Published online Nov 18, 2017. doi: 10.5312/wjo.v8.i11.829
Peer-review started: March 10, 2017
First decision: June 30, 2017
Revised: July 5, 2017
Accepted: September 12, 2017
Article in press: September 13, 2017
Published online: November 18, 2017
Processing time: 255 Days and 0.4 Hours
To reduce post treatments of kyphoplasty, as a common treatment for osteoporotic vertebrae.
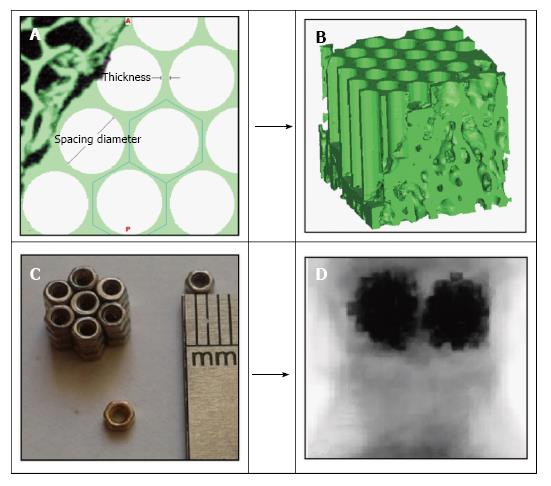
This study suggests a new method for treating vertebrae by setting the hexagonal porous structure instead of the rigid bone cement mass in the kyphoplasty (KP). The KP procedure was performed on the fresh ovine vertebra of the level L1. Micro finite element modeling was performed based on micro computed tomography of ovine trabecular cube. The hexagonal porous structure was set on one cube instead of the bone cement mass. For the implant designing, two geometrical parameters were considered: Spacing diameter and thickness.
The results of micro finite element analyses indicated the improvement in the mechanical behavior of the vertebra treated by the hexagonal porous structures, as compared to those treated by vertebroplasty (VP) and KP under static loading. The improvement in the mechanical behavior of the vertebra, was observed as 54% decrease in the amount of maximum Von Misses stress (improvement of stress distribution), in trabecular cube with embedded hexagonal structure, as compared to VP and KP. This is comparable to the results of the experimental study already performed; it was shown that the improvement of mechanical behavior of the vertebra was observed as: 83% increase in the range of displacements before getting to the ultimate strength (increasing the toughness) after setting hexagonal pearls inside vertebrae. Both the material and geometry of implant influenced the amount of Von Mises stress in the structure.
The new proposed method can be offered as a substitute for the KP. The implant geometry had a more obvious effect on the amount of Von Mises stress, as compared to the implant material.
Core tip: By embedding the hexagonal porous structure with two variable parameters including spacing diameter and thickness, as a substitute for the bone cement mass in the vertebral kyphoplasty, lower levels of maximum Von Mises stress could be achieved, thereby indicating the reduction of stress concentration in the interface area between the bone cement mass and the cancellous bone, as well as the reduction of post treatments. Furthermore, setting porous structures with different geometries inside vertebrae could provide the possibility of bone regeneration, the transfer of growth factors and recreation of mechanical properties.
- Citation: Faradonbeh SAH, Jamshidi N. Biomechanical assessment of new surgical method instead of kyphoplasty to improve the mechanical behavior of the vertebra: Micro finite element study. World J Orthop 2017; 8(11): 829-835
- URL: https://www.wjgnet.com/2218-5836/full/v8/i11/829.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i11.829
Vertebroplasty (VP) and kyphoplasty (KP) as minimally invasive surgeries, consume poly methyl methacrylate (PMMA) and calcium phosphate as the bone cement for the treatment of patients with osteoporotic disorders in vertebral column. But one important challenge affecting the quality of procedure is the leakage of bone cement. Also the mechanical behavior of treated vertebra can affect biomechanics of the whole spine[1-3]. It is known that the KP does not guarantee the stoppage of fractures. Based on the studies of Polikeit et al[4], the strength of the treated vertebrae can be regained by cement augmentation, while it increases the endplate bulge and generates some altered load transfer in adjacent vertebrae. Then the rigid cement augmentation could facilitate the subsequent collapse of adjacent vertebrae.
According to a careful literature review performed by Wilcox[5], the main factors affecting the spine performance after cement augmentation can be classified in three groups: (1) the cement properties and volume; (2) the features of connection between the structure and vertebra; and (3) the spine properties. The bone and cement interface is important in providing the longer term stability of the construction. Cement augmentation improves motion segment stiffness, while, it alters the bone stress distributions in the treated and adjacent segments.
Keller et al[6], indicated that cement injection affects the stress distribution in both the vertebra and adjacent segments. Rohlmann et al[7] proved that the wedge shaped vertebral body could alter the center of the gravity of the upper body. This shift was compensated by the KP leading to a lower muscle force and an increase in the spinal load. The cement augmentation increases the intradiscal pressure in adjacent discs with a slight increase in Von Mises stress in vertebral endplates. Based on attempts of Liang et al[8], the asymmetrical cement distribution inside the treated vertebra led to the unrelieved pain after percutaneous vertebral augmentation. The insufficient distribution of the bone cement increased the displacement of augmented vertebral body. Tschirhart et al[9] and Xu et al[10], both emphasized that in the case of severe fractures, cement augmentation could worsen the fracture, leading to the cement leakage with subsequent problems; this indicated the uncertainty in the results of VP. Baroud et al[11], emphasized that both experimental study and finite element modeling are often focused on the effect of type of bone cement and volume. To reduce the risk of adjacent fracture after cement augmentation, Boger et al[12] suggested consuming the low modulus cement (consisting of the regular bone cement (PMMA) and the low-modulus cement prepared with Vertecem by the addition of an aqueous fraction of 35% sodium hyaluronate).
Kinzl et al[13], emphasized that stress concentration in trabecular bone is based on cement distribution. Basically, in the KP, the bone cement and trabecular structure are separated. So the whole structure is not homogeneous and the stress concentration can be seen in the interface area which is the reason of occurring micro fractures. The result of studies of Kosmopoulos et al[14] and Kettler et al[15], indicated that the main problems with the cement augmentation are: (1) stress concentration in the interface area; and (2) asymmetrical cement distribution. Baroud et al[16], proved that the wedge shaped vertebrae, induced a shift in the center of gravity of the upper body and therefore increased the intradiscal pressure and stress on endplates. Compensation of this shift by the KP procedure decreased the erector spinae force and also, the axial force in spinal column. Armsen et al[17], emphasized that in the case of the long term stability of the structure build up from the bone cement and trabecular bone, two main factors must be considered. First, it should be noted that the cytotoxicity of poly methyl methacrylate prevents the osteointegration; second, the osteoporosis as a progressive disease weakens the structure of the trabecular bone. Considering the necessity of setting a structure to solve mentioned problems, Garzón-Alvarado et al[18], suggested setting porous structures. Verhulp et al[19], emphasized that the structural complexity in trabecular bone could be the reason of existence of a wide range of differences in the amount of Von Misses stress in the structure. Landgraft et al[20], attempted to simulate the generation of microstructural models of human trabecular bone and the acrylic bone cement injection with the Finite Element Method (FEM) of cement curing inside vertebrae based on micro computed tomography (μCT) scanning.
Considering the results of all studies conducted to address the limitations of cement augmentation and the problems that patients may encounter after the VP and KP, the main drawbacks of these procedures can be classified as: (1) asymmetrical cement distribution inside the vertebra; (2) stress concentration in the interface area between cement mass and bone in the KP; (3) the risk of occurring fractures in the adjacent vertebrae; (4) the risk of cement leakage while augmenting; and (5) as an important case, different outcomes of patients due to ignoring the morphological parameters of trabecular bone such as trabecular thickness (Tb. Th) and trabecular spacing (Tb. Sp) in treating osteoporotic vertebrae.
In this study, the hexagonal porous structure with the low rate of mass/volume and high stability, is presented with defined geometrical parameters including thickness and spacing diameter, as determining factors to build the implant with optimum design and therefore, to reduce post treatments. Furthermore, setting porous structures with different geometries inside the vertebra, could provide the possibility of tissue regeneration, the transfer of growth factors and the recreation of mechanical properties.
Two L1 ovine vertebral bodies were chosen for the VP and KP procedures. Cement augmentation was performed according to common instructions already brought in the literature. The VP and KP procedures were performed by needle insertion through the pedicle. The PMMA was used as the bone cement. The volume fraction for the consumed PMMA was 20% of the whole vertebral volume. The cement distribution was checked by CT scanning of the samples after cement augmentation.
To reconstruct three dimensional micro structure of trabecular bone, a micro computed tomography (μCT 100, SCANCO Medical AG, Switzerland) was used for a specimen treated by VP and KP. To evaluate the regional variations of stress distribution inside the vertebrae, each specimen was subdivided to 9 smaller cubes with the size of 5 mm × 5 mm × 5 mm in 3 layers (Figure 1). Then the model was imported into the analytical software ABAQUS 6.14.
The hexagonal porous structure was set on one cube instead of the bone cement mass. For designing the implant, two geometrical parameters were considered as: Spacing diameter and thickness (Figure 2). The implant design was performed in four groups with different geometries. The geometrical characteristics of the hexagonal structure including thickness and spacing diameter, are presented in Table 1. There was also a pattern among the designed implants: The thickness of the structure was decreased simultaneously with growing the spacing diameter from the models 1 to 4. The hexagonal porous implants were designed in the way to replace the cement mass on one cube in four models with two groups of materials: Steel and titanium. The porous structure is set in the space already occupied by the cement mass. The symmetric circle pattern was used to construct the hexagonal structure equivalent to the experimental study[21], previously performed. Material properties including elastic modulus and Poisson ratio used in the FEM are shown in Table 2.
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Thickness (mm) | 0.5 | 0.4 | 0.3 | 0.2 |
| Spacing diameters (mm) | 1 | 1.5 | 2 | 2.3 |
| Components | Elastic modulus (MPa) | Poisson ratio |
| Trabecular bone | 30 | 0.2 |
| Bone cement | 2530 | 0.2 |
| Steel | 200e3 | 0.3 |
| Titanium | 110e3 | 0.3 |
In accordance with micro finite element analyses (μFEA) performed by Gong et al[22], to simulate the experimental testing conditions, a displacement load was applied as 1% compressive strain on the longitudinal direction with the full constraints at the bottom of each trabecular cube.
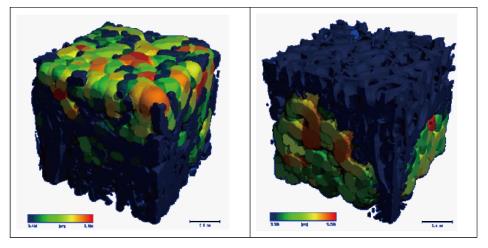
The results of μFEA of cubes related to VP and KP indicated that the maximum Von misses stress in the cubes of the VP was less than that of the KP. Basically, the mechanical behavior of a construction made of the bone cement and trabecular structure is based on the cement distribution. As shown in Figure 3, the cement distribution in trabecular cubes is totally different in the VP and KP.
The results of μFEA of the cubes surrounding the injected cement mass in both VP and KP indicated the low regional variations in the amount of maximum Von Misses stress in the cubes 1 to 6 with the average value of 21.7 MPa and 36.6 MPa for the VP and KP, respectively. Also, in the third layer without cement penetration, for cubes 7 to 9, the difference between the amounts of maximum Von Misses stress in the cubes was not considerable and had the average value of 7.3 and 7.21 MPa for the VP and KP.
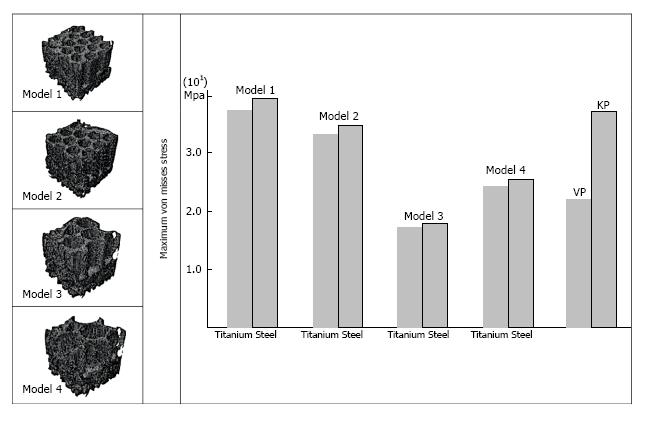
The low regional variation in the cubes with combination of bone cement and trabecular bone in the KP, made the selection of each cube for embedding the hexagonal structure, a reasonable statistical one. Therefore, the results of analyses for the selected cube were generalizable to the whole vertebra. The maximum Von misses stress in the cube 2, with the embedded hexagonal porous structure with different geometries, instead of the cement mass, is shown in Figure 4. The maximum Von Misses stress in the cube with hexagonal structure was more than that of the same cube treated by KP procedures at first. But after implementing different geometrical parameters of the hexagonal structure, the maximum Von Misses stress was decreased in the whole cube. This was the sign of altered stress distribution inside vertebrae.
For the model 1, with the thickness of 0.5 mm and spacing diameter of 1 mm, the maximum Von-Misses stress for the implant material of steel and titanium was 38.2 and 37.8 MPa, respectively. For the model 2, with 0.4 and 1.5 mm for the thickness and spacing diameter, the maximum Von-Misses stress reduced to 34 and 32.2 MPa for steel and titanium implants, respectively. This decreasing trend in the amount of Von Misses stress was continued until reaching to 16.5 and 16.4 MPa for the steel and titanium implants in the model 3, with the 0.3 and 2 mm for the thickness and spacing diameters. But after this, increasing the spacing diameter and decreasing the thickness in the hexagonal structure, caused the enhancement of Von Misses stress in model 4.
The diagrams of vertebrae with hexagonal structure, indicated two main points: (1) after implementing geometrical parameters of the hexagonal structure, the amount of maximum Von Misses stress in the construction was decreased; and (2) the influence of type of material (steel or titanium) on the amount of maximum Von Misses stress was less obvious than the impact of implant geometry.
This study was set to compare the mechanical behavior of the vertebrae treated by the VP and KP with the one treated by the hexagonal porous structure having different geometries. In the case of VP, the bone cement filled most of the porous space of the trabecular structure, such that the whole construction was almost homogeneous. This was the reason for increasing the stress in endplates, pressure inside disks and the bulge of adjacent endplates leading to occurring adjacent fractures[4]. On the other hand, in the KP, the bone cement mass and the trabecular structure were separated and the whole structure was not homogeneous. So the stress concentration could be seen in the interface area. The stress concentration in the separation region caused high amounts of Von Misses stress in the construction.
The results of an experimental study addressing the differences between the mechanical behavior of the vertebrae treated by hexagonal pearls embedded inside vertebrae and that of the VP and KP[21], confirmed the results of μFEA in this study. Based on the results of mechanical tests, by setting the hexagonal porous structure, the toughness of the vertebra was enhanced substantially in the form of increased range of displacement of the vertebrae (83%) before getting to the ultimate strength under static loading. Also, the effect of the type of material in increasing the toughness was less obvious when compared to the effect of implant shape. In the KP, the separation area between the rigid cement mass and the trabecular bone was the most susceptible region for occurring fractures because the stress distribution in the boundary region was not homogeneous. The hexagonal porous structure rendered better stress distribution in the boundary region and reduced the risk of fracture in the future.
The complicated geometry of trabecular bone requires an algorithm to determine the amount and the position of bone cement or any other structure. The improvement in stress distribution inside treated vertebrae leads to the reduction of stress in endplates[6]. So it could be inferred that the treated vertebrae by the hexagonal structure is likely to encounter a less amount of stress in endplates. As the treated vertebrae by hexagonal structure had better stress distribution, it could be predicted that in the term of long term stability, those vertebrae might show a better performance.
The decreasing trend in the maximum Von Misses stress in the cube with the hexagonal porous structure can be obviously seen in Figure 4. The improvement in the mechanical behavior of the whole vertebra treated by new method, as compared to the common procedures, could be achieved by validating the theoretical results through experimental tests. The reduction of Von Misses stress in the construction after implementing geometrical parameters is comparable to the results of the experimental tests already performed, thereby showing the increased range of displacement before getting to the ultimate strength in the vertebrae treated by the hexagonal structure; this represented the better stress distribution inside the vertebrae under the uniaxial compressive load. The increasing trend in Von Misses stress in the model 4 indicated the existence of an optimal amount for the geometrical parameters of hexagonal structure. Considering the morphological parameters of trabecular cube such as Tb. Th and Tb. Sp, it seems that there is a relationship between geometrical parameters of the embedded structure and morphological parameters of trabecular bone. Therefore, optimizing the design of implants is related to those morphological parameters.
The more dependence of stress distribution on different geometries of implant, compared to the variation of material in the results of μFEA, was also observed in the experimental study[21], this showed that the influence of the material of hexagonal structure in increasing the range of displacements before getting to the ultimate strength (improved stress distribution), as compared to the impact of implant geometry, is of secondary importance.
Future studies must be focused on optimizing the geometry of the hexagonal implants based on morphological parameters of trabecular structure in order to provide clear surgical instructions for each patient. In addition to the improvement of mechanical properties of the treated vertebrae, setting porous scaffolds might help better bone regeneration, cell migration and bone repair.
In conclusion, the new method presented here is based on using hexagonal implants instead of the rigid cement mass. The results of treated vertebrae by hexagonal structures showed that the improvement of the stress distribution inside vertebrae could lead to increasing the toughness and reducing stress in endplates. Also, because the hexagonal porous structure was symmetrical and geometrically optimized, it could solve the problem of asymmetrical cement distribution, a common problem in the VP and KP. The results of this study indicated that a wide range of material could be selected in providing implants due to the low dependence of stress distribution, relative to the implant material variance.
Future studies must be focused on evaluating geometrically different models of implants based on defined parameters of this study: Thickness and spacing diameter; this could lead to optimizing the stress distribution considering the morphological parameters of trabecular bone for each patient. Also, advanced techniques to facilitate the insertion of porous structures inside vertebrae must be considered. In the case of long term stability, in-vivo studies could be an effective method to assess the bone repair and evaluating the durability.
The vertebroplasty (VP) and kyphoplasty (KP) are known as common procedures in treating osteoporotic vertebrae. Although post treatments of KP are less than those of VP, but the stress concentration in the interface area of bone cement and trabecular bone could be regarded as the main reason of occurring micro fractures, pain and aseptic loosening.
Previous researches have already proved that cement augmentation increases the interadiscal pressure in adjacent discs with a slight increase in Von Mises stress in vertebral endplates. Basically, the main drawbacks of cement augmentation are: (1) stress concentration in the interface area; and (2) asymmetrical cement distribution.
This is the first study addressing the hexagonal porous structure with defined geometrical parameters including spacing diameter and thickness. It was conducted to alter the mechanical behavior of the osteoporotic vertebrae.
The hexagonal porous structures could be considered as a substitute for bone cement with the variety of geometrical parameters. Furthermore, setting porous structures with different geometries inside vertebrae may provide the possibility of bone regeneration, transfer of the growth factors and recreation of mechanical properties.
The hexagonal porous structure could be considered as a substitute for bone cement mass due to lowering the level of the maximum Von Mises stress and reducing the stress concentration and post treatments. Within defined geometrical parameters, thickness and spacing diameter, the structure could be optimized as well.
Good study, subject addressed in this article is worthy of investigation.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country of origin: Iran
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chowdhury FH, Elgafy H S- Editor: Cui LJ L- Editor: A E- Editor: Lu YJ
| 1. | Ruiz Santiago F, Santiago Chinchilla A, Guzmán Álvarez L, Pérez Abela AL, Castellano García Mdel M, Pajares López M. Comparative review of vertebroplasty and kyphoplasty. World J Radiol. 2014;6:329-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Drampalos E, Nikolopoulos K, Baltas C, Balanika A, Galanos A, Papaioannou N, Pneumaticos S. Vertebral fracture assessment: Current research status and application in patients with kyphoplasty. World J Orthop. 2015;6:680-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Kosmopoulos V, Keller TS. Damage-based finite-element vertebroplasty simulations. Eur Spine J. 2004;13:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Polikeit A, Nolte LP, Ferguson SJ. The effect of cement augmentation on the load transfer in an osteoporotic functional spinal unit: finite-element analysis. Spine (Phila Pa 1976). 2003;28:991-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 264] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Wilcox RK. The biomechanics of vertebroplasty: a review. Proc Inst Mech Eng H. 2004;218:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Keller TS, Kosmopoulos V, Lieberman IH. Vertebroplasty and kyphoplasty affect vertebral motion segment stiffness and stress distributions: a microstructural finite-element study. Spine (Phila Pa 1976). 2005;30:1258-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Rohlmann A, Zander T, Bergmann G. Spinal loads after osteoporotic vertebral fractures treated by vertebroplasty or kyphoplasty. Eur Spine J. 2006;15:1255-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Liang D, Ye LQ, Jiang XB, Yang P, Zhou GQ, Yao ZS, Zhang SC, Yang ZD. Biomechanical effects of cement distribution in the fractured area on osteoporotic vertebral compression fractures: a three-dimensional finite element analysis. J Surg Res. 2015;195:246-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Tschirhart CE, Roth SE, Whyne CM. Biomechanical assessment of stability in the metastatic spine following percutaneous vertebroplasty: effects of cement distribution patterns and volume. J Biomech. 2005;38:1582-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Xu G, Fu X, Du C, Ma J, Li Z, Ma X. Biomechanical effects of vertebroplasty on thoracolumbar burst fracture with transpedicular fixation: a finite element model analysis. Orthop Traumatol Surg Res. 2014;100:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Baroud G, Yahia FB. A finite element rheological model for polymethylmethacrylate flow: analysis of the cement delivery in vertebroplasty. Proc Inst Mech Eng H. 2004;218:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Boger A, Heini P, Windolf M, Schneider E. Adjacent vertebral failure after vertebroplasty: a biomechanical study of low-modulus PMMA cement. Eur Spine J. 2007;16:2118-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Kinzl M, Boger A, Zysset PK, Pahr DH. The mechanical behavior of PMMA/bone specimens extracted from augmented vertebrae: a numerical study of interface properties, PMMA shrinkage and trabecular bone damage. J Biomech. 2012;45:1478-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Kosmopoulos V, Keller TS, Schizas C. Early stage disc degeneration does not have an appreciable affect on stiffness and load transfer following vertebroplasty and kyphoplasty. Eur Spine J. 2009;18:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Kettler A, Schmoelz W, Shezifi Y, Ohana N, Ben-Arye A, Claes L, Wilke HJ. Biomechanical performance of the new BeadEx implant in the treatment of osteoporotic vertebral body compression fractures: restoration and maintenance of height and stability. Clin Biomech (Bristol, Avon). 2006;21:676-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Baroud G, Nemes J, Heini P, Steffen T. Load shift of the intervertebral disc after a vertebroplasty: a finite-element study. Eur Spine J. 2003;12:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 17. | Armsen N, Boszczyk B. Vertebro/kyphoplasty history, development, results. Euro J Trauma. 2005;31:433-441. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Garzón-Alvarado DA, Velasco MA, Narváez-Tovar CA. Modeling porous scaffold microstructure by a reaction-diffusion system and its degradation by hydrolysis. Comput Biol Med. 2012;42:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Verhulp E, Van Rietbergen B, Muller R, Huiskes R. Micro-finite element simulation of trabecular-bone post-yield behaviour--effects of material model, element size and type. Comput Methods Biomech Biomed Engin. 2008;11:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Landgraft R, Ihlemannl J, Kolmeder S, Lion A, Lebsack H, Kober C. Modelling and simulation of acrylic bone cement injection and curing within the framework of vertebroplasty. J Appl Math Appl Mech. 2015;95:1530-1545. [DOI] [Full Text] |
| 21. | Hosseini Faradonbeh SA, Jamshidi N. The Experimental Assessment of Hexagonal Porous structure in Vertebroplasty and Kyphoplasty. Iranian Journal of Orthopedic Surgery. 2015;13:168-176. |
| 22. | Gong H, Zhang M, Qin L, Hou Y. Regional variations in the apparent and tissue-level mechanical parameters of vertebral trabecular bone with aging using micro-finite element analysis. Ann Biomed Eng. 2007;35:1622-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |