Published online Sep 18, 2015. doi: 10.5312/wjo.v6.i8.567
Peer-review started: January 26, 2015
First decision: March 6, 2015
Revised: June 10, 2015
Accepted: July 16, 2015
Article in press: July 17, 2015
Published online: September 18, 2015
Processing time: 238 Days and 15.8 Hours
The drastic changes in body composition following spinal cord injury (SCI) have been shown to play a significant role in cardiovascular and metabolic health. The pattern of storage and distribution of different types of adipose tissue may impact metabolic health variables similar to carbohydrate, lipid and bone metabolism. The use of magnetic resonance imaging provides insights on the interplay among different regional adipose tissue compartments and their role in developing chronic diseases. Regional adipose tissue can be either distributed centrally or peripherally into subcutaneous and ectopic sites. The primary ectopic adipose tissue sites are visceral, intramuscular and bone marrow. Dysfunction in the central nervous system following SCI impacts the pattern of distribution of adiposity especially between tetraplegia and paraplegia. The current editorial is focused primarily on introducing different types of adipose tissue and establishing scientific basis to develop appropriate dietary, rehabilitation or pharmaceutical interventions to manage the negative consequences of increasing adiposity after SCI. We have also summarized the clinical implications and future recommendations relevant to study adiposity after SCI.
Core tip: The focus of this current editorial is to introduce different adipose tissue types that may impose significant health risks to individuals with spinal cord injury (SCI). Accurate measuring of this depot of ectopic adipose tissue may require special knowledge; however, it is important considering the dramatic changes in body composition and the extensive loss in skeletal muscle tissue below the level of injury. The clinical implications of studying adipose tissue may encourage further research to decipher the mechanistic links with the metabolic profile after SCI. Our current knowledge is limited and rehabilitation strategies are still pre-mature in targeting ectopic adiposity after SCI.
- Citation: Gorgey AS, Wells KM, Austin TL. Adiposity and spinal cord injury. World J Orthop 2015; 6(8): 567-576
- URL: https://www.wjgnet.com/2218-5836/full/v6/i8/567.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i8.567
Adipose tissue is one of the main types of connective tissue in the body and is the storage site of triglycerides. It is not only served as an energy reservoir, but it has been recently considered as the largest endocrine gland in the body[1,2]. The endocrine properties are related to the secretion of regulatory proteins similar to leptin, cytokines and adiponectin that are likely to influence whole body metabolism, energy intake as well as insulin sensitivity[2]. Inflammatory biomarker factors similar to interlukin 6, tumor necrosis factor alpha and C-reactive proteins which are likely to interact with various systems can trigger insulin resistance[2,3]. The inflammatory nature of adipose tissue has been linked to other comorbidities similar to type 2 diabetes mellitus, cancer and cardiovascular disease. Additionally, adipose tissues secrete number of active peptides called adipokines[3].
Interest in studying adipose tissue physiology and anatomical distribution has stemmed from its closest association with disturbance in metabolic profile including carbohydrate, lipid, and bone metabolism[3-7]. Most recently the link between adipose tissue and bone health has been recognized after spinal cord injury (SCI)[7]. This link may contribute to our understanding of different pathways that lead to the development of obesity and osteoporosis. Moreover, the role of exercise in adipose tissue utilization as a primary source of fuel is an active area of continuous research investigation[8-11]. Excessive adipose tissue accumulation imposes significant health risks to populations with physical disability leading to poor quality of life and short life-span. Imbalance between energy intake and energy expenditure may lead to excessive adipose tissue accumulation, a phenomenon which is referred to as obesity[12]. Obesity is further complicated by other factors similar to physical activity, genetic, socio-economical and educational factors. According to a survey conducted by the Center for Disease Control, more than one-third (34.9%) of adults in the United States are considered obese[13].
SCI is a medical condition resulting from direct or indirect aggravation or insult to the neural pathway located in the vertebral column. The disruption in the efferent and afferent neural transmission between the cortex and the periphery leads to the paralysis of skeletal and smooth muscles as well as somatosensory loss of pain, touch and temperature sensation below the level of injury. A person with SCI is considered to be on the lowest end of the spectrum of physical activity as determined by the lowest oxygen uptake during peak exercise activity[14]. The low level of physical activity, significant muscle loss with changes in regional and total body composition, dysfunction in the autonomic nervous system and diminished anabolic hormones are a typical phenotype profile for a person with SCI[4,15,16].
The significant loss in lean mass within the first year of injury is accompanied with continuous increase in adipose tissue accumulation representing significant health risks after SCI[17]. Time since injury is strongly associated with a greater loss of lean tissue after SCI[17]. A strong effect of time since injury was identified in the legs and total body of monozygotic twins with paraplegia compared to their non-disabled (ND) co-twins, thereby eliminating age and genetic factors, essentially isolates the effect of SCI[18].
The purpose of this editorial is to introduce different types of adipose tissue distribution and their link to health consequences after SCI[19-22]. Considering the role of adipose tissue in the metabolic health variables after SCI, we sought to summarize the available evidence on different forms of adipose tissue. We highlighted the basic findings relevant to ectopic adipose tissue [visceral fat (VAT), intramuscular fat (IMF) and bone marrow fat (BMF)] in persons with SCI and the technical procedures regarding the use of magnetic resonance imaging (MRI) in measuring ectopic adipose tissue[19,22]. The difference in terminology between intermuscular (IeMF) and IMF was addressed as well as the significance of measuring visceral and bone marrow adiposity (Figures 1 and 2).
Finally, the clinical implications and future directions of measuring different types of adipose tissue and cardio-metabolic health were acknowledged. We have also realized the significance of exercise and dietary interventions in addressing the complications of reduction in level of physical activity after SCI. Encouragement to expand leisure time physical activity may help to counteract reduced level of energy expenditure and balanced their daily caloric intake.
For this editorial, we would like to distinguish between the terms “obesity” and “adiposity” relevant to the SCI population. Obesity is simply a metabolic disorder resulted from an imbalance between energy intake and expenditure. Obesity is simply defined using body mass index (BMI), waist and abdominal circumferences. These anthropometric criteria have not been well validated in the field of body composition and SCI, with a considerable debate about its efficacy in identifying those at risks of developing cardio-metabolic complications. It is well established that BMI underestimates the percentage fat mass (FM) in persons with SCI, with a continuous effort to develop a population specific BMI criteria[17]. The term adiposity refers to infiltration or storage of adipose tissue in subcutaneous or ectopic sites due to inactivity, disruption in hormonal secretion, altered body composition and poor nutritional choices after SCI. Studying whole and regional adiposity may need specialized body composition assessment techniques to accurately quantify adiposity after SCI. Although the prevalence of obesity may easily exceed two-thirds of the SCI population[23]; we believe that that the SCI population suffers from excessive adiposity that exceeds 30% of the whole body mass[6,17]. This may be true in 50% of the SCI population despite having normal BMI, which leads to significant metabolic and health implications[6].
Advances in imaging technology similar to the use of MRI, ultrasound and dual-energy X-ray absorptiometry (DXA) facilitate the study of the distribution of regional adiposity[19-22]. DXA scans are commonly used in the clinical settings to measure total, regional body composition and bone mineral density or content. However, DXA is limited in distinguishing between subcutaneous adipose tissue (SAT) and ectopic adipose tissue[6]. The anatomy and distribution of adipose tissue is of particular interest to the metabolic health after SCI[5-7,16]. Triglycerides can be either stored in subcutaneous or ectopic sites similar to the peritoneum, visceral adipose tissue (VAT), in the liver (steatosis), in the muscle such as IMF or IeMF and in the bone marrow as BMF. The mechanisms by which triglycerides are stored in subcutaneous or ectopic sites are poorly understood; however, it is linked to genetic and lifestyle factors. Adopting an active lifestyle including routine exercise is likely to reduce ectopic adipose tissue storage in the general population as well as in persons with SCI[9,10]. This is also accompanied with mitigating the negative consequences of ectopic adipose tissues on the metabolic profile. The role of exercise on SAT is still questionable[9].
Compared with non-disabled (ND) individuals, Spungen et al[17] showed that the individuals with SCI were 13% fatter per unit of BMI (kg/m2). Moreover, the total body fat was 10% greater in the group with tetraplegia and 12% greater in the group with paraplegia compared to ND individuals. Also, percent FM in the arms of tetraplegics was 8% greater than individuals with paraplegia[17]. Persons with SCI are likely to have more than 30% FM of the total body mass; that are stored at the central or peripheral site[23]. The central distribution of adipose tissue may represent up to 25% of total body FM[22]. Gorgey and Gater[6] have highlighted the significance of studying regional and relative adiposity and their associations to metabolic health variables after SCI.
The central adipose tissue refers to quantifying trunk FM which can further be sub-divided into SAT and VAT. In analyzing VAT and SAT volumes, our laboratory has taken advantage of analyzing multiple axial slices acquired during MRI. We have previously shown that using a single axial slice may inaccurately reflect the true volumetric distribution of VAT and SAT[22]. After measuring the CSA of series of axial images, the volume of VAT or SAT can be calculated by summing up all the measured areas. The volume (cm3) is calculated using the following equation = (A1d1-2 + A2d2-3 + A3d3-4.........Andn-n+1) after considering the thickness of the slice. The “A” letter refers to the CSA of a single axial image and “d” refers to the distance (cm) of inter-space between two corresponding axial images.
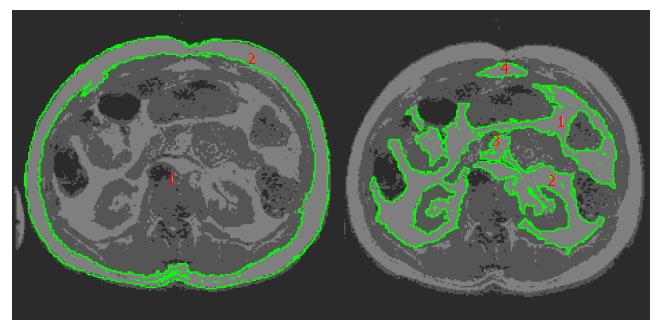
SAT: SAT is layered superficial to the outer muscular wall of the abdominal region and skeletal muscle, and immediately below the dermal layer of the skin[5,22]. Abdominal SAT is superficial to the abdominal and back muscles and lies beneath the hypodermis. SAT is measured by tracing the outer edge of the abdominal region (hypodermis) and along the outermost point of the muscular abdominal wall. The area in between these two traces will be considered the cross-sectional area (CSA) in cm2 of this slice (Figure 3).
The level of injury strongly determines the severity of SCI as well as the associated changes in body composition. Individuals with tetraplegia population have a greater leg FM/trunk FM (45%) and leg FM/body FM (26%) and lower trunk FM/body FM (29%) ratios than individual with paraplegia[6]. This may suggest that persons with tetraplegia have the tendency to accumulate greater leg FM compared to those with paraplegia. Surprisingly, persons with tetraplegia have a lower ratio of trunk FM to whole body FM compared to persons with SCI[6]. These findings were confirmed when MRI was used to separate trunk VAT from SAT. The SAT CSA was non-significantly greater in persons with paraplegia compared to tetraplegia[24]. The hyperactivity of sympatho-adrenergic peripheral innervation to SAT may be responsible for the lower trunk adipose tissue in persons with tetraplegia and may explain the tight association that was documented between SAT CSA and lipid profile[24,25].
VAT: VAT is defined as intra-abdominal fat bound by parietal peritoneum or transversalis fascia, excluding the vertebral column and paraspinal muscles[5,22]. VAT may extend from the xyphoid process of the sternum to both greater trochanters of the femoral bones. VAT is measured by tracing out the innermost muscles of the abdominal cavity and the other abdominal organs present in a single slice. The area is quantified in cm2 which shows the amount of VAT present within the abdominal organs.
It is important to be meticulous and not trace over any fat tissue as it will yield inaccurate results. Increase in VAT accumulation has been identified as an independent risk factor of developing cardiovascular disease, insulin resistance and mortality[26]. This is mainly because of the close proximity of the VAT to the portal circulation. Further analysis using MRI showed that about 6% of whole body FM is VAT and 10% is SAT[22]. Persons with SCI have 58% and 48% greater VAT CSA and VAT/SAT CSA compared to ND controls[27]. It is apparent that VAT and increase VAT/SAT ratio are likely to have detrimental effects on the metabolic profile after SCI[5].
The pattern of VAT distribution may vary greatly among individuals with SCI. It is likely to be impacted by the level of injury and the de-innervation of the anterior abdominal muscles[24]. Further analysis based on the level of the injury did not reveal major differences in VAT between persons with paraplegia and tetraplegia[24]. However, the sample size was limited and future studies need to consider large sample size to study the differences in central adiposity based on the level of injury.
There are several points that need to be considered when using MRI to measure central adiposity in persons with SCI including the type of the injury, date of the injury, spinal fusion, and ability of the persons to hold his or her breath. All of these factors may influence the quality of the scan and may interfere with the ability of the examiner to accurately analyze the images[5,22,24]. For example, a gunshot wound may lead to bullet fragments in the spinal canal that will be of high risk to be exposed to a magnetic field. This bullet fragments may accidently move and lead to further damage to the intact cord or the surrounding blood supplies. A person with high level of injury, similar to C5, may lack the ability to hold his breath for 20-22 s to capture the whole trunk region. This may require the examiner to break the whole scan into multiple scans to allow short breath holding time that does not exceed 10 s. Other limitations may include the exact positioning of the participant inside the magnet mainly because of pelvic or trunk obliquity or uncontrolled spasticity that may be triggered before or during the scans and require special handling from positioning and stabilizing lower extremities, this is specifically important to accurately match the pre and post-MRI images in a longitudinal study.
The scans are captured from the xyphoid process to both greater trochanters. It becomes apparent that using a flexible trunk coil compared to the whole body magnet improves the quality and resolution of the captured images as well as higher signal to noise ratio. It is highly recommended that multiple axial images are sequenced to ensure the appropriate anatomical organization before the analysis. This is a crucial step before measuring the volume of the SAT or VAT. Different software programs may be available allowing appropriate segmentation of VAT and SAT in a single axial slice to measure the CSA without including other visceral regions similar to the intestines or the blood vessels in the region of interest.
Studying SAT and VAT distribution have revealed two body composition phenotypes[5,6]. The first phenotype is likely to store adipose tissue in subcutaneous compared to visceral sites and this phenotype may protect against developing metabolic disorders. The second phenotype has a greater tendency to store adipose tissue in visceral sites with the tendency to develop insulin resistance, dyslipidemia and other metabolic disorders. The exact mechanisms behind these two phenotypes have yet to be deciphered. Anecdotal evidence from our laboratory suggests that gender may play a definite role in the distribution of VAT and SAT after SCI. Women with SCI have greater SAT and smaller VAT CSAs than men with SCI (Gorgey et al unpublished results). This may shed the light on the significance of studying hormonal differences and adiposity after SCI.
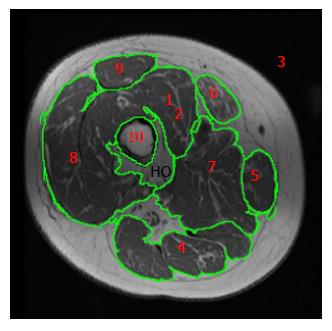
The peripheral distribution may vary between arms and legs. Legs are likely to have on average 34%-38% of the whole body FM[6,17]. The increase storage of adipose tissue is primarily accompanied with process of lower-extremity disuse, skeletal muscle atrophy and autonomic nervous system dysfunction[16]. This will eventually contribute to infiltration and accumulation of adipose tissue in non-subcutaneous sites (muscle and bone). This ectopic accumulation of adipose tissue leads to serious metabolic consequences after SCI. The use of MRI manages to segment and compartmentalize adipose tissue based on its high signal intensity from the atrophic skeletal muscles, allowing accurate quantification of muscle size in response to disuse and exercise[9,10,16]. In our laboratory (Figures 1 and 2), we have managed to distinguish between IMF, IeMF, subfacial and SAT accumulation in persons with SCI[9,10].
The storage of triglycerides in ectopic locations is linked with chronic inflammation, impaired glucose tolerance, increased total cholesterol, and decreased strength and mobility[1,2]. There is growing interest in studying different peripheral adipose tissue compartments to determine its link to metabolic profile after SCI[6,26]. Moreover, there is anecdotal evidence supporting the notion that nutritional status and dietary habits may influence these compartments; because persons with SCI are likely to consume high fat diet, which is close to 40% of the total caloric intake[9,28].
IMF vs IeMF: Previous studies have focused on the metabolic impact of intramyocellular lipid (IMCL) content (i.e., lipid droplets stored in the cytoplasm of muscle cells) and extramyocellular lipid (EMCL) content, which can reliably be assessed by proton magnetic resonance spectroscopy (1 H-MRS). The deposition of IMCL could be altered in prediabetic or obese subjects, and could probably be associated with insulin resistance[29,30]. A study that compared persons with paraplegia to ND controls showed that IMCL was not different in the paralyzed muscles. However, the study noted greater EMCL in the paraplegic muscles, which was negatively associated with insulin sensitivity. The same study noted a 57% lower succinate dehydrogenase activity in person with SCI compared to controls[29].
The definition of IMF refers to infiltration of adipose tissue within individual muscle. It is measured by determining the attenuation property of computerized tomography (CT) or the signal intensity of MRI[31,32]. The storage of IMF is dramatically elevated after SCI and it is closely linked to other fat compartments, especially to VAT (Figures 2 and 3). It seems that IMF and VAT share an analogous pattern in distribution and association with insulin sensitivity[5,26,27,31,32]. Using MRI, Gorgey and Dudley[16] showed that IMF was 126% greater in persons with incomplete SCI compared to the matched ND controls. IMF CSA continued to increase in the 3-mo follow-up MRI scan[16]. Elder et al[31] reported that IMF and skeletal muscle atrophy in the thigh accounted for 70% of glucose intolerance after SCI. The same study noted that IMF and subfacial fat appeared to be increased in chronic individuals with SCI when compared to ND controls[31]. It is interesting to note that unlike SAT, IMF decreases in response to spasticity and exercise activity similar to resistance training[9,33].
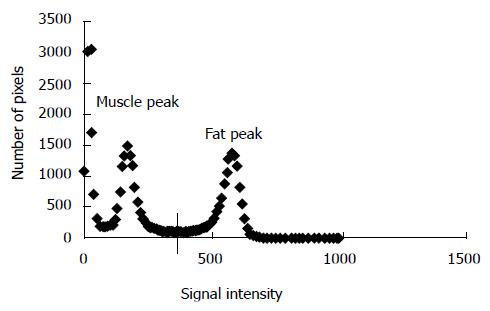
Although IMF is likely to be visualized, it is nearly impossible to be traced in order to be quantified. IeMF refers to fatty infiltration of adipose tissue between individual muscle groups[30]. This fatty infiltration is highly accumulated in the posterior compartment of the thigh and visually can be traced or separated based on its high signal intensity (Figure 2). A controversial area of debate is whether MRI can be used to quantify IMF. The histographic analysis has helped differentiating between IeMF and IMF infiltration (Figure 1).
Reliance on histographic analysis can help distinguish between muscle from fat voxels (i.e., the volume of a pixel). This histographic analysis allows previous quantification of IMF in individuals’ thigh muscles. We have previously felt more comfortable referring to adipose tissue as IMF and not IeMF because it composed both the fat infiltrated within the muscle as well as the fat infiltrated between individual muscles. This may further shed the light on the significance of separating IMF from IeMF accumulation and the associated links with metabolic consequences after SCI.
The histogram (pixel number versus signal intensity) produced from analysis of the whole thigh (muscle, fat and bone) showed two distinct muscle and fat peaks based on the variations in the signal intensity to the magnetic field[9,10,16,33]. The ranges and values for these peaks differ from individual to individual. The left peak was distinct, thin, and representing skeletal muscle area. This is due to the lower signal intensity that skeletal muscle is represented through MRI. The higher signal intensity peak is on the right side and representing the fat peak (Figure 1).
There is a greater infiltration of IMF most notably in the areas of the quadriceps, hamstrings, and adductor muscles. After determining the cut-off point between muscle and fat based on the signal intensity, the percentage of IMF within each individual muscle can be accurately measured. A crucial point that needs to be considered is measuring muscle size after accounting for IMF percentage. Failure to separate IMF from the measured muscle CSA overestimates the actual skeletal muscle size. For example, measuring the muscle CSA of the three vasti equated to 29.34 cm2. After applying the muscle-fat cut off technique, the percentage IMF was 26.25% with an area of 7.702 cm2. This means that failure to account for IMF overestimated muscle size by more than 26%. The accumulation of IMF within muscle has been previously considered in measuring muscle size in individuals with SCI[9,10,16,33].
From the rehabilitation point of view, IMF may impede the progression of the current in the exercising muscles during neuromuscular electrical stimulation[34]. This may result in an unnecessary increase of the amplitude of the current of neuromuscular electrical stimulation and lead to rapid muscle fatigue of the paralyzed muscles.
Bone marrow adipose tissue: The relationship between the alarming prevalence of obesity and osteoporosis is considered an area of a growing research interest. It is unclear whether there is a common origin from where excessive adiposity and continuous loss in bone mass originates after SCI. The mesenchymal progenitor stem cells (MSC) can be differentiated based on the mechanical stimulus applied into either bone and muscle cells or adipose tissue[35-37]. This differentiation may be very important in providing the appropriate mechanical stimulus necessary for the development of muscle and bone tissue compared to adipose tissues after SCI. Our initial observation showed that persons with motor complete SCI had 2-3 times bone marrow adipose tissue compared to matched able-bodied controls; primarily because of unloading on lower extremities[7]. This is accompanied with 1.5-2 times lower cortical bone CSA in persons with SCI compared to able-bodied controls[7]. Moreover, the bone marrow adipose tissue was inversely related to the bone mineral density and bone mineral content as measured by DXA. This preliminary evidence suggests that there is inverse relationship between increasing bone marrow adiposity and both cortical bone as well as bone mineral density[7]. This may suggest that MSC need to be considered as a therapeutic anatomical target for future interventions to cease the progress in adiposity and osteoporosis after SCI.
Anecdotal evidence based on recent imaging analysis showed that MSC differentiation into bone marrow adiposity can leak outside and contribute to the development of intermuscular adipose tissue or IMF infiltration in the atrophic skeletal muscle. In Figure 3, we have traced an abnormal growth of heterotrophic ossification (HO) within the thigh region. It is clear that this HO has been developed as a leakage from the bone marrow region. This abnormal bone growth within the skeletal muscle has a signal intensity that it is closely related to the signal intensity of the yellow bone marrow and lower than that of adipose tissue. This may speculatively suggest that HO development started as an abnormal fatty infiltration from that yellow marrow followed by calcium deposition in this region.
It has been discovered that humans contain both white and brown adipose tissue which possess two distinct developmental origins and functions[38-42]. The brown adipose tissue has yet to be studied in humans with SCI; however, its potential contribution to increase the metabolic rate is important considering the prevalence of obesity after SCI.
White adipose tissue: White adipose tissue takes on a critical role in maintaining energy homeostasis throughout the entire body. Homeostasis is maintained by storing triglycerides when energy is in surplus, releasing fatty acids as fuel during energy storage, and secreting adipokines which regulate glucose and lipid metabolism[2,38]. Recent studies indicate that white adipose tissue serves as an active endocrine organ which secretes numerous hormones, cytokines, and chemokines that are essential for regulating energy homeostasis in the body[2]. Two specific hormones released by white adipose tissue are leptin and adiponectin. Leptin is positively associated with white adipose tissue and serves to suppress food intake and increase energy expenditure[2]. The hormone adiponectin is inversely correlated with white adipose tissue and has been considered a promising biomarker for the indication of insulin sensitivity[2,39]. In addition to these secreting hormones, white adipose tissue can also secrete proinflammatory factors including tumor necrosis factor alpha, interleukin-6, and monocyte chemotactic protein-1[2]. The various adipose tissue compartments, such as subcutaneous and visceral depots follow a distinct pattern of hormone and adipokine secretion[39]. The adipocytes in subcutaneous depots have a higher capacity for adipogenesis and differentiate more rapidly compared to visceral depots. White adipose tissue in the visceral abdominal region has been shown to increase insulin resistance, thereby, it is less harmful to store white adipose tissue in subcutaneous compartment compared to the visceral region[38].
Brown adipose tissue: Brown adipose tissue contains lipid droplets and is rich in mitochondria[40-42]. The thermogenetic adipocytes increase energy expenditure through the uncoupling of oxidative metabolism from ATP production[41]. Thermogenesis of the brown adipose tissue is stimulated by the the sympathetic nerve terminals in the extensive vascular and nerve supply. Uncoupled thermogenesis is highly active metabolically and predominately utilizes lipid as fuel, but may also take up glucose as well[41,42]. Activation of brown adipose tissue has anti-obesity as well as glucose- and lipid- lowering effects.
Stimulation of the sympathetic nervous system below the level of SCI is impaired in this population. Therefore, the activation of brown adipose tissue in SCI may be limited. In response to exercise, the sympathetic nervous system in able-bodied individuals releases epinephrine to initiate the process of lipolysis as a source of fuel in cellular respiration[42]. In the SCI population, this process is altered and it is thought that muscle hypertrophy may trigger lipolysis instead of epinephrine release[9,10].
Uncoupling protein in brite adipose tissue: The phenomenon known as the “browning” effect occurs when white adipose tissue begins to accumulate the uncoupling protein (UCP 1) that characterizes brown adipose tissue. This transformed white adipose tissue is referred to as brite/beige adipose tissue[41,42]. Although it possesses the UCP 1 which essentially mediates adaptive thermogenesis, it is unclear as to how effective the mitochondria with UCP 1 in beige adipose tissue are in performing thermogenesis[41,42]. This is especially significant in the medical field as a further understanding of this phenomenon could lead to new therapeutic strategies for obesity, diabetes, and other metabolic disorders. Due to the lack of sympathetic nervous system in SCI and decrease in energy expenditure, finding a therapeutic approach of transforming adipose tissue into thermogenetic cells may allow SCI individuals to decrease adipose tissue accumulation.
Pharmaceutical techniques which may activate the UCP 1 in beige adipose tissue may cause significant declines in white adipose tissue, further benefiting the metabolic profile and body composition after SCI.
The rapid loss in muscle mass following SCI leads to serious metabolic consequences similar to extensive decline in basal metabolic rate (BMR), insulin resistance and impaired glucose tolerance. The evidence suggests that there is up to 22%-40% decline BMR in persons with SCI based on their level of injury and about 50%-75% suffer from impaired glucose tolerance or type II diabetes mellitus[43,44]. Dyslipidemia, as manifested by decreased level of circulating high density lipoprotein-cholesterol and increased levels of triglycerides and low density lipoprotein-cholesterol, contributes to an accelerating atheorgenic process to the cardiovascular system after SCI[4,45]. The economic impact and burden of these comorbidities may be of paramount significance to study the regional and ectopic adipose tissue changes after SCI.
The disruption in the energy balance process predisposes these individuals to become fat building machines with a fat storage capacity that exceeds more than 30% of their total body mass[23]. There are also substantial shifts in substrate utilization from reliance on fat as a source of energy to the exercised muscles to be dependent on the short-term energy supply of the glycogen storage. This is primarily impacted by the transformation of slow oxidative to fast fatigable muscle fibers[46]. Talmadge et al[46] estimated that by 24 wk, the vastus lateralis, gastrocnemius, and soleus muscles, approximately 90% of muscle fibers, are fast twitch fibers compared to 6 wk at baseline. The process typically manifests between 4 and 7 mo post-injury and can continue up to 70 mo post-injury before plateauing into a steady state of predominantly type IIx, fast-glycolytic twitch muscle fibers[46]. This transformation renders the skeletal muscle to be highly fatigable and susceptible to skeletal muscle damage after exercise.
Disruption in the process of lipolysis as a result of injury to the sympathetic chain may be another important factor that needs to be considered[25]. An acute bout of functional electrical stimulation cycling has failed to increase delivery of the circulating fatty acids to the exercised muscles[11]. We have shown that 12 wk of evoked resistance training using neuromuscular electrical stimulation and ankle weights resulted in decrease of IMF and VAT without changing SAT[9].
We should acknowledge that persons with SCI consume close to 40% of their dietary intake as fat; which is likely to be a major source of the continuous and longitudinal changes in body FM[9,28]. This is accompanied with low consumption (less than 20%) of dietary protein intake[9,47]. Dietary intake can be manipulated to enable more effective utilization of macronutrients[47]. The American diet is especially prone to consume excessive carbohydrates and fats which alter blood glucose levels and increase adipose tissue if not expended. Individuals generally consume a high-fat diet that may further disrupt their metabolism. Therefore, dietary intake is equally important as an exercise after SCI. Dietary habits can be more easily manipulated and controlled than exercise intervention due to various existing exercise barriers in the SCI population[48].
Administering of pharmaceutical interventions similar to testosterone replacement may be another vehicle that can be utilized to overcome that reduced anabolic level after SCI. Using DXA to measure body composition, Bauman et al[49] did not observe changes in whole body and regional body FM after administration of 1 year (5-10 mg) of Testosterone replacement in hypogonadal men with chronic SCI. The study noted signifcant increase in lean mass and resting energy expenditure[49]. It is yet to be studied the long-term effects testosterone administration on ectopic adipose tissue sites and the interaction with exercise after SCI.
Individuals with SCI are limited in their physical activity to the muscle mass above the level of injury. According to guidelines generated by an expert panel, adults with SCI should engage in at least 20 min of aerobic exercise training twice weekly prescribed at moderate-vigorous intensity or 3 sets of 8-10 repetitions of resistance training to the major muscle groups[50].
Once weekly of exercise training in SCI has also shown to maintain overall body composition, combating the otherwise inevitable increase in adipose tissue characterized by this disease. Our research found that once weekly of upper extremity circuit resistance training or neuromuscular electrical stimulation did not impact body FM after SCI[51,52]. Limited transportation interferes with accessing clinical settings; home-based training may be possible to allow for training programs especially with advancing in tele-health communication similar to the video conferencing.
Different adipose tissue compartments have been highlighted in the current review. Their full contribution to the metabolic health after SCI is not fully understood. The use of sophisticated imaging techniques allow to distinguish these compartments and to quantify their changes in response to SCI and training. Different rehabilitation interventions similar to exercise, electrical stimulation, bionic suits, dietary and pharmaceutical interventions may be available; however, their effects on subcutaneous and ectopic adipose tissues are yet to be studied and their effects may be attenuated by the disruption of autonomic nervous system and limited access to these rehabilitation interventions. Strategies targeted towards skeletal muscle hypertrophy and gain in lean mass are likely to improve the basal energy expenditure, reduce adipose tissue stacking and improve metabolic profile after SCI. Therefore, individuals with SCI may require a very personalized treatment plan in a multidisciplinary approach to prevent the excessive gain of adipose tissue and loss of lean muscle mass. This interdisciplinary approach is most beneficial and may be of great benefit in attenuating the changes in whole and regional body composition after SCI.
We would like to thank Hunter Holmes McGuire Research Institute, SCI Services and Disorders and the Radiology Service for providing the environment to conduct clinical human research trials.
P- Reviewer: Berra LV, Daniels AH, Kahveci R S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK
| 1. | Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol. 2014;2014:309570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 450] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 2. | Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3336] [Article Influence: 158.9] [Reference Citation Analysis (0)] |
| 3. | Manns PJ, McCubbin JA, Williams DP. Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch Phys Med Rehabil. 2005;86:1176-1181. [PubMed] |
| 4. | Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile - part I. J Spinal Cord Med. 2014;37:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 5. | Gorgey AS, Mather KJ, Gater DR. Central adiposity associations to carbohydrate and lipid metabolism in individuals with complete motor spinal cord injury. Metabolism. 2011;60:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Gorgey AS, Gater DR. Regional and relative adiposity patterns in relation to carbohydrate and lipid metabolism in men with spinal cord injury. Appl Physiol Nutr Metab. 2011;36:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Gorgey AS, Poarch HJ, Adler RA, Khalil RE, Gater DR. Femoral bone marrow adiposity and cortical bone cross-sectional areas in men with motor complete spinal cord injury. PM R. 2013;5:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, Ivy JL. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol. 2009;19:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Gorgey AS, Mather KJ, Cupp HR, Gater DR. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc. 2012;44:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 139] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Gorgey AS, Shepherd C. Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: case report. J Spinal Cord Med. 2010;33:90-95. [PubMed] |
| 11. | Kjaer M, Dela F, Sørensen FB, Secher NH, Bangsbo J, Mohr T, Galbo H. Fatty acid kinetics and carbohydrate metabolism during electrical exercise in spinal cord-injured humans. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1492-R1498. [PubMed] |
| 12. | Gater DR. Obesity after spinal cord injury. Phys Med Rehabil Clin N Am. 2007;18:333-351, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | National Center for Chronic Disease Prevention and Health Promotion. Adult Obesity Facts. Available from: http://www.cdc.gov/obesity/data/adult.html. |
| 14. | Hagerman F, Jacobs P, Backus D, Dudley GA. Exercise responses and adaptations in rowers and spinal cord injury individuals. Med Sci Sports Exerc. 2006;38:958-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Castro MJ, Apple DF, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 280] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45:304-309. [PubMed] |
| 17. | Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Waters RL, Bauman WA. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 2003;95:2398-2407. [PubMed] |
| 18. | Spungen AM, Wang J, Pierson RN, Bauman WA. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol. 2000;88:1310-1315. [PubMed] |
| 19. | Modlesky CM, Bickel CS, Slade JM, Meyer RA, Cureton KJ, Dudley GA. Assessment of skeletal muscle mass in men with spinal cord injury using dual-energy X-ray absorptiometry and magnetic resonance imaging. J Appl Physiol (1985). 2004;96:561-565. [PubMed] |
| 20. | Gorgey AS, Chiodo AE, Zemper ED, Hornyak JE, Rodriguez GM, Gater DR. Relationship of spasticity to soft tissue body composition and the metabolic profile in persons with chronic motor complete spinal cord injury. J Spinal Cord Med. 2010;33:6-15. [PubMed] |
| 21. | Gorgey AS, Dolbow DR, Gater DR. A model of prediction and cross-validation of fat-free mass in men with motor complete spinal cord injury. Arch Phys Med Rehabil. 2012;93:1240-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Gorgey AS, Mather KJ, Poarch HJ, Gater DR. Influence of motor complete spinal cord injury on visceral and subcutaneous adipose tissue measured by multi-axial magnetic resonance imaging. J Spinal Cord Med. 2011;34:99-109. [PubMed] |
| 23. | Gorgey AS, Gater DR. Prevalence of obesity after spinal cord injury. Top Spinal Cord Inj Rehabil. 2007;12:1-7. |
| 24. | Gorgey AS, Gater DR. A preliminary report on the effects of the level of spinal cord injury on the association between central adiposity and metabolic profile. PM R. 2011;3:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Karlsson AK, Elam M, Friberg P, Sullivan L, Attvall S, Lönnroth P. Peripheral afferent stimulation of decentralized sympathetic neurons activates lipolysis in spinal cord-injured subjects. Metabolism. 1997;46:1465-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1643] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 27. | Edwards LA, Bugaresti JM, Buchholz AC. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr. 2008;87:600-607. [PubMed] |
| 28. | Khalil RE, Gorgey AS, Janisko M, Dolbow DR, Moore JR, Gater DR. The role of nutrition in health status after spinal cord injury. Aging Dis. 2013;4:14-22. [PubMed] |
| 29. | Jonkers RA, Dirks ML, Nabuurs CI, De Feyter HM, Praet SF, Nicolay K, van Loon LJ, Prompers JJ. Myofibrillar distribution of succinate dehydrogenase activity and lipid stores differs in skeletal muscle tissue of paraplegic subjects. Am J Physiol Endocrinol Metab. 2012;302:E365-E373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Boettcher M, Machann J, Stefan N, Thamer C, Häring HU, Claussen CD, Fritsche A, Schick F. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. J Magn Reson Imaging. 2009;29:1340-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 31. | Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury--a cross-sectional study. Spinal Cord. 2004;42:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 169] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885-892. [PubMed] |
| 33. | Gorgey AS, Dudley GA. Spasticity may defend skeletal muscle size and composition after incomplete spinal cord injury. Spinal Cord. 2008;46:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Gorgey AS, Cho GM, Dolbow DR, Gater DR. Differences in current amplitude evoking leg extension in individuals with spinal cord injury. NeuroRehabilitation. 2013;33:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412:603-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 454] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 36. | Rubin C, Turner AS, Mallinckrodt C, Jerome C, McLeod K, Bain S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002;30:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 202] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal V, Rosen CJ, Pessin JE, Judex S. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci USA. 2007;104:17879-17884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 38. | Feng B, Zhang T, Xu H. Human adipose dynamics and metabolic health. Ann N Y Acad Sci. 2013;1281:160-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci. 2009;30:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 40. | Booth A, Magnuson A, Foster M. Detrimental and protective fat: body fat distribution and its relation to metabolic disease. Horm Mol Biol Clin Investig. 2014;17:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 659] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 42. | Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013;5:1196-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 506] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 43. | Buchholz AC, McGillivray CF, Pencharz PB. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr. 2003;77:371-378. [PubMed] |
| 44. | Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 286] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 45. | Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24:266-277. [PubMed] |
| 46. | Talmadge RJ, Castro MJ, Apple DF, Dudley GA. Phenotypic adaptations in human muscle fibers 6 and 24 wk after spinal cord injury. J Appl Physiol. 2002;92:147-154. [PubMed] |
| 47. | Groah SL, Nash MS, Ljungberg IH, Libin A, Hamm LF, Ward E, Burns PA, Enfield G. Nutrient intake and body habitus after spinal cord injury: an analysis by sex and level of injury. J Spinal Cord Med. 2009;32:25-33. [PubMed] |
| 48. | Gorgey AS. Exercise awareness and barriers after spinal cord injury. World J Orthop. 2014;5:158-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Bauman WA, Cirnigliaro CM, La Fountaine MF, Jensen AM, Wecht JM, Kirshblum SC, Spungen AM. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res. 2011;43:574-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Ginis KA, Hicks AL, Latimer AE, Warburton DE, Bourne C, Ditor DS, Goodwin DL, Hayes KC, McCartney N, McIlraith A. The development of evidence-informed physical activity guidelines for adults with spinal cord injury. Spinal Cord. 2011;49:1088-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 51. | Fisher JA, McNelis M, Gorgey AS, Dolbow DR, Goetz LL. Does Upper Extremity Training Influence Body Composition after Spinal Cord Injury? Aging and Disease. 2015;In Press. |
| 52. | Gorgey AS, Caudill C, Khalil RE. Effects of once weekly of NMES training on knee extensors fatigue and body composition in a person with spinal cord injury. J Spinal Cord Med. 2015;Jan 23; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |