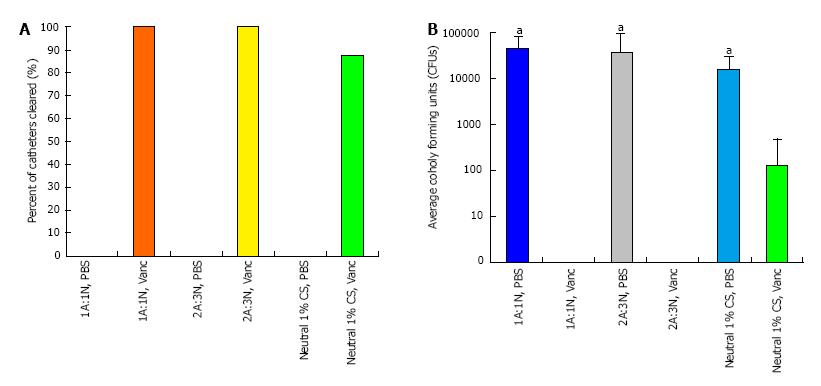
CPP and sponges proved effective in preventing S. aureus from contaminating implanted catheters. Vancomycin-loaded CPP resulted in 100% clearance rate of bacterial contamination on the catheters, while vancomycin-loaded sponges cleared bacteria from seven of eight catheters (88%) (Figure 5A). Additionally, PBS-only paste samples were ineffective in preventing bacterial contamination in all catheters; containing CFU levels between 103 and 104 (P < 0.001) (Figure 5B).
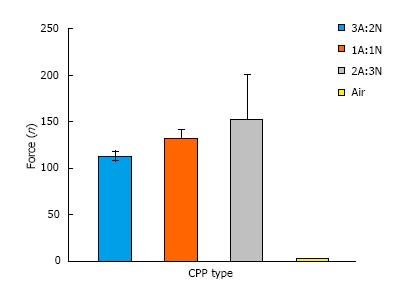
Average ejection forces for all CPP variations were less than or around 150N, and easily injectable with a handheld repeater pipette (Figure 6). It can be concluded the ratio of acidic to neutral components of the CPP may lead to a significant difference in the ejection force. It can also be concluded that acidity of paste components is inversely related to ejection force.
Adhesion tests illustrated that all variations of CPP and the control chitosan sponges were capable of adhering to both soft and hard tissue long enough to allow proper wound closure and prevent the paste from moving in order to elute antibiotics at the intended location. The completely acidic chitosan paste proved incapable of adhering to either type of tissue for a significant amount of time.
Complex musculoskeletal wound infections, especially those complicated by biofilm-forming bacteria, increase treatment duration, surgeries, total cost, and patient morbidity[1,6,8,9]. Systemic antibiotic therapy efficacy is substantially reduced at musculoskeletal wound sites with reduced bacteria-clearing ability. Delivery of very high dosages of antibiotics are effective but there is potential of causing adverse effects[4,5]. The clinical need for local drug delivery devices that effectively eliminate contaminating bacteria while also being biocompatible, biodegradable, and capable of antibiotic application at the time of surgical intervention resulted in chitosan and chitosan/PEG sponge development[16-20]. Paste development from these sponges was a modification designed to address device migration and wound coverage while maintaining the beneficial degradability, biocompatibility, and drug elution qualities[19,26]. The research evaluated whether chitosan/PEG blended sponges fabricated into an injectable form could result in a local antibiotic delivery device that will satisfy clinical needs. The preliminary study evaluates if chitosan/PEG in a paste form can perform as an effective localized delivery device of antibiotics with needed biocompatibility, degradability, antibiotic elution, and the functional in vivo antibacterial properties.
There are limitations of this preliminary study that should be noted. Primarily, the in vivo animal study of antibiotic activity did not investigate CPP loaded with amikacin against the bacterial contamination prevention of P. aeruginosa or polymicrobial infections. Second, the timeline of the in vitro drug delivery qualities of the CPP (i.e., degradation, antibiotic elution, biocompatibility) could be lengthened, which would further support the biocompatibility and degradation qualities of CPP. Third, the interaction of CPP with complex musculoskeletal wounds or orthopedic hardware is needed for a more accurate representation of a large, complex, and infected clinical musculoskeletal wound. The results of the in vitro and screening model for biofilm formation warrant further investigation into local delivery of antibiotics adjunctive to systemic delivery to prevent infection, especially in at-risk patients. In past studies using the murine catheter model, antibiotics administered systemically were only partially effective in preventing biofilm formation[35]. Therefore, to avoid confounding effects and also reduce animal number, systemic delivery of antibiotics was avoided in the present functional model. Since a portion of the CPP variations are acidic, degradation of the acidic components will inevitably lower the pH of the surrounding environment. There is potential for the lowered pH to affect the efficacy of vancomycin and amikacin. However, the in vitro antibiotic activity tests revealed there was not a significant effect on the efficacy of the antibiotics in question, suggesting that CPP has the potential to prevent and treat polymicrobial infections in musculoskeletal injuries.
Studies investigating local antibiotic delivery in gels and paste, other than chitosan derivatives, have reported similar results. Overstreet et al[38] investigated the local delivery of gentamicin from a hydrogel composed of polyN-isopropylacrylamide-co-dimethyl-γ-butyrolactone acrylate-co-Jeffamine® M-1000 acrylamide (PNDJ). It was discovered the gentamicin loaded PNDJ hydrogel was capable of releasing effective levels of gentamicin over 7 d and preventing infection in an in vivo model for up to 4 wk. However, there was no significant in vivo degradation, meaning the hydrogel may have to be surgically removed after a period of time. Additionally, renal dysfunction was observed at higher doses of applied gentamicin[38]. Pritchard et al[39] investigated the efficacy of a silk-fibroin based hydrogel as a local antibiotic delivery device, specifically, for use in avascular wounds. In vitro elution profiles of silk hydrogels loaded with ampicillin and penicillin produced eluates active against S. aureus for up to 72 h and 48 h, respectively. The in vivo efficacy of the ampicillin loaded silk hydrogel was tested on a S. aureus infected murine model over 24 h. Results indicated effective infection prevention from the silk hydrogel compared to the control. However, there was no significant difference from local injection of ampicillin alone due to the short test time period. Additionally, the study did not directly test the degradation qualities of the silk hydrogel. It was also noted antibiotics with low water solubility were difficult to conventionally load onto the silk hydrogel[39].
Studies have been published highlighting the drug delivery qualities of chitosan/PEG blended biomaterial. One study investigated a thermosensitive chitosan/PEG hydrogel drug delivery device administered as a nasal spray[40]; another developed an injectable chitosan-PEG-tyramine hydrogel to be used as tissue adhesives for wound healing[41]; while others have explored injectable PEG-grafted-chitosan thermosensitive hydrogels for sustained protein release[42] and drug delivery[43]. Many of the researched studies did not explicitly examine injectability. The devices were characterized as in situ forming hydrogels injectable from a needle, but the CPP would be injected through a larger cannula device due to higher viscosity. A study found a hydrogel made from tyramine modified polyethylene glycol grafted onto the backbone of a chitosan molecule exhibited similar adhesion to porcine skins as CPP did to porcine vertebral tissue[41]. Karn et al[44] determined the cationic nature of chitosan at pH below 6.5-7 is responsible for its mucoadhesion, the strong charge attraction between chitosan and mucins, negatively charged glycosylated proteins highly concentrated on tissue surfaces such as pulmonary, corneal, intestinal, and gastric mucosal tissues. Sogias et al[45] reported the mucoadhesive qualities of chitosan can be linked to the electrostatic attraction of the primary amino groups to the negatively charged mucins, as well as the attractive forces caused by hydrogen bonding between the chitosan and the mucosal membranes. Chitosan can adhere to a musculoskeletal wound via negatively charged molecules in the tissue such as the proteoglycans in connective tissue.
There are studies addressing degradation of chitosan/PEG hydrogel. Parker et al[18] developed neutral chitosan/PEG sponges for drug delivery utilizing 6000 g/mol PEG. In vitro degradation studies reported 55%-75% sponge remaining after 10 d of mass-based degradation, significantly higher than CPP with 0%-24% remaining, and 99%-100% of their neutral chitosan sponge remaining after 10 d, comparable to the neutral sponge (100% remaining). Acidic variations of chitosan, compared to neutralized, contain a protonated amine group. In aqueous solution, the proton dissociates from the amine group to join the water molecules; facilitating the solubilization of the chitosan molecules. Therefore, the lower degradation rates of neutral chitosan, compared to acidic, can be attributed to the lack of protonation with the surrounding environment[46]. Based on previous in vivo results[18,47] and the rapid in vitro degradation experienced by CPP, rapid degradation of CPP should be expected in vivo. Sample degradation was not measured in the in vivo studies due to the short time course of the subcutaneous catheter infection model, but ongoing evaluations will characterize time course of in vivo degradation. In previous in vitro studies, multiple chitosan/PEG hydrogels were reported to be biocompatible without eliciting significant cytotoxicity[40,43,48] along with various chitosan hydrogels[47,49] and chitosan/PEG sponges[17,18]. Biocompatibility studies involving indirect exposure of CPP to cell culture models demonstrated similar cell viability to control sponges, comparable to results of other in vitro studies[40,43,48]. Based on previously reported in vivo biocompatibility for other chitosan/PEG devices, a minimal[40,41,47] to moderate[18,43] inflammatory response is expected, which is comparable to other implanted devices[50].
Other chitosan/PEG hydrogels were successful in releasing bovine serum albumin and cyclosporin A[42,48]. Although in vitro release or activity of antibiotics was not investigated in the studies, the release profiles characterized by PEG content are still relevant. Bhattarai et al[42] concluded that a hydrogel with a PEG weight percentage of at least 40% demonstrated a burst release of albumin within the first 5 h followed by a steady linear release for approximately 70 h. Tsao et al[48] reported the grafting of a methoxy-poly(ethylene glycol) onto the hydrophobic chitosan results in a hydrophilic composite material. The hydrophobic-hydrophilic balance among the components instills a multi-stimuli-responsive property into the hydrogel. The degradation of the hydrogel can be controlled by stimuli involving salt concentration, solute concentration, temperature, and pH. Jiang et al[43] investigated the release of cyclosporin A from a PEG/chitosan hydrogel; reporting an absence of any significant burst effect and a sustained release at an effective concentration for three weeks in vitro and more than five weeks in vivo. Parker et al[17] reported an initial burst release of vancomycin from neutral chitosan/PEG and chitosan sponges after 1 h, with significant decrease in eluted antibiotic thereafter. Noel et al[16] investigated in vitro release of vancomycin from chitosan sponges made with lactic and acetic acid, showing an initial burst release after 1 h with 98% of the loaded vancomycin released after 72 h. The chitosan sponges used as controls in the present study experienced a similar initial burst release of vancomycin after 1 h with a sharp decrease to minimal levels thereafter. The CPP samples displayed a more extended release through 72 h, eluting 35% of the total loaded vancomycin after 72 h. While vancomycin concentrations measured by Parker et al[17] from chitosan/PEG blend sponges were above the MIC through 24 h, eluates utilized for turbidity testing only remained active against S. aureus through 6 h. Noel et al[16] found levels of vancomycin remained above the MIC and eluates remained active through 72 h, similar to findings with CPP. Noel et al[16] also reported amikacin eluates from chitosan sponges remaining active through 48 h while amikacin eluates from CPP remained active through 24 h. The PEG/chitosan sponges may not absorb as much solution as the sponges made of chitosan alone. The studies by Parker et al[17] and Noel et al[16] did not report the amount of antibiotics absorbed by the sponges while soaking in excess solution; highlighting the limitations of varying elution protocols as well as the need for confirmation of in vitro antibiotic elution results in functional animal models. The difference between the elution profiles of vancomycin compared to amikacin within chitosan drug delivery systems may be due to differences in antibiotic structure or charge. It is possible that vancomycin may associate with the chitosan/PEG leading to a more extended release.
Other chitosan/PEG hydrogels were successful in in vivo delivery of insulin[40] and cyclosporin A at effective levels[43], but none tested the in vivo activity of these therapeutic devices. Our control sponge cleared bacteria from 87.5% of catheters, while Parker et al[17] only reported a 50% clearance rate; however, the number of remaining viable CFUs in both studies was reduced compared to controls. All groups of vancomycin-loaded CPP displayed 100% bacterial clearance of catheter. Longer durations of study are necessary to determine the long-term infection prevention efficacy, as although bacterial counts may be reduced by local delivery of antibiotics, remaining viable bacteria could rebound and lead to infection.
In conclusion, CPP is an injectable, bioadhesive, biodegradable, and biocompatible material with potential to allow variable antibiotic loading at the time of surgical intervention, and active, local antibiotic release to prevent bacterial contamination. In vitro studies confirm CPP is more readily degradable, and displays similar cytocompatibility compared to other chitosan and chitosan/PEG drug delivery devices. CPP also demonstrated a uniform and extended antibiotic release over the course of 72 h. Antibiotic loaded CPP proved to be more effective than chitosan sponges in preventing biofilm formation in a murine catheter model. CPP, unlike other chitosan/PEG hydrogels that form in situ[40,43], must be injected through a larger cannula device, and exhibited adhesion to representative musculoskeletal tissues. The CPP formulations were found to have comparable antimicrobial effects to the control chitosan sponge devices and also infection prevention devices studied in previous studies. Future studies will modify the formulation to improve material properties, such as injectability, and to further evaluate the drug delivery devices in expanded preclinical models of complex extremity trauma, both with and without implanted hardware.
We thank Diego Velasquez Pulgarin for his help with cell culture among other contributions, Michael Harris for his help with HPLC, Carlos Wells for helping with the in vivo mouse model surgeries at the University of Arkansas for Medical Sciences, Marsalas Whitaker for his help with degradation solution changes, Daniel Ahn for his help with adhesion testing, and Drs Keaton Smith and Ashley Parker for their guidance in the laboratory and studies preceding this research.
Complex musculoskeletal wounds are highly susceptible to polymicrobial infections; severely impairing the healing process and threatening patient health. Systemic delivery of antibiotics can be ineffective in preventing bacterial infection due to the lack of vascularity surrounding a severe complex musculoskeletal wounds. Therefore, a method of locally delivering antibiotics to the wound could prove effective in the prevention of a bacterial infection. Utilizing previously developed chitosan sponges, a polyethylene glycol-chitosan blended paste was investigated as a local delivery device. Chitosan/PEG paste (CPP) was evaluated as an injectable, adhesive device to determine biodegradability, biocompatibility, antibiotic elution and activity, and in vivo efficacy.
Chitosan is being researched as a drug delivery device because it is biocompatible, degradable by enzymes found in the body such as lysozyme, and adhesive to mucins on the surface of visceral tissues. It can be loaded with different types of drugs and applied directly to a wound as a paste, hydrogel, or wound dressing to locally deliver the designated drugs to the surrounding injured tissue. The diversity of drugs chitosan is capable of delivering make it a versatile component to be used in drug delivery devices. The addition of polyethylene glycol to chitosan increases the rate of degradation and also improves the drug releasing ability of the drug delivery device.
The local delivery of antibiotics provides an effective concentration of infection preventing molecules sustained at a more steady concentration over an extended amount of time compared to the peaks and troughs associated systemic delivery. The decrease in bioavailability of antibiotics during breaks from systemic delivery leaves the wound vulnerable to a bacterial infection. The bacterial population can become resistant to a particular antibiotic if it is being exposed to levels below the minimum inhibitory concentration. Antibiotic loaded CPP has shown to be capable of locally releasing antibiotics while maintaining biocompatibility and being completely biodegradable; qualities that are advantages compared to common methods of local drug delivery such as calcium sulfate and polymethylmethacrylate beads.
In vitro test results suggest CPP is capable of being loaded with amikacin and successfully inhibiting the growth of Pseudomonas aeruginosa (P. aeruginosa), a representative Gram negative bacteria. Both in vitro studies and in vivo functional models proved the bacterial prevention efficacy of vancomycin-loaded CPP against Staphylococcus aureus (S. aureus), a representative Gram positive bacteria. Therefore, the CPP is being researched as an adjunctive method of bacterial contamination prevention in wounds; particularly in complex musculoskeletal wounds that are inherently at a higher risk of becoming infected.
Chitosan is the deacetylated form chitin, the most abundant naturally occurring amino-polysaccharide found in arthropod exoskeletons. Polyethylene glycol is a hydrophilic polyether compound; blended with chitosan to increase the dissociation rate of the paste in an aqueous environment. Biofilm forms when bacteria or other microorganisms attach to a surface, such as a metal implant or damaged tissue, and causes infection that is highly resistant to antibiotics or immune system clearance. S. aureus and P. aeruginosa are among the common pathogens that cause infections in the musculoskeletal system with the formation of biofilm.
The paper is of interest and well-organized. The aims and objectives are clear, the hypothesis is sound and the data are well presented.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Campo GM, Cui Q S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Andersson GB, Bouchard J, Bozic KJ. The burden of musculoskeletal diseases in the United States: Prevalence, societal and economic cost. Rosemont, IL: American Academy of Orthopaedic Surgeons 2008; . |
| 2. | Bernstein J. Musculoskeletal Medicine. Rosemont, IL: American Academy of Orthopaedic Surgeons 2003; . |
| 3. | Park H, Copeland C, Henry S, Barbul A. Complex wounds and their management. Surg Clin North Am. 2010;90:1181-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Hanssen AD. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin Orthop Relat Res. 2005;91-96. [PubMed] |
| 5. | Skinner HB. Current diagnosis and treatment in orthopedics. New York: Lange Medical Books/McGraw-Hill Medical Pub. Div 2006; . |
| 6. | Esterhai JL, Gristina AG, Poss R. Musculoskeletal infection. Park Ridge, IL: American Academy of Orthopaedic Surgeons 1992; . |
| 7. | Zalavras CG, Patzakis MJ, Holtom PD, Sherman R. Management of open fractures. Infect Dis Clin North Am. 2005;19:915-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Davis JS. Management of bone and joint infections due to Staphylococcus aureus. Intern Med J. 2005;35 Suppl 2:S79-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol. 2008;52:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 10. | Diefenbeck M, Mückley T, Hofmann GO. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury. 2006;37 Suppl 2:S95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Mlynek KD, Callahan MT, Shimkevitch AV, Farmer JT, Endres JL, Marchand M, Bayles KW, Horswill AR, Kaplan JB. Effects of Low-Dose Amoxicillin on Staphylococcus aureus USA300 Biofilms. Antimicrob Agents Chemother. 2016;60:2639-2651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Gitelis S, Brebach GT. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. J Orthop Surg (Hong Kong). 2002;10:53-60. [PubMed] |
| 13. | McLaren AC. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin Orthop Relat Res. 2004;101-106. [PubMed] |
| 14. | Robinson D, Alk D, Sandbank J, Farber R, Halperin N. Inflammatory reactions associated with a calcium sulfate bone substitute. Ann Transplant. 1999;4:91-97. [PubMed] |
| 15. | Bronzino JD. The biomedical engineering handbook. Boca Raton: CRC Press 1995; . |
| 16. | Noel SP, Courtney HS, Bumgardner JD, Haggard WO. Chitosan sponges to locally deliver amikacin and vancomycin: a pilot in vitro evaluation. Clin Orthop Relat Res. 2010;468:2074-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Parker AC, Beenken KE, Jennings JA, Hittle L, Shirtliff ME, Bumgardner JD, Smeltzer MS, Haggard WO. Characterization of local delivery with amphotericin B and vancomycin from modified chitosan sponges and functional biofilm prevention evaluation. J Orthop Res. 2015;33:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Parker AC, Rhodes C, Jennings JA, Hittle L, Shirtliff M, Bumgardner JD, Haggard WO. Preliminary evaluation of local drug delivery of amphotericin B and in vivo degradation of chitosan and polyethylene glycol blended sponges. J Biomed Mater Res B Appl Biomater. 2016;104:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Stinner DJ, Noel SP, Haggard WO, Watson JT, Wenke JC. Local antibiotic delivery using tailorable chitosan sponges: the future of infection control? J Orthop Trauma. 2010;24:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Parker AC, Jennings JA, Bumgardner JD, Courtney HS, Lindner E, Haggard WO. Preliminary investigation of crosslinked chitosan sponges for tailorable drug delivery and infection control. J Biomed Mater Res B Appl Biomater. 2013;101:110-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Dash M, Chiellini F, Ottenbrite RM, Chiellini E. Chitosan - A versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci. 2011;36:981-1014. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1921] [Cited by in RCA: 1614] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 22. | Noel SP, Courtney H, Bumgardner JD, Haggard WO. Chitosan films: a potential local drug delivery system for antibiotics. Clin Orthop Relat Res. 2008;466:1377-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Smith JK, Moshref AR, Jennings JA, Courtney HS, Haggard WO. Chitosan sponges for local synergistic infection therapy: a pilot study. Clin Orthop Relat Res. 2013;471:3158-3164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Phaechamud T, Charoenteeraboon J. Antibacterial activity and drug release of chitosan sponge containing doxycycline hyclate. AAPS PharmSciTech. 2008;9:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60:655-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 394] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 26. | Jones LC, Frondoza C, Hungerford DS. Effect of PMMA particles and movement on an implant interface in a canine model. J Bone Joint Surg Br. 2001;83:448-458. [PubMed] |
| 27. | Vårum KM, Myhr MM, Hjerde RJ, Smidsrød O. In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr Res. 1997;299:99-101. [PubMed] |
| 28. | Owens BD, White DW, Wenke JC. Comparison of irrigation solutions and devices in a contaminated musculoskeletal wound survival model. J Bone Joint Surg Am. 2009;91:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Sattur AP, Lee JH, Song KB, Panda T, Kim CH, Rhee SK, Gokul B. Analytical techniques for vancomycin - A review. Biotechnol Bioproc E. 2000;5:153-158. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | McClain JB, Bongiovanni R, Brown S. Vancomycin quantitation by high-performance liquid chromatography in human serum. J Chromatogr. 1982;231:463-466. [PubMed] |
| 31. | Omar MA, Ahmed HM, Hammad MA, Derayea SM. Validated spectrofluorimetric method for determination of selected aminoglycosides. Spectrochim Acta A Mol Biomol Spectrosc. 2015;135:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Ericsson H, Tunevall G, Wickman K. The paper disc method for determination of bacterial sensitivity to antibiotics. Relationship between the diameter of the zone of inhibition and the minimum inhibitory concentration. Scand J Clin Lab Invest. 1960;12:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3777] [Cited by in RCA: 3125] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 34. | Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ, Blevins JS, Smeltzer MS. Global gene expression in Staphylococcus aureus biofilms. J Bacteriol. 2004;186:4665-4684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 450] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 35. | Weiss EC, Zielinska A, Beenken KE, Spencer HJ, Daily SJ, Smeltzer MS. Impact of sarA on daptomycin susceptibility of Staphylococcus aureus biofilms in vivo. Antimicrob Agents Chemother. 2009;53:4096-4102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012;54:755-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 396] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 37. | Nakamura A, Hosoda M, Kato T, Yamada Y, Itoh M, Kanazawa K, Nouda H. Combined effects of meropenem and aminoglycosides on Pseudomonas aeruginosa in vitro. J Antimicrob Chemother. 2000;46:901-904. [PubMed] |
| 38. | Overstreet D, McLaren A, Calara F, Vernon B, McLemore R. Local gentamicin delivery from resorbable viscous hydrogels is therapeutically effective. Clin Orthop Relat Res. 2015;473:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Pritchard EM, Valentin T, Panilaitis B, Omenetto F, Kaplan DL. Antibiotic-Releasing Silk Biomaterials for Infection Prevention and Treatment. Adv Funct Mater. 2013;23:854-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 40. | Wu J, Wei W, Wang LY, Su ZG, Ma GH. A thermosensitive hydrogel based on quaternized chitosan and poly(ethylene glycol) for nasal drug delivery system. Biomaterials. 2007;28:2220-2232. [PubMed] |
| 41. | Lih E, Lee JS, Park KM, Park KD. Rapidly curable chitosan-PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta Biomater. 2012;8:3261-3269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 42. | Bhattarai N, Ramay HR, Gunn J, Matsen FA, Zhang M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J Control Release. 2005;103:609-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 446] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 43. | Jiang G, Sun J, Ding F. PEG-g-chitosan thermosensitive hydrogel for implant drug delivery: cytotoxicity, in vivo degradation and drug release. J Biomater Sci Polym Ed. 2014;25:241-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Karn PR, Vanić Z, Pepić I, Skalko-Basnet N. Mucoadhesive liposomal delivery systems: the choice of coating material. Drug Dev Ind Pharm. 2011;37:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Sogias IA, Williams AC, Khutoryanskiy VV. Why is chitosan mucoadhesive? Biomacromolecules. 2008;9:1837-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 511] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 46. | Rinaudo M. Chitin and chitosan: Properties and applications. Prog Polym Sci. 2006;31:603-632. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5089] [Cited by in RCA: 4040] [Article Influence: 212.6] [Reference Citation Analysis (0)] |
| 47. | De Souza R, Zahedi P, Allen CJ, Piquette-Miller M. Biocompatibility of injectable chitosan-phospholipid implant systems. Biomaterials. 2009;30:3818-3824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Tsao CT, Hsiao MH, Zhang MY, Levengood SL, Zhang M. Chitosan-PEG hydrogel with sol-gel transition triggerable by multiple external stimuli. Macromol Rapid Commun. 2015;36:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Tan H, Chu CR, Payne KA, Marra KG. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30:2499-2506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 881] [Cited by in RCA: 674] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 50. | Anderson JM. Inflammatory response to implants. ASAIO Trans. 1988;34:101-107. [PubMed] |