Published online Oct 18, 2015. doi: 10.5312/wjo.v6.i9.712
Peer-review started: February 13, 2015
First decision: June 9, 2015
Revised: July 27, 2015
Accepted: August 13, 2015
Article in press: August 14, 2015
Published online: October 18, 2015
Processing time: 249 Days and 19 Hours
AIM: To investigate the effectiveness of two-stage reimplantation using antibiotic-loaded bone cement (ALBC) and the risk factors associated with failure to control periprosthetic joint infection (PJI).
METHODS: We retrospectively reviewed 38 consecutive hips managed using two-stage reimplantation with ALBC. The mean follow-up period was 5.4 years (range: 2.5-9 years).
RESULTS: The causative pathogens were isolated from 29 patients (76%), 26 of whom were infected with highly virulent organisms. Sixteen patients (42%) underwent at least two first-stage debridements. An increased debridement frequency correlated significantly with high comorbidity (P < 0.001), a lower preoperative Harris hip score (HHS; P < 0.001), antimicrobial resistance, and gram-negative and polymicrobial infection (P = 0.002). Of the 35 patients who underwent two-stage reimplantation, 34 showed no signs of recurrence of infection. The mean HHS improved from 46 ± 12.64 to 78 ± 10.55 points, with 7 (20%), 12 (34%), 11 (32%) and 5 (14%) patients receiving excellent, good, fair and poor ratings, respectively.
CONCLUSION: The current study demonstrated that two-stage reimplantation could successfully treat PJI after hip arthroplasty. However, the ability of ALBC to eradicate infection was limited because frequent debridement was required in high-risk patients (i.e., patients who are either in poor general health due to associated comorbidities or harbor infections due to highly virulent, difficult-to-treat organisms). Level of evidence: Level IV.
Core tip: Two-stage revision with antibiotic-laden bone cement for periprosthetic infection after total hip arthroplasty is generally recognized as the gold-standard treatment. Two-stage revision usually comprises removal of all components, including cement, and radical debridement of all suspected infectious, necrotic tissues and bone. However, despite the success of two-stage revision for the treatment of infected primary hip arthroplasties, not all causative organisms can be successfully eradicated, especially multidrug-resistant virulent microorganisms; therefore we evaluated the efficacy of two-stage reimplantation with antibiotic-laden bone cement against difficult-to-treat microorganisms as well as the risk factors associated with failure to control infection.
- Citation: Yoon YC, Lakhotia D, Oh JK, Moon JG, Prashant K, Shon WY. Is two-stage reimplantation effective for virulent pathogenic infection in a periprosthetic hip? A retrospective analysis. World J Orthop 2015; 6(9): 712-718
- URL: https://www.wjgnet.com/2218-5836/full/v6/i9/712.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i9.712
Infection following total hip arthroplasty (THA) is a serious obstacle that incurs high costs and considerably affects the physical and mental health of the patient, and causes stress for the treating surgeon. The general incidence of periprosthetic joint infection (PJI) is 1%-2% in most centers, despite the use of new antimicrobial agents and sterilization appliances in the operating room[1].
Two-stage reimplantation with antibiotic loaded bone cement (ALBC) is the most widely performed THA procedure and has a high success rate[2,3]. This procedure has several advantages, including a reduced infective burden, exposure of residual bacteria under the biofilm, and antibiotic elution at a high concentration to assist with infection control. Moreover, articulating cement spacers help to preserve the limb length and normal tensile forces of the soft-tissues, thereby providing partial joint mobility[4]. Despite the success of this technique, however, not all infections are successfully eradicated[5]. A significant number of patients still experience difficult-to-treat complex infections, despite being treated after two-stage reconstruction[6]. The reported success rates are lower among patients harboring gram-negative organisms, polymicrobial infections, Enterococci, methicillin-resistant Staphylococci, or any organism that elaborates a glycocalyx, which are considered highly virulent[7,8]. Some authors recommend against the use of spacers in patients with highly virulent, difficult to treat microorganisms[9].
The potential associations of an increased failure rate with the virulence of the infecting organism[10-12], clinical effectiveness of ALBC[13,14], and the etiology of treatment failure in patients with PJI[15] remains controversial. The aim of this study was to assess the efficacy of two-stage reimplantation with ALBC against virulent difficult-to-treat microorganisms and the risk factors associated with failure to control infection.
This retrospective study involved 43 patients treated between 2003 and 2011. Five patients were excluded because of death unrelated to the relevant surgery. The mean age of the remaining 38 patients (24 men and 14 women) was 63.97 ± 12.66 years (range 41-89 years). The patients had undergone primary arthroplasty (16 THA and 15 bipolar hemiarthroplasty) or revision THA (seven patients). The mean follow-up duration was 5.4 years (range 2.5-9 years). Patient comorbidity data are shown in Table 1.
| Comorbidities | No. of cases |
| Heart diseases, including hypertension | 15 |
| Uncontrolled diabetes mellitus | 11 |
| Liver cirrhosis | 6 |
| Repeated urinary tract infection | 6 |
| Brain lesions including stroke | 4 |
| Chronic gout | 3 |
| Psoriasis | 2 |
| Systemic lupus erythematosus | 1 |
| Chronic renal failure | 2 |
| Autoimmune hepatitis | 1 |
| Cushing’s syndrome | 1 |
| Asthma | 1 |
| Chronic obstructive pulmonary disease | 1 |
A PJI was confirmed if at least two of the following criteria were present 3 mo after arthroplasty: (1) a chronic, discharging sinus in communication with the prosthesis; (2) clinical symptoms and signs of unexplainable pain, persistent local erythema, swelling, and a high erythrocyte sedimentation rate (ESR ≥ 30 mm/h) and C-reactive protein level (CRP ≥ 10 mg/L); (3) isolation of a microorganism in one culture; and (4) the presence of purulence in the affected joint or > 5 neutrophils per high-powered field upon histopathological examination of at least two different sites[16,17].
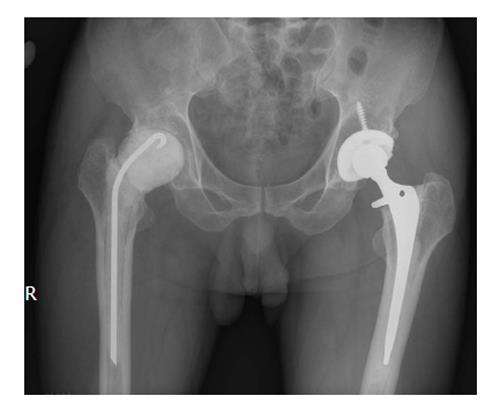
The first stage consisted of sinus excision and abscess drainage, if present, prosthesis removal, and extensive debridement, followed by insertion of ALBC (Figure 1). The type of antibiotics loaded in the cement was based on the result of presurgical bacterial cultures. In cases with gram-positive and negative bacterial cultures, 3 g of vancomycin was used; in cases with gram-negative or mixed bacterial infections a combination of 2-3 g of vancomycin and 2 g of fortimicin was mixed with 40 g of bone cement (Surgical Simplex-P; Stryker, Allendale, NJ, United States). For one patient infected with tubercle bacillus and Pseudomonas aeruginosa, a combination of 2 g each of vancomycin, fortimicin, and streptomycin was used.
Intravenous antibiotics were given for 4-8 wk depending on the bacterial cultures. In patients with increased inflammatory markers associated with clinical findings despite a 6-8 wk antibiotic course, repeated debridement was performed. Secondary reimplantation was scheduled when the ESR and CRP levels remained within normal limits with no evidence of persistent infection for two consecutive reviews.
All patients were examined at 1, 2, 3 and 6 mo, and then yearly. Clinical evaluations including Harris hip score (HHS) and hematological evaluations, were performed at every follow-up. Acetabular loosening was defined as a shift of the acetabulum > 4 mm, change in the inclination angle of > 5°, and a radiolucent line demarcation of > 1 mm in all three zones[18]. The Harris criteria were used to determine loosening of the cemented femoral stem[19]. Subsidence or radiolucency was evaluated for the cementless femoral component[20].
A data analysis was conducted using SPSS software, version 20.0 (SPSS, Inc., Chicago, IL, United States); a modified Kruskal-Wallis test was used, as a nonparametric method to analyze factors influencing the frequency of debridement and cure rate of treatment with ALBC. The statistical significance was set at P < 0.05.
Microorganisms were identified in 29 patients (76%), including four cases with methicillin-sensitive bacteria, nine cases with methicillin-resistant bacteria, three cases with vancomycin-resistant Enterococcus (VRE), nine with multiple bacterial organisms (including methicillin-resistant and tubercular bacillus), and four cases with gram-negative bacteria such as Pseudomonas and Salmonella (Table 2).
| Variable | No. of debridements (38 hips) | P value | ||
| 1 (n = 22) | 2 (n = 10) | > 3 (n = 6) | ||
| Mean age (yr) | 61.09 | 68.50 | 68.33 | 0.168 |
| Infecting organism (n) | 0.002 | |||
| Methicillin sensitive | 2 | 2 | ||
| Resistant Staphylococcus | 6 | 2 | 1 | |
| Vancomycin-resistant Enterococcus | 2 | 1 | ||
| Pseudomonas | 1 | 2 | ||
| Salmonella | 1 | |||
| Polymicrobial | 1 | 3 | 5 | |
| Negative culture | 9 | |||
| Comorbidities (n) | < 0.001 | |||
| 0 | 10 | 1 | ||
| 1 | 8 | 2 | 1 | |
| 2 | 2 | 5 | 2 | |
| 3 | 2 | 2 | 1 | |
| 4 | 1 | |||
| 5 | 1 | |||
| Preoperative HHS | < 0.001 | |||
| 20-29 | 1 | |||
| 30-39 | 1 | 3 | ||
| 40-49 | 1 | 6 | 2 | |
| 50-59 | 7 | 1 | ||
| 60-69 | 8 | 2 | ||
| 70-80 | 6 | |||
Repeated debridement was performed in 16 patients (Table 3). Ten, one, four, and 1 patient required a second, third, fourth and seventh debridement, respectively. Ten of the 16 patients were infected with resistant bacteria (methicillin-resistant Staphylococcus epidermidis, VRE, and methicillin-resistant Staphylococcus aureus). Six patients with nine polymicrobial infections harbored resistant bacteria (Table 3) and underwent multiple debridements (two or more). The remaining four patient harboring resistant bacteria had monobacterial infections and underwent multiple debridements. A 66-year-old woman with methicillin-resistant organisms, VRE, and Pseudomonas aeruginosa required seven debridements, with methicillin-resistant organisms isolated during the second, third, and fourth debridements and Pseudomonas during the seventh debridement.
| Patient | Microorganism | No. of debridements |
| 1 | MRSA | 2 |
| 2 | Streptococcus parasanguis | 2 |
| 3 | Staphylococcus haemolyticus | 2 |
| 4 | Enterococcus faecalis (VRE) | 2 |
| 5 | MRSE | 2 |
| 6 | Pseudomonas aeruginosa | 2 |
| 7 | Pseudomonas stutzeri | 2 |
| 8 | MRSA, Acinetobacter baumannii | 2 |
| 9 | Enterococcus faecium (VRE), MRSA | 2 |
| 10 | Serratia marcescens, Acinetobacter baumannii | 2 |
| 11 | MRSE, Escherichia coli, Staphylococcus haemolyticus | 3 |
| 12 | MRSA | 4 |
| 13 | Salmonella, MRSE, MRSA | 4 |
| 14 | Acinetobacter baumannii, Proteus penneri, MRSA, Enterococcus faecium (VRE) | 4 |
| 15 | Pseudomonas aeruginosa, Acinetobacter baumannii | 4 |
| 16 | MRSA, MRSE, Pseudomonas aeruginosa, Enterococcus faecium (VRE) | 7 |
The average period of parenteral antibiotic administration was 5.6 wk (range: 4-8 wk) in 22 patients who underwent one debridement. Patients who underwent more than two debridements were subjected to antibiotic therapy for variable durations. The mean duration between primary and secondary surgeries was 5.4 mo (range: 1.2-24 mo). Secondary reimplantation could not be performed in three patients. One of these patients had a poor general condition and the other patients refused to undergo the procedure despite successful control of the infection.
Thirty-four (97.1%) of 35 patients did not show any sign of recurrence after secondary reimplantation. In one case, Escherichia coli infection recurred after 4 years. In that case an initial THA was performed to treat a pelvic-acetabular fracture, along with diversion colostomy.
The rate of repeated debridement was higher in patients with associated comorbidities (P < 0.001), a low preoperative HHS (P < 0.001), and infection with resistant, gram-negative, and polymicrobial organisms (P = 0.002). We did not find any statistically significant disparity in the frequency of debridement with respect to patient age (P = 0.168).
The mean HHS improved from 46 ± 12.64 (range: 27-67 points) to 78 ± 10.35 points (range: 60-98 points), with 7 (20%), 12 (34%), 11 (32%), and five (14%) patients categorized as excellent, good, fair, and poor, respectively. During follow-up, a demarcation line was observed on the acetabular side in zones 1 and 2 in one patient and in zones 2 and 3 in another patient. Both of these patients presented with mild pain but required no further treatment.
Posterior hip dislocation in three patients was successfully treated via closed reduction. Three patients experienced periprosthetic femoral fractures (Vancouver type C in one and B1 in two) that were successfully managed via open reduction and internal fixation. Limb length discrepancies of > 2 cm were observed in two patients.
Two-stage reimplantation with ALBC is a well-established procedure with a success rate exceeding 95% in recent studies[21,22]. However, other studies have reported methicillin-resistant Staphylococcus infection control rates of 50%-80% with this procedure[5,11,17]. In the present study, we achieved equivalent or better results, although first-stage debridement had to be repeated at least twice in 16 patients (42%), thus raising doubts about the effectiveness of two-stage revision with ALBC, especially for the control of virulent pathogens.
First-stage debridement was declared a failure after 6-8 wk for the following reasons: (1) a 6-8 wk course of an appropriate systemic antibiotic is sufficient for the eradication of pathogens[23]; (2) ALBC becomes surrounded by reactive fibrous tissue, which reduces its therapeutic efficacy[24]; and (3) repeated debridement represents the only possibility of eradicating local infection.
Studies have demonstrated persistent infection in 6%-28% of patients after first-stage debridement, thus requiring repeated debridements[5,14,25,26]. Biofilm formation is a well-known and important step in the pathogenesis of polymer-associated infections[27], necessitating a 1000-fold higher dose of antibiotics than that needed to eradicate a planktonic population[28]. Although some studies demonstrated that the antibiotic levels remain well above the minimum inhibitory concentration for common microbes even several weeks after implantation[29], in reality, the hydrophobic nature of bone cement permits effective elution of only 10% of the antibiotic[30]. Furthermore, ALBC has a favorable surface for bacterial growth, and long-term antibiotic use at a sub-inhibitory concentration facilitates the development of mutational resistance[31,32].
Many studies have emphasized the emergence of bacterial strains with altered resistance profiles following adherence to ALBC in both in vivo and in vitro models[32,33]. Therefore, the development of resistant organisms on cement spacers that lack suitable antibiotics is a matter of concern, and Giulieri[34] suggested that, in patients with infections due to difficult-to-treat organisms such as Enterococcus or other multidrug-resistant organisms, two-stage reimplantation without the use of ALBC is preferred.
The most recently published success rates for a two-stage exchange without the use of ALBC were between 80% and 90%, comparable to the results of the current standard procedures that include ALBC (Table 4). The reasons for the high failure rate in our series during the first-stage remain unclear, although the overall treatment success rate was 97% despite that 26 (68%) patients were infected with virulent organisms. We found that the virulence of the causative microorganisms, including resistant microorganisms, gram-negative microorganisms (Pseudomonas, salmonella), and polymicrobial microorganisms, was associated with the persistence of infection and need for repeated debridement. Other important prognostic factors in our study were the presence of comorbidities and a low preoperative HHS. Medical conditions that impair host immunity, such as uncontrolled diabetes mellitus and liver cirrhosis, also increase the risk of persistent infection. We believe that a low HHS might predict the severity and duration of PJI.
| Ref. | Local cement device | Follow up (mo) | Infection control after first debridement | Infection control after reimplantation | Hips with resistant organisms | Successful control of infection |
| Hofmann et al[3] | Spacer | 76 | 26/42 (62%) | 26/27 (96%) | NA | NA |
| Salgado et al[11] | Spacer | 6 | 28/45 (62%) | 18/25 (72%) | 12 (MRSA-12) | 6/12 (50%) |
| Lim et al[35] | Spacer | 54 | 38/45 (84%) | 35/42 (83%) | 24 (MRSA-14 MRCNS-10) | 16/24 (67%) |
| Fink et al[2] | Spacer | 35 | 40/40 (100%) | 40/40 (100%) | 3 (MRSA-3) | 3/3 (100%) |
| Toulson et al[36] | Spacer | 64.8 | 80/84 (95%) | 80/84 (95%) | 21 (MRSA-7, MRSE-12, VRE-2) | 21/21 (100%) |
| Parvizi et al[25] | Spacer | 12 | 61/127 (57%) | 49/72 (67%) | 66 (MRSA-34, MRSE-32) | 48/66 (75%) |
| Romano et al[22] | Spacer | 48 | 98/102 (96%) | 98/102 (96%) | NA | NA |
| Leung et al[5] | Spacer | 58 | 30/38 (79%) | 30/38 (79%) | 38 (MRSA-10, MRSE-26, Both-2) | 30/38 (79%) |
| Uchiyama et al[12] | Spacer | 48 | 33/36 (92%) | 21/31 (67%) | 15 (MRSA-10, MRCNS-4) | 10/14 (71%) |
| Volin et al[10] | No spacer | 48 | 43/46 (93%) | 43/46 (93%) | 9 (MRSA-2, MRCNS-7) | 8/9 (89%) |
| Cordero-Ampuero et al[37] | No spacer | 53 | 30/36 (83%) | 20/20 (100%) | 19 (MRSA-19) | 17/19 (89%) |
| Van Diemen et al[26] | No spacer | 96 | 118/136 (87%) | 118/138 (87%) | 7 (MRSA-7) | 5/7 (71%) |
| Current study 2015 | Spacer | 64 | 37/38 (97%) | 34/35 (97%) | 26 (MRSA-4, MRSE-5, VRE-3, 1Poly-9) | 25/26 (96%) |
Our study has several limitations. First, this was a retrospective study and most patients were referred; accordingly, there may have been variations in the data collection methods as well as referral bias. Second, the sample size was small, making it difficult to draw definitive conclusions. Third, we could not determine the respective contributions of ALBC and systemic antimicrobial therapy to infection control. However, our analysis yielded some significant results. We believe that our findings are remarkable because we included an adequate number of patients for achieving statistically significant results, and the same surgeon performed all operations by using a standardized protocol.
Based on the results of this study, two-stage reimplantation can successfully treat PJI after hip arthroplasty, but the ability of ALBC to eradicate infection is limited because frequent debridements are required in some patients who are either in poor general health due to associated comorbidities or harbor infections with highly virulent, difficult-to-treat organisms.
Two-stage reimplantation is the preferred treatment protocol for chronic periprosthetic infection. However, the effectiveness of antibiotic-loaded cement spacers for chronic periprosthetic infection with difficult-to-treat microorganisms remains controversial. This study aimed to investigate the clinical effectiveness of two-stage reimplantation with antibiotic cement spacers for chronic periprosthetic hip infection and the risk factors associated with failure to control infection.
Effectiveness of two-stage reimplantation with antibiotic-loaded bone cement and risk factors associated with failure to control periprosthetic joint infection.
Infection was controlled in 37 patients (97.3%) after the first stage. Second-stage reimplantation was possible in 35 patients (92%), and there was no evidence of infection recurrence in 34 (97.1%). Two or more first-stage debridements were performed in 16 patients (42%). A mean of 1.8 (range: 1-7) debridements were required to control infection. An increase frequency of debridement correlated significantly with increase comorbidity (P < 0.001), a low preoperative Harris hip score (P < 0.001), antibiotic resistance, and polymicrobial culture results (P < 0.001).
More repeated debridements were required in patients with chronic periprosthetic infections caused by resistant organisms, as well as in the patients with medical comorbidities at the time of two-stage reimplantation with antibiotic-loaded cement spacers.
Although a few studies have demonstrated the maintenance of antibiotic levels above the minimum inhibitory concentration for common pathogens at several months after implantation, the relative hydrophobicity of bone cement allows the effective elution of only 10% of the antibiotic. Furthermore, the antibiotic-loaded cement spacer has an optimum surface for colonization, and prolonged exposure to antibiotics at sub-inhibitory levels facilitates mutational resistance.
This is an well-written paper.
P- Reviewer: Erkan S, Isidro A S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. 2009;24:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 2. | Fink B, Grossmann A, Fuerst M, Schäfer P, Frommelt L. Two-stage cementless revision of infected hip endoprostheses. Clin Orthop Relat Res. 2009;467:1848-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Hofmann AA, Goldberg TD, Tanner AM, Cook TM. Ten-year experience using an articulating antibiotic cement hip spacer for the treatment of chronically infected total hip. J Arthroplasty. 2005;20:874-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007;89:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Leung F, Richards CJ, Garbuz DS, Masri BA, Duncan CP. Two-stage total hip arthroplasty: how often does it control methicillin-resistant infection? Clin Orthop Relat Res. 2011;469:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Kalra KP, Lin KK, Bozic KJ, Ries MD. Repeat 2-stage revision for recurrent infection of total hip arthroplasty. J Arthroplasty. 2010;25:880-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Garvin KL, Hanssen AD. Infection after total hip arthroplasty. Past, present, and future. J Bone Joint Surg Am. 1995;77:1576-1588. [PubMed] |
| 8. | Kilgus DJ, Howe DJ, Strang A. Results of periprosthetic hip and knee infections caused by resistant bacteria. Clin Orthop Relat Res. 2002;116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 193] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Trampuz A, Zimmerli W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr Infect Dis Rep. 2008;10:394-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Volin SJ, Hinrichs SH, Garvin KL. Two-stage reimplantation of total joint infections: a comparison of resistant and non-resistant organisms. Clin Orthop Relat Res. 2004;94-100. [PubMed] |
| 11. | Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Uchiyama K, Takahira N, Fukushima K, Moriya M, Yamamoto T, Minegishi Y, Sakai R, Itoman M, Takaso M. Two-stage revision total hip arthroplasty for periprosthetic infections using antibiotic-impregnated cement spacers of various types and materials. ScientificWorldJournal. 2013;2013:147248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Cabo J, Euba G, Saborido A, González-Panisello M, Domínguez MA, Agulló JL, Murillo O, Verdaguer R, Ariza J. Clinical outcome and microbiological findings using antibiotic-loaded spacers in two-stage revision of prosthetic joint infections. J Infect. 2011;63:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Disch AC, Matziolis G, Perka C. Two-stage operative strategy without local antibiotic treatment for infected hip arthroplasty: clinical and radiological outcome. Arch Orthop Trauma Surg. 2007;127:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Lee J, Kang CI, Lee JH, Joung M, Moon S, Wi YM, Chung DR, Ha CW, Song JH, Peck KR. Risk factors for treatment failure in patients with prosthetic joint infections. J Hosp Infect. 2010;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Bori G, Soriano A, García S, Gallart X, Mallofre C, Mensa J. Neutrophils in frozen section and type of microorganism isolated at the time of resection arthroplasty for the treatment of infection. Arch Orthop Trauma Surg. 2009;129:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Parvizi J, Ghanem E, Menashe S, Barrack RL, Bauer TW. Periprosthetic infection: what are the diagnostic challenges? J Bone Joint Surg Am. 2006;88 Suppl 4:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;20-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 215] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Harris WH, McCarthy JC, O’Neill DA. Femoral component loosening using contemporary techniques of femoral cement fixation. J Bone Joint Surg Am. 1982;64:1063-1067. [PubMed] |
| 20. | Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987;69:45-55. [PubMed] |
| 21. | Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma. 2004;56:1247-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Romanò CL, Romanò D, Logoluso N, Meani E. Long-stem versus short-stem preformed antibiotic-loaded cement spacers for two-stage revision of infected total hip arthroplasty. Hip Int. 2010;20:26-33. [PubMed] |
| 23. | Bernard L, Legout L, Zürcher-Pfund L, Stern R, Rohner P, Peter R, Assal M, Lew D, Hoffmeyer P, Uçkay I. Six weeks of antibiotic treatment is sufficient following surgery for septic arthroplasty. J Infect. 2010;61:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Streuli JC, Exner GU, Reize CL, Merkofer C, Scott CP, Zbinden R. In vitro inhibition of coagulase-negative staphylococci by vancomycin/aminoglycoside-loaded cement spacers. Infection. 2006;34:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Parvizi J, Azzam K, Ghanem E, Austin MS, Rothman RH. Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res. 2009;467:1732-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 26. | van Diemen MP, Colen S, Dalemans AA, Stuyck J, Mulier M. Two-stage revision of an infected total hip arthroplasty: a follow-up of 136 patients. Hip Int. 2013;23:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Antoci V, Adams CS, Parvizi J, Davidson HM, Composto RJ, Freeman TA, Wickstrom E, Ducheyne P, Jungkind D, Shapiro IM. The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials. 2008;29:4684-4690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Cerca N, Martins S, Sillankorva S, Jefferson KK, Pier GB, Oliveira R, Azeredo J. Effects of growth in the presence of subinhibitory concentrations of dicloxacillin on Staphylococcus epidermidis and Staphylococcus haemolyticus biofilms. Appl Environ Microbiol. 2005;71:8677-8682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Masri BA, Panagiotopoulos KP, Greidanus NV, Garbuz DS, Duncan CP. Cementless two-stage exchange arthroplasty for infection after total hip arthroplasty. J Arthroplasty. 2007;22:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | DiCicco M, Duong T, Chu A, Jansen SA. Tobramycin and gentamycin elution analysis between two in situ polymerizable orthopedic composites. J Biomed Mater Res B Appl Biomater. 2003;65:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Thomes B, Murray P, Bouchier-Hayes D. Development of resistant strains of Staphylococcus epidermidis on gentamicin-loaded bone cement in vivo. J Bone Joint Surg Br. 2002;84:758-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Kendall RW, Duncan CP, Beauchamp CP. Bacterial growth on antibiotic-loaded acrylic cement. A prospective in vivo retrieval study. J Arthroplasty. 1995;10:817-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | König DP, Schierholz JM, Hilgers RD, Bertram C, Perdreau-Remington F, Rütt J. In vitro adherence and accumulation of Staphylococcus epidermidis RP 62 A and Staphylococcus epidermidis M7 on four different bone cements. Langenbecks Arch Surg. 2001;386:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Giulieri SG, Graber P, Ochsner PE, Zimmerli W. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection. 2004;32:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 161] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Lim SJ, Park JC, Moon YW, Park YS. Treatment of periprosthetic hip infection caused by resistant microorganisms using 2-stage reimplantation protocol. J Arthroplasty. 2009;24:1264-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Toulson C, Walcott-Sapp S, Hur J, Salvati E, Bostrom M, Brause B, Westrich GH. Treatment of infected total hip arthroplasty with a 2-stage reimplantation protocol: update on “our institution’s” experience from 1989 to 2003. J Arthroplasty. 2009;24:1051-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468:3268-3277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |