Published online Jun 18, 2016. doi: 10.5312/wjo.v7.i6.392
Peer-review started: July 23, 2015
First decision: October 17, 2015
Revised: January 23, 2016
Accepted: March 24, 2016
Article in press: March 25, 2016
Published online: June 18, 2016
Processing time: 331 Days and 6.3 Hours
AIM: To evaluate whether anterior cruciate ligament (ACL) allograft irradiation is effective for sterility without compromising graft integrity and increasing failure rate.
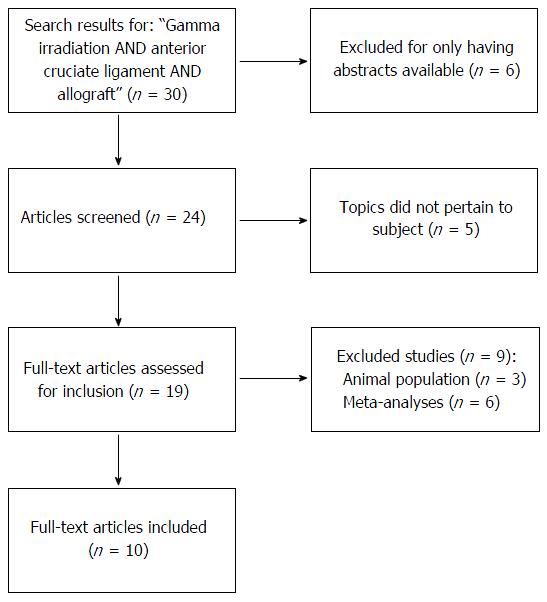
METHODS: A literature search was conducted using PubMed, Cochrane, and Google. The following search terms were used: “Gamma irradiation AND anterior cruciate ligament AND allograft” with a return of 30 items. Filters used included: English language, years 1990-2015. There were 6 hits that were not reviewed, as there were only abstracts available. Another 5 hits were discarded, as they did not pertain to the topic of interest. There were 9 more articles that were excluded: Three studies were performed on animals and 6 studies were meta-analyses. Therefore, a total of 10 articles were applicable to review.
RESULTS: There is a delicate dosing crossover where gamma irradiation is both effective for sterility without catastrophically compromising the structural integrity of the graft. Of note, low dose irradiation is considered less than 2.0 Mrad, moderate dose is between 2.1-2.4 Mrad, and high dose is greater than or equal to 2.5 Mrad. Based upon the results of the literature search, the optimal threshold for sterilization was found to be sterilization at less than 2.2 Mrad of gamma irradiation with the important caveat of being performed at low temperatures. The graft selection process also must include thorough donor screening and testing as well as harvesting the tissue in a sterile fashion. Utilization of higher dose (≥ 2.5 Mrad) of irradiation causes greater allograft tissue laxity that results in greater graft failure rate clinically in patients after ACL reconstruction.
CONCLUSION: Allograft ACL graft gamma irradiated with less than 2.2 Mrad appears to be a reasonable alternative to autograft for patients above 25 years of age.
Core tip: The dose of gamma irradiation is directly correlated with increased failure rate of allograft in both in vitro and in vivo studies. Optimal gamma irradiation dose is less than 2.2 Mrad and should be performed in the setting of a low temperature.
- Citation: Dashe J, Parisien RL, Cusano A, Curry EJ, Bedi A, Li X. Allograft tissue irradiation and failure rate after anterior cruciate ligament reconstruction: A systematic review. World J Orthop 2016; 7(6): 392-400
- URL: https://www.wjgnet.com/2218-5836/full/v7/i6/392.htm
- DOI: https://dx.doi.org/10.5312/wjo.v7.i6.392
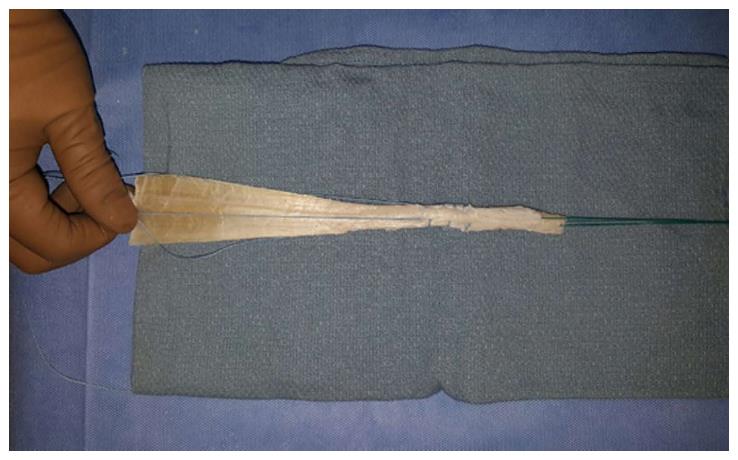
Rupture of the anterior cruciate ligament (ACL) has been reported to have an incidence of 100000 to 200000 in the United States with about 400000 ACL reconstructions performed worldwide annually[1,2]. ACL reconstruction is a common procedure in an orthopaedic sports medicine practice and has been shown to have favorable return to play outcomes and preservation of knee function. Both autograft (from the patient) and allograft (cadaver) can be used for the ACL reconstruction procedure[3]. Advantages of autograft include lower graft failure rate in the young (< 25 years old) and active patient population, lower infection rate, and no risk of disease transmission or immune reaction[4-7]. Alternatively, advantages of allograft include no donor site morbidity with decreased operative time, earlier return to sports and lower postoperative pain. In the older population, allograft has comparable outcomes compared to autograft reconstruction with a decrease in patient morbidity, surgical time, and smaller incision[8]. Since using allograft tissue for ACL reconstruction (Figure 1) has proven to be successful in older patients with less physical demands, determining the most favorable processing method of the allograft tissue while minimizing catastrophic failure rates is of paramount importance.
For allograft tissue currently used, it must first undergo a detailed sequence of procedures that include medically and serologically screening donors to rule out viral contamination via nucleic acid testing. The Food and Drug Administration and the American Association of Tissue Banks have set industry standards for donor eligibility and tissue preparation. The donor is subjected to a rigorous physical and medical examination and an array of serological tests to detect antibodies for human immunodeficiency virus 1 and 2, hepatitis C virus, hepatitis B antigen, and syphilis[9,10]. The rigorous donor selection process serves to eliminate specific contaminants by evaluating the allograft tissue’s physical composition, including uniformity in shape and density as well as its biological properties to assess the level of microbial burden and risk for disease transmission[9,11,12].
After appropriate graft donor selection has occurred, the next step is to sterilize the graft to a sterility assurance level (SAL) of 10-6 organism[9,11,12]. There have been numerous preparations tested to determine the optimal sterilization method including peracetic acid and ethylene oxide, but many of the methods were abandoned after they were found to have detrimental effects on the mechanical properties of the graft and/or cause an inflammatory response in recipients[13,14]. Other methods of sterilization, such as gamma irradiation, have proven to be more promising. This process uses a source emitting high-frequency electromagnetic radiation to disrupt the DNA (nucleic acids) of living organisms on the tissue to eliminate microbes and inactivating viruses[15]. The International Atomic Energy Agency has developed a set of standards that govern the proper radiation sterilization of tissue allografts. Their protocol incorporates the principles that were put in place by the International Organization for Standardization to guide the radiation sterilization process of industrially produced health care products[16].
Some authors cite that anywhere from 0.92-2.5 Mrads is needed to eliminate bacterial bioburden and fungal spores to achieve SAL of 10-6 on musculoskeletal allografts[2,9,12,17,18]. However, the effect of gamma irradiation on viruses is controversial. It has been reported that, in order to inactivate human immunodeficiency virus (HIV) and hepatitis, doses as high as 4.0-5.0 Mrad are required[17,18].
Unfortunately, gamma irradiation can have destructive effects on allografts by disrupting the polypeptide chain sequence and inducing minor crosslinking without interfering with collagen’s normal banding pattern[19]. There have been reports of gamma irradiation of 3 Mrad causing a reduction on the mechanical properties of the allograft tissue[20]. Additionally, the temperature at which the gamma irradiation is performed is also important to consider. The mechanical destruction has been reported to be lessened when grafts were irradiated at low temperatures (i.e., on dry ice) when compared to the same process performed at room temperature[17,21]. However the production of free radicals has also been cited to be a benefit of performing irradiation at higher temperature as the free radicals have anti-microbial effects[17].
The balance between sterilization of the allograft tissue without compromising the biomechanical properties, strength and functional outcomes is a topic of debate. The purpose of this systematic review article is to further explore the effect of gamma irradiation on the catastrophic failure rate and functional outcomes in patients after ACL reconstruction with allograft tissue.
A literature search was performed using PubMed, Cochrane, and Google with the following search terms with a return of 30 items: “Gamma irradiation AND anterior cruciate ligament AND allograft”. Filters used included: English language, years 1990-2015. There were 6 items that were not reviewed, as there were only abstracts available. Another 5 articles were discarded, as they did not pertain to the topic of interest. There were 9 more articles that were excluded: Three studies were performed on animals and 6 studies were meta-analyses. Therefore, a total of 10 clinical articles were applicable to review (Figure 2).
With regards to the effects from gamma irradiation, there are several articles that have extensively studied the effects of irradiation on ACL grafts (Tables 1 and 2). There were four prospective, randomized trials by the same author who attempted to answer this question. The results of these studies all demonstrated that patients who had allografts exposed to > 2.5 Mrad of irradiation had a significantly greater laxity than autograft or non-irradiated allografts; however there were no significant differences in any of these studies on the International Knee Documentation Committee (IKDC) outcome scores or range of motion[22-25]. Unfortunately, none of these studies report graft temperature during irradiation and therefore should be interpreted with caution. As stated earlier, it is important that the grafts be irradiated at low temperatures to decrease the free radical formation as to not weaken the allograft biomechanical properties[17,21].
| Ref. | Year | Type of study | Graft type | Irradiation dose | No. of patients (at final follow-up/enrolled) | Male | Female | Average age (yr) | Follow-up length | Findings | Weaknesses |
| Rihn et al[26] | 2006 | Retrospective | BPTB - allograft | 2.5 Mrad | 39 | 27 | 12 | 44 ± 8.4 | 4.2 yr | Significant difference between irradiated allograft and allograft with laxity, Lachman, and pivot shift clunk | Age difference in populations |
| BPTB - autograft | None | 63 | 43 | 20 | 25.3 ± 9.3 | 4.6 yr | No significant difference on range of motion, effusion, IKDC, KT-1000 when adjusted for age, IKDC physical exam rating, and return to sport | No mention of temperature when irradiation performed | |||
| Rappé et al[18] | 2007 | Cohort | Achilles - allograft | 2.0-2.5 Mrad | 33/45 | N/A | N/A | 26 (ranage 14-59) | 6 mo | Significantly more clincal failures in irradiated allograft vs non-irradiated allograft groups with failures occuring about 9 mo earlier in irradiated group (failure of graft = 5 mm or greater on KT-1000 compared to contralateral side, positive Lachman, or magnetic resonance imaging) | Large loss of follow-up in irradiated group |
| Achilles - allograft | None | 42/45 | 27 (range 14-57) | No mention of temperature when irradiation performed | |||||||
| Sun et al[22] | 2009 | Prospective, randomized | BPTB - allograft | 2.5 Mrad | 32/33 | 24 | 8 | 30.1 ± 6.1 | 31 mo (2% lost to follow-up) | Significant difference between irradiated allograft and autograft with greater laxity on KT-2000, side to side difference on KT-2000, pivot shift grade II or III, anterior drawer test grade II or III, and Lachman test grade II or III | No mention of temperature when irradiation performed |
| BPTB - autograft | None | 33/33 | 24 | 9 | 29.7 ± 7.2 | No significant difference on overall IKDC, range of motion, Harner's vertical jump test, Daniel's one-leg hip test, subjective IKDC, Cincinnati knee score, Lysholm score, and Tegner score | |||||
| Sun et al[23] | 2009 | Prospective, randomized | BPTB - allograft | 2.5 Mrad | 32 | 24 | 8 | 30.1 ± 6.1 | 31 mo (1% lost to follow-up - treatment group not mentioned) | Significant difference between irradiated allograft, non-irradiated allograft, and autograft with greater laxity on KT-2000, side to side difference on KT-2000, pivot shift grade II or III, anterior drawer test grade II or III, and Lachman test grade II or III | No mention of temperature when irradiation performed |
| BPTB - allograft | None | 34 | 22 | 12 | 31.8 ± 6.9 | ||||||
| BPTB - autograft | None | 33 | 24 | 9 | 29.7 ± 7.2 | No significant difference on overall IKDC, range of motion, Harner's vertical jump test, Daniel’s one-leg hip test, subjective IKDC, Cincinnati knee score, Lysholm score, and Tegner score | |||||
| Sun et al[24] | 2011 | Prospective, randomized | Hamstring - allograft | 2.5 Mrad | 31/37 | 24 | 7 | 30.3 ± 7.9 | 42.2 mo (11% lost to follow-up) | Significant difference between irradiated allograft and autograft with greater laxity on KT-2000, side to side difference on KT-2000, pivot shift grade II or III, anterior drawer test grade II or III, and Lachman test grade II or III | No mention of temperature when irradiation performed |
| Hamstring - autograft | None | 36/38 | 28 | 8 | 30.9 ± 8.7 | No significant difference on overall IKDC, range of motion, Harner's vertical jump test, Daniel's one-leg hip test, subjective IKDC, Cincinnati knee score, Lysholm score, and Tegner score | |||||
| Sun et al[25] | 2012 | Prospective, randomized | Hamstring - allograft | 2.5 Mrad | 31/38 | 24 | 7 | 30.3 ± 7.9 | 42.5 mo (9.1% lost to follow-up) | Significant difference between irradiated allograft and non-irradiated allograft with greater laxity on KT-2000, side to side difference on KT-2000, pivot shift grade II or III, anterior drawer test grade II or III, and Lachman test grade II or III | No mention of temperature when irradiation performed |
| Hamstring - allograft | None | 38/39 | 31 | 7 | 31.7 ± 7.8 | No significant difference on overall IKDC, range of motion, Harner's vertical jump test, Daniel's one-leg hip test, subjective IKDC, Cincinnati knee score, Lysholm score, and Tegner score |
| Ref. | Year | Type of study | Graft type | Irradiation dose | Temperautre when irradiated | No. of samples | Age (yr) | Findings |
| Balsly et al[9] | 2008 | Laboratory | BPTB - low dose | 1.83-2.18 Mrad | - 20 °C to -50 °C | 9 | 18-55 | There was a significant difference for: (1) BPTB - tensile strength in the moderate dose irradiation vs control groups (2) Fascia lata - modulus of elasticity in the moderate dose irradiation vs control groups |
| BPTB - moderate dose | 2.4-2.85 Mrad | 9 | ||||||
| BPTB - control | None | N/A | 9 controls for low dose | |||||
| 9 controls moderate dose | ||||||||
| Anterior Tibialis - low dose | 1.83-2.18 Mrad | - 20 °C to -50 °C | 10 | 23-64 | No significant difference between the tensile strength and modulus of elasticity for all other groups for low dose irradiation vs control and moderate dose irradiation vs control (other than stated above) | |||
| Anterior Tibialis - moderate dose | 2.4-2.85 Mrad | 10 | ||||||
| Anterior Tibialis - control | None | N/A | 10 controls for low dose | |||||
| 10 controls for moderate dose | ||||||||
| Semitendinosus - low dose | 1.83-2.18 Mrad | - 20 °C to -50 °C | 8 | 16-54 | ||||
| Semitendinosus - moderate dose | 2.4-2.85 Mrad | 10 | ||||||
| Semitendinosus - control | None | N/A | 10 controls for low dose | |||||
| 10 controls for moderate dose | ||||||||
| Fascia Lata - low dose | 1.83-2.18 Mrad | - 20 °C to -50 °C | 10 | 19-48 | ||||
| Fascia Lata - moderate dose | 2.4-2.85 Mrad | 10 | ||||||
| Fascia Lata - control | None | N/A | 10 controls for low dose | |||||
| 10 controls for moderate dose | ||||||||
| Greaves et al[17] | 2008 | Laboratory | Tibialis - single strand irradiated (age < 45) | 1.46-1.8 Mrad | Dry ice temperatures | 10 irradiated | < 45 | No significant difference in failure loads for irradiated vs non-irradiated for each of the three age groups (midsubstance failure = any rupture within graft substance, grip failure = slip from 1 of tendon grips exposing serrated portion of tendon) |
| Tibialis - single strand non-irradiated (age < 45) | 10 non-irradiated | |||||||
| Tibialis - double strand irradiated (age < 45) | 10 irradiated | |||||||
| Tibialis - double strand non-irradiated (age < 45) | 10 non-irradiated | |||||||
| Tibialis - single strand irradiated (age 46-55) | 13 irradiated | 46-55 | ||||||
| Tibialis - single strand non-irradiated (age 46-55) | 13 non-irradiated | |||||||
| Tibialis - double strand irradiated (age 46-55) | 10 irradiated | |||||||
| Tibialis - double strand non-irradiated (age 46-55) | 10 non-irradiated | |||||||
| Tibialis - single strand irradiated (age 56-65) | 10 irradiated | 56-65 | ||||||
| Tibialis - single strand non-irradiated (age 56-65) | 10 non-irradiated | |||||||
| Tibialis - double strand irradiated (age 56-65) | 10 irradiated | |||||||
| Tibialis - double strand non-irradiated (age 56-65) | 10 non-irradiated | |||||||
| Baldini et al[34] | 2012 | Laboratory | Tibialis | 2.0-2.8 Mrad | Not reported | 15 | 41.8 | There were no significant difference in stiffness, failure to load, and failure stress between the irradiated vs non-irradiated groups |
| None | 12 | 47.4 | ||||||
| Yanke et al[35] | 2013 | Laboratory | BPTB | 1.0-1.2 Mrad | Not reported | 10 | 52 ± 11 | There was a significant difference in stiffness between the irradiated vs non-irradiated groups but none found in strain and elongation |
| None | 10 |
Rappé et al[18] conducted a cohort study comparing non-irradiated Achilles allografts to irradiated Achilles allografts (2.5 Mrad) with the primary outcome of clinical failure (positive Lachman exam, magnetic resonance imaging, and/or side-to-side difference of 5 mm or greater on KT-1000 exam). They found that there was a significant difference in clinical failures between the irradiated (33.3%) and non-irradiated groups (2.4%; P < 0.01)[18]. However, there are weaknesses to their study, including a large loss of follow-up in the irradiated group (27%) compared to the non-irradiated group (7%); additionally there was no mention of the temperature at which the irradiation process was performed.
Rihn et al[26] compared the outcomes of bone-patella-tendon-bone (BTB) autograft to BTB allograft that underwent 2.5 Mrad of irradiation. They found no differences in the rate of return to sports or the IKDC scores; however, objectively, the allograft group had significantly more laxity on KT-1000, Lachman exam, and pivot shift clunk. However, similar to Rappé et al[18], this study made no mention of temperature of the graft preparation and had significant differences in the mean age of the two study groups with the allograft group having an older mean age.
The increase in laxity and failure rates was also noted in the studies by Sun et al[22-25] with 34% failure seen in the irradiated allograft group (2.5 Mrad), 6.4% in the autograft group and 8.8% in the nonirradiated allograft group. Sun et al[23] compared hamstring autograft to irradiated allograft with over 2.5 years of follow-up and reported the rate of laxity was 32% higher in the irradiated vs autograft group (8.3%). In addition, anterior and rotational stability also decreased significantly in the irradiated allograft group; however, the IKDC functional scores were very similar between the two groups. In a subsequent follow-up randomized controlled trial comparing irradiated to nonirradiated hamstring allograft for ACL reconstruction, Sun et al[24] found that allograft irradiation is responsible for increased anterior and rotational instability. Furthermore, the knees that had an ACL reconstruction done with the irradiated graft has significantly more osteoarthritis compared to the nonirradiated group.
In biomechanical testing, Roche et al[27] evaluated the effect on gamma irradiation (1.55 Mrad on dry ice or low temperature) with BTB and fascia lata allografts. The authors found no significant difference between the irradiated group and the non-irradiated groups when testing the grafts’ tensile strength. Balsly et al[9] also reported on the effect of low (1.8-2.2 Mrad) vs high dose (2.4-2.8 Mrad) irradiation on the biomechanical properties of allograft tissue (BTB, anterior tibialis, semitendinosus, and fascia lata allografts). All irradiation processing was performed at low temperatures. For the low dose irradiation groups for all types of allografts mentioned, there were no significant difference found between the control groups and the low dose irradiation groups when the tensile strength or modulus of elasticity of the grafts was tested. For the moderate irradiation group, there was either a significant difference or a trend towards having a significant difference when compared to the controls in these same measures.
Fideler et al[28] also demonstrated that the initial biomechanical strength of allografts was reduced 15% when compared to controls after 2 Mrad of irradiation. He also showed a dose dependent effect on the integrity of the allograft tissue with increasing gamma irradiation at 3 and 4 Mrads. Curran et al[29] reported the average load to failure of irradiated patellar tendon grafts vs nonirradiated grafts was 1965 ± 512 N vs 2457 ± 647 N, respectively. Furthermore, with cyclic loading, the irradiated grafts elongated 27% more than the nonirradiated grafts (P < 0.05).
The choice of graft for ACL reconstruction is contingent upon many factors including the age, baseline level of activity, and planned level of future activities[2,3,30]. Much of the debate about whether to use allograft or autograft for ACL reconstruction is related to the morbidity and complications related to each option. Autograft procedures have the disadvantage of increased surgical time due to graft harvesting, which can translate into higher procedural costs (i.e., operating room time). Additional autograft harvesting risks include quadriceps or hamstring weakness from quadriceps/hamstring grafts and anterior knee pain from the BTB procedures. The benefits of using autograft include minimal risk of infection/disease transmission from donated tissue, no possibility of immune reaction to the graft, no cost of the graft (other than increased OR time), and an overall decreased rerupture rate in younger patients under the age of 25 years[2,3,11,22,31].
Reconstruction using allograft has its own unique risks. There are risks of immunogenic reaction, bacterial infection, and disease transmission from the graft donor. However, it has been reported that HIV or hepatitis transmission is 1 in 1.6 million[2,9,17,18,21,26,31,32]. The possibility of an immune reaction stems from the body’s response to foreign tissues via interactions to human leukocyte antigens (HLAs) on donor cells. Fortunately, recent studies have found that an immune response to allograft tissue has not been a common issue, since the allograft tissue processing (i.e., the freezing process) essentially eliminates active HLA markers[2,31]. Another cited disadvantage of allograft use is increased laxity over time, which can result in knee laxity and failure to return to previous level of activities despite an “intact” graft[31]. Some of the cited advantages of using allograft include smaller incisions, reduced postoperative pain/less donor site morbidity (since no graft harvesting is required), larger graft availability, earlier postoperative knee range of motion, and decreased surgical time[2,11].
A discussion with patients about graft integrity is important when allograft is being used for ACL reconstruction. There have been reports about having decreased allograft strength after gamma irradiation, which clinically manifests as laxity and/or catastrophic graft failure. This has been a controversial topic with conflicting studies attributed to the lack of details about how grafts were processed including the irradiation dose and the graft temperature during irradiation[9,17,18,21,26,32]. Our review of the literature found that utilizing the higher dose (≥ 2.5 Mrad) of irradiation causes greater allograft tissue laxity and subsequently increased graft failure rate. However, in the subset of patients that did not have catastrophic failures from the irradiated graft, overall functional outcome as measured by the IKDC scores were similar to the nonirradiated allograft or autograft groups. Ghodadra et al[33], compared BTB autograft and Patellar tendon allograft (nonirradiated and low dose irradiation - 1.0 to 1.3 Mrad) using a retrospective cohort and found no differences in postoperative laxity (KT-1000) or failure rates at 6 wk and 1 year. Additionally, the authors found no difference in the laxity between the patellar tendon groups that had the low dose irradiation vs no irradiation.
The choice to use an irradiated graft is contingent upon many factors. The important details to know when choosing an irradiated graft are: How the graft was prepared, the dose range of irradiation used, and the temperature of the graft when the irradiation was performed. The results from our systematic review suggest that grafts that are irradiated at low temperatures with 1.8 to 2.2 Mrad of irradiation do not appear to have deleterious effects on the allograft tissue tensile strength or elasticity modulus. However, moderate to high doses of gamma radiation (≥ 2.5 Mrad) will have a major impact on the allograft tissue biomechanical properties which may result in increased laxity that may compromise clinical outcomes and increase rates of functional failure. These above studies suggest that grafts irradiated at low temperatures with less than 2.2 Mrad of irradiation are an acceptable choice to optimize the benefits of sterility and without affecting rate of functional or catastrophic structural failure.
There have been large advancements in allograft tissue processing for ACL reconstruction over the past several decades. There are many advantages of using allograft for ACL reconstruction in the older and less active population when compared to autograft with similar functional outcomes. The concerns of infection with allografts have been mitigated by the changes in the tissue bank facility practices with improved donor tissue screening and use of gamma irradiation. Irradiation has proven to be successful at reducing the bioburden found on allografts (and possibly viral contamination) and appears to not have an effect on the rate of functional failure if it is performed with low dose irradiation (< 2.2 Mrad) at low temperatures. Grafts prepared with higher dose irradiation (≥ 2.5 Mrad) may be weakened and the additional irradiation may compromise the graft’s biomechanical properties and clinical outcomes resulting in unacceptable failure rates.
Anterior cruciate ligament (ACL) reconstruction is a common procedure in the orthopaedic sports medicine practice. The surgery involves using tissue either from the patient (autograft) and/or from cadaver (allograft). It has been shown that the failure rates when comparing allograft to autograft tissue decreases with increased age.
One of the main concerns with the use of allograft is the balance between the process of graft sterilization and its potential impact on graft integrity. It has been suggested that there is an association between increased gamma irradiation dosage and an adverse impact on the biomechanical properties of the allograft tissue, although controversy remains with regards to dosing thresholds that will compromise the strength of the allograft tissue.
There has been a trend toward increased allograft use in older and lower demand patients in recent years in an effort to decrease the morbidity associated with autograft harvest. With increased allograft use, appropriate graft irradiation exposure has been investigated. Four prospective, randomized trials demonstrated that patients who had allografts exposed to greater than or equal to 2.5 Mrad of irradiation had a significantly greater laxity and clinical failure rates than autograft or non-irradiated (< 2.2 Mrad) allografts (all different studies), but temperature at time of irradiation was not recorded. It is also important that the grafts be irradiated at low temperatures to decrease the free radical formation as to not weaken the allograft.
The authors used a systematic review of the currently available literature to determine that there is a delicate dosing crossover where gamma irradiation is both effective for sterility without catastrophically compromising the graft structural integrity.
Gamma irradiation is a means of allograft sterilization, which uses a source emitting high-frequency electromagnetic radiation to disrupt the DNA (nucleic acids) of living organisms on the tissue.
This is a useful systematic review on the use of allograft for ACL reconstruction particularly focusing on the effect of the sterilization process on its biomechanical properties and clinical outcomes. The authors introduce the reader to the ACL reconstruction graft options, sterilization process and associated clinical and laboratory results. This review gives readers the opportunity to implement to their practice a better use and understanding of gamma irradiation of allograft tissues for ACL reconstruction.
P- Reviewer: Drampalos E, Metzger PD S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Rayan F, Nanjayan SK, Quah C, Ramoutar D, Konan S, Haddad FS. Review of evolution of tunnel position in anterior cruciate ligament reconstruction. World J Orthop. 2015;6:252-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 2. | Klimkiewicz JJ, Brian J, Samsell BJ, Riff A, DeBarardino TM, Moore MA. Comparison of human tendon allografts and autografts used in knee reconstruction. Current Orthopaedic Practice. 2011;22:494-502. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Kaeding CC, Aros B, Pedroza A, Pifel E, Amendola A, Andrish JT, Dunn WR, Marx RG, McCarty EC, Parker RD. Allograft Versus Autograft Anterior Cruciate Ligament Reconstruction: Predictors of Failure From a MOON Prospective Longitudinal Cohort. Sports Health. 2011;3:73-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 363] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 4. | Arnoczky SP, Warren RF, Ashlock MA. Replacement of the anterior cruciate ligament using a patellar tendon allograft. An experimental study. J Bone Joint Surg Am. 1986;68:376-385. [PubMed] |
| 5. | Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Crawford C, Kainer M, Jernigan D, Banerjee S, Friedman C, Ahmed F, Archibald LK. Investigation of postoperative allograft-associated infections in patients who underwent musculoskeletal allograft implantation. Clin Infect Dis. 2005;41:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Malinin TI, Levitt RL, Bashore C, Temple HT, Mnaymneh W. A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy. 2002;18:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Barrett G, Stokes D, White M. Anterior cruciate ligament reconstruction in patients older than 40 years: allograft versus autograft patellar tendon. Am J Sports Med. 2005;33:1505-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Title 21 - Food and Drugs: Part 1271 Human Cells, Tissues, and Cellular and Tissue-Based Products. 2014 April 1, 2014. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1271. |
| 11. | McKee J. Autograft or Allograft for ACL Reconstruction? 2012. [Accessed 26 October 2015]. Available from: http://www.aaos.org/news/aaosnow/apr12/cover1.asp. |
| 12. | Baker TF, Ronholdt CJ, Bogdansky S. Validating a low dose gamma irradiation process for sterilizing allografts using ISO 11137 method 2B. Cell Tissue Bank. 2005;6:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Scheffler SU, Scherler J, Pruss A, von Versen R, Weiler A. Biomechanical comparison of human bone-patellar tendon-bone grafts after sterilization with peracetic acid ethanol. Cell Tissue Bank. 2005;6:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Scheffler SU, Gonnermann J, Kamp J, Przybilla D, Pruss A. Remodeling of ACL allografts is inhibited by peracetic acid sterilization. Clin Orthop Relat Res. 2008;466:1810-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Mikhael MM, Huddleston PM, Zobitz ME, Chen Q, Zhao KD, An KN. Mechanical strength of bone allografts subjected to chemical sterilization and other terminal processing methods. J Biomech. 2008;41:2816-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Radiation sterilization of tissue allografts-requirements for validation and routine control A code of practice. IAEA 2007: 55. Available from: http://www-pub.iaea.org/MTCD/publications/PDF/Pub1307_web.pdf. |
| 17. | Greaves LL, Hecker AT, Brown CH. The effect of donor age and low-dose gamma irradiation on the initial biomechanical properties of human tibialis tendon allografts. Am J Sports Med. 2008;36:1358-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Rappé M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35:1653-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Schwartz HE, Matava MJ, Proch FS, Butler CA, Ratcliffe A, Levy M, Butler DL. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34:1747-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Samsell BJ, Moore MA. Use of controlled low dose gamma irradiation to sterilize allograft tendons for ACL reconstruction: biomechanical and clinical perspective. Cell Tissue Bank. 2012;13:217-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17:464-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. ACL reconstruction with BPTB autograft and irradiated fresh frozen allograft. J Zhejiang Univ Sci B. 2009;10:306-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Sun K, Zhang J, Wang Y, Xia C, Zhang C, Yu T, Tian S. Arthroscopic anterior cruciate ligament reconstruction with at least 2.5 years’ follow-up comparing hamstring tendon autograft and irradiated allograft. Arthroscopy. 2011;27:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Sun K, Zhang J, Wang Y, Zhang C, Xia C, Yu T, Tian S. A prospective randomized comparison of irradiated and non-irradiated hamstring tendon allograft for ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2012;20:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Rihn JA, Irrgang JJ, Chhabra A, Fu FH, Harner CD. Does irradiation affect the clinical outcome of patellar tendon allograft ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2006;14:885-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Roche C, Gaskins B, Kuhn C, Moore M. The effects of gamma irradiation on the biomechanical properties of soft tissue allografts. 2005; Available from: http://www.aatb.org/files/2005Abstract37.pdf. |
| 28. | Fideler BM, Vangsness CT, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med. 1995;23:643-646. [PubMed] |
| 29. | Curran AR, Adams DJ, Gill JL, Steiner ME, Scheller AD. The biomechanical effects of low-dose irradiation on bone-patellar tendon-bone allografts. Am J Sports Med. 2004;32:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Gifstad T, Foss OA, Engebretsen L, Lind M, Forssblad M, Albrektsen G, Drogset JO. Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med. 2014;42:2319-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 31. | Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Moore MA. Inactivation of enveloped and non-enveloped viruses on seeded human tissues by gamma irradiation. Cell Tissue Bank. 2012;13:401-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Ghodadra NS, Mall NA, Grumet R, Sherman SL, Kirk S, Provencher MT, Bach BR. Interval arthrometric comparison of anterior cruciate ligament reconstruction using bone-patellar tendon-bone autograft versus allograft: do grafts attenuate within the first year postoperatively? Am J Sports Med. 2012;40:1347-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Baldini T, Caperton K, Hamkins M, McCarty E. Effect of a novel sterilization method on biomechanical properties of soft tissue allografts. Knee Surg Sports Traumatol Arthrosc. 2014; Epub ahead of print. [PubMed] |
| 35. | Yanke AB, Bell R, Lee AS, Shewman E, Wang VM, Bach BR. Central-third bone-patellar tendon-bone allografts demonstrate superior biomechanical failure characteristics compared with hemi-patellar tendon grafts. Am J Sports Med. 2013;41:2521-2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |