Published online Apr 18, 2015. doi: 10.5312/wjo.v6.i3.351
Peer-review started: January 24, 2015
First decision: February 7, 2015
Revised: February 12, 2015
Accepted: March 5, 2015
Article in press: March 9, 2015
Published online: April 18, 2015
Processing time: 75 Days and 4.1 Hours
Complications associated with prone surgical positioning during elective spine surgery have the potential to cause serious patient morbidity. Although many of these complications remain uncommon, the range of possible morbidities is wide and includes multiple organ systems. Perioperative visual loss (POVL) is a well described, but uncommon complication that may occur due to ischemia to the optic nerve, retina, or cerebral cortex. Closed-angle glaucoma and amaurosis have been reported as additional etiologies for vision loss following spinal surgery. Peripheral nerve injuries, such as those caused by prolonged traction to the brachial plexus, are more commonly encountered postoperative events. Myocutaneous complications including pressure ulcers and compartment syndrome may also occur after prone positioning, albeit rarely. Other uncommon positioning complications such as tongue swelling resulting in airway compromise, femoral artery ischemia, and avascular necrosis of the femoral head have also been reported. Many of these are well-understood and largely avoidable through thoughtful attention to detail. Other complications, such as POVL, remain incompletely understood and thus more difficult to predict or prevent. Here, the current literature on the complications of prone positioning for spine surgery is reviewed to increase awareness of the spectrum of potential complications and to inform spine surgeons of strategies to minimize the risk of prone patient morbidity.
Core tip: This review addresses the complications of prone positioning for spine surgery, which is an important and relatively underrepresented topic in the literature. Here, we address the wide range of complications by system, covering the most common complications, current understanding of pathophysiology, and strategies for prevention. Individual cases of very rare complications are also addressed. This article provides increased awareness and understanding of the risks of prone positioning, which is important for patient morbidity.
- Citation: DePasse JM, Palumbo MA, Haque M, Eberson CP, Daniels AH. Complications associated with prone positioning in elective spinal surgery. World J Orthop 2015; 6(3): 351-359
- URL: https://www.wjgnet.com/2218-5836/full/v6/i3/351.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i3.351
Prone surgical positioning is commonly utilized for procedures requiring the posterior approach to the spine. Prone positioning is associated with several important and potentially catastrophic complications which can result in permanent disability. Complications include hemodynamic changes resulting in hypoperfusion, a range of ophthalmologic conditions, central nervous system lesions, peripheral nerve compression injuries, compartment syndrome, and pressure ulcers. Other complications include airway swelling and peripheral arterial compression. Though most of these complications are rare, familiarity with the spectrum of potential complications and strategies for prevention can limit morbidity in prone spine surgery.
A comprehensive literature review utilizing PubMed was performed identifying relevant articles that addressed complications associated with prone surgical positioning in spine surgery. Search terms in the primary search were MESH terms “spine” and “prone position.” Current and relevant literature was then reviewed. Reference lists from papers retrieved from the primary search were also searched for additional current and relevant work.
The prone position poses unique hemodynamic challenges. Compression of the abdomen may restrict blood flow through the inferior vena cava. The resultant engorgement of the paravertebral and epidural veins can increase bleeding in the surgical field. Because there is baseline postural hypotension and decreased cardiac function in the prone position, hypovolemia in the prone patient can exacerbate hypoperfusion to multiple organ systems and may increase the likelihood of acute kidney injury, especially during procedures with high blood loss[1,2].
In order to minimize intra-abdominal pressure and blood loss, currently available tables and frames have been engineered to leave the abdomen free and prevent an increase in intra-abdominal pressure. The various tables and frames differ in their effect on spinal alignment, location of pressure points, and hemodynamic derangement in the prone position. In 1969, Relton and Hall described a frame that possessed two parallel but separate V-shaped supports, one under the upper thoracic cage and the other under the pelvis. These lateral supports provide stability for deformity correction/scoliosis procedures. This frame allows for a free abdomen, but can potentially hyperextend the vertebral column[3].
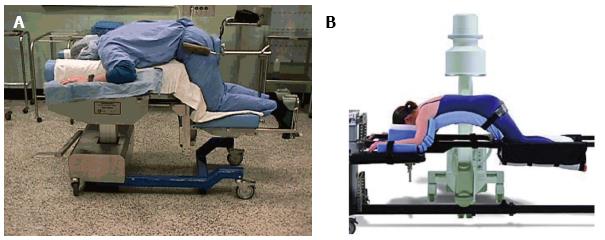
The Andrews and the Wilson frames were both designed for prone lumbar spine surgery (Figure 1). The Andrews frame positions the patient in a modified knee-chest position allowing for some control of lumbar sagittal plane alignment. By adjusting hip flexion with a mobile tibial support this frame creates relative kyphosis of the lumbar spine. The resultant increase in interlaminar distance facilitates access to the spinal canal during decompressive procedures.
The Wilson frame has two parallel, longitudinal curved pads which support both sides of the patient’s torso. The degree of curvature of the bolsters is adjustable, which also allows the surgeon to alter the patient’s lumbar sagittal plane alignment. The C-arm fluoroscopy machine can be integrated with both frames, with the Wilson frame providing a full 360 degrees of radiolucency when placed on the Jackson table[3]. The Jackson table also offers complete axial rotation capability for combined approaches. Both the Andrews and the Wilson frame can produce substantial hemodynamic compromise as a result of decreased preload and increased afterload, respectively[2].
Ophthalmologic complications of prone positioning were first reported in 1948 by Slocum et al[4] who described a case of blindness resulting from malpositioning on a Bailey headrest. Subsequent reports established the causal relationship between increased intraocular pressure, decreased tissue perfusion, and perioperative visual loss (POVL). The rarity of this devastating complication has limited research[5].
The incidence of POVL after spine surgery ranges from 0.019% to 0.2%, with an increased risk during procedures involving prone positioning and in surgery for spinal deformity[6-8]. There is also an increased risk in patients with comorbidities such as diabetes mellitus with end organ damage, coagulopathy, neurologic disorders, and paralysis[6].
Despite the absence of large prospective studies, several etiologies of POVL have been described, including anterior and posterior ischemic optic neuropathy (AION and PION), central retinal artery occlusion (CRAO), and cortical blindness[5] (Table 1). Other rare etiologies have also been reported, including POVL associated with acute closed-angle glaucoma and amaurosis[9,10].
| Complication | Risk factors |
| Ophthalmological complications | |
| ION[14] | Obesity, male sex, Wilson frame use, longer anesthetic duration, greater estimated blood loss, increased ratio of crystalloid to colloid |
| Posterior ION[13] | Blood loss greater than 4 L, persistent relative hypotension |
| Anterior ION[16] | Atherosclerosis, diabetes |
| Central retinal artery occlusion[5,13] | External compression of the eye |
| Cortical blindness[13] | Profound hypotension, prolonged hypoxia, cardiac arrest, thromboembolism |
| Neurologic complications | |
| Acute cervical myelopathy[20] | Cervical spondylosis, neck extension, paralytic anesthesia |
| Brachial plexopathy[21] | Extension, external rotation, and abduction of the arm, ipsilateral rotation and lateral flexion of neck, shoulder braces |
| Ulnar nerve palsy[30] | Obesity, inadequate elbow padding |
| Myocutaneous complications | |
| Compartment syndrome[34-36] | Padding directly over the compartment, obesity |
| Pressure ulcers[37,38] | Procedure duration, advanced age, obesity, steroid administration |
| Femoral head avascular necrosis[42] | Pressure over the groin, hypotension |
Ischemic optic neuropathy (ION) is thought to be the most common cause of POVL following prone spine surgery. ION was cited as the etiology of 89% of cases of POVL in the American Society of Anesthesiologists’ Postoperative Visual Loss Registry[11]. However, only 28% of the cases in a recent NIS analysis of POVL after spinal fusions were attributed to ION[6]. ION is categorized anatomically based on the location of the ischemia. AION affects the optic disc supplied by the posterior ciliary arteries. PION affects the retrobulbar or intracanalicular optic nerve supplied by penetrating vessels from the pial circulation. Both forms of ION can be further subdivided into arteritic and non-arteritic types. Arteritic ION is not seen as a surgical complication. Non-arteritic ION is most often caused by hypovolemia or anemia, although vascular disease can also cause spontaneous AION[5].
ION during spine surgery is often due to a combination of hypotension, blood loss and increase in orbital venous pressure. Compression of the abdomen during prone positioning, Trendelenburg positioning, and placement of the head below the heart can all raise orbital venous pressure; reverse Trendelenburg positioning reduces orbital pressure[12]. Increased orbital venous pressure worsens interstitial edema, compressing perforating vessels and decreasing tissue perfusion[5,13]. A multivariate analysis of the American Society of Anesthesiologists (ASA) Postoperative Visual Loss Registry demonstrated that independent risk factors for ION after spine surgery in the prone position include obesity, male sex, Wilson frame use, longer anesthetic duration, greater estimated blood loss, and decreased percent colloid administration[14]. This analysis supports the theory that decreased perfusion as a result of an altered pressure gradient is the primary mechanism for development of ION. On the other hand, a retrospective case-control study by Holy et al[15] reported no difference in blood loss and perioperative hemodynamics, including vasopressor use, in patients who developed ION versus control patients.
Although AION is more common in general, PION is more common following spine surgery[13]. PION was implicated in 60% of the cases in the ASA Postoperative Visual Loss Registry[11]. PION is associated with large intraoperative blood loss, especially with blood loss over 4 L, as well as relative hypotension extended over the duration of the case[13]. Patients with PION report vision loss upon waking from general anesthesia which is often bilateral and can result in complete blindness[16]. Some patients may report a prodrome of blurred vision[13]. Fundoscopic examination is initially normal; optic nerve atrophy and pallor is visible after four to six weeks. The vision loss is often permanent with no effective treatment available[16].
AION is a multifactorial condition that is triggered by acute hypoperfusion or occlusion of the posterior ciliary arteries[16]. Patients with atherosclerosis and diabetes are at increased risk for this complication. In contrast to PION, fundoscopic examination reveals segmental or diffuse edema of the optic disc and occasionally splinter hemorrhages[5]. Visual loss resulting from AION can also be permanent, though there may be a role for treatment with high-dose corticosteroids[16].
CRAO is the second most commonly encountered cause of POVL after prone spine surgery. In 1954 Hollenhorst et al[17] first reported CRAO in neurosurgical patients who were placed in a horseshoe-shaped headrest. CRAO subsequently became known as “headrest syndrome”. In the ASA Visual Loss Registry, CRAO accounted for 11% of the cases of POVL with perioperative eye trauma noted in seven of the ten cases[11]. Proposed mechanisms of CRAO include thromboembolism or increased intraocular pressure from direct compression of the globe[13] which in turn causes decreased perfusion of the retina.
Patients with CRAO most often suffer severe visual loss unilaterally, and they may have other evidence of extended periods of unilateral compression, such as periorbital ecchymosis and edematous slera[13]. Fundoscopic examination reveals a cherry-red spot on the macula. There is no effective treatment; the visual loss is almost always irreversible.
Cortical blindness results from decreased perfusion of the visual cortex in the occipital lobes (which receive blood from the posterior cerebral arteries). It is the most common form of POVL after spinal fusions or spinal deformity surgery, according to a recent analysis of data from the NIS[6]. Etiologies include profound hypotension, prolonged hypoxia, cardiac arrest, or thromboembolism[13]. Patients may have very subtle symptoms and may have difficulty recognizing their visual impairment. However, these patients cannot react to threat or respond to optokinetic stimulation[13]. Fundoscopic examination and papillary reaction to light is normal, but visual field defects corresponding with the affected cortical area will be noted on ophthalmologic examination or visual field testing. Unilateral cortical blindness most commonly results in contralateral homonymous hemianopsia. Bilateral cortical blindness may cause complete peripheral vision loss. Symptoms often improve from the initial ischemic insult without specific treatment, but complete recovery is rare[5].
POVL have been described infrequently as a consequence of acute angle-closure glaucoma and amaurosis[9,10]. Provocative tests in the office have demonstrated that acute angle-closure glaucoma can result from facedown positioning in susceptible individuals. Singer et al[10] reported a case of bilateral acute angle-closure glaucoma after spine surgery. The patient reported nausea and eye pain; exam demonstrated diffuse corneal edema and fixed, mid-dilated pupils. The patient was treated with aqueous suppressants and steroid drops with improvement in the intraocular pressure[10]. Definitive treatment is laser peripheral iridotomy.
Similar to PION, amaurosis represents the loss of vision without an apparent ocular lesion. It may be permanent or transient (amaurosis fugax). Amaurosis may result from thrombosis of the ophthalmic artery or remote plaque rupture, commonly at the carotid artery bifurcation, causing an embolic event in the ophthalmic artery. Zimmerer et al[9] reported a case of amaurosis during lumbar disc surgery in the prone position which was complicated by an episode of hypotension requiring catecholamines and the need for Trendelenburg positioning[9]. Immediately after the surgery, the patient complained of impaired vision bilaterally which worsened to complete visual loss in the right eye six hours post-operatively. The patient was ultimately diagnosed with complete occlusion of the right ophthalmic artery. Investigation revealed that the patient’s antihypertensive pharmacologic regimen had been increased two days prior to surgery. The authors concluded that the combination of medication, arteriosclerosis, and intraoperative hypotension resulted in the amaurosis[9].
Subconjunctival hemorrhage is an uncommon ophthalmologic complication of spine surgery[18]. It does not cause visual loss. Akhaddar et al[18] reported a case of a patient who developed a painless subconjunctival hemorrhage after lumbar disc surgery in the prone position. The patient’s visual acuity and fundoscopic examination were normal. Subconjunctival hemorrhages present as blood trapped beneath the transparent conjunctiva and white sclera. Though the impressive appearance can be concerning to patients and health care workers, these hemorrhages are asymptomatic and resolve without treatment.
Although POVL can occur even under optimal conditions, several strategies can be used to reduce the risk of occurrence (Table 2). As noted above, the increase in orbital venous pressure or intraocular pressure and concurrent decrease in perfusion pressure is the most common etiology for POVL, thus methods for reducing this shift in pressures may reduce the risk of POVL. Reverse Trendelenburg positioning and frames that allow for a free abdomen can limit the increase in orbital venous pressure. Additionally, the use of Gardner-Wells tongs on a Jackson table with 10-15 pounds of weight prevents any pressure on the face, which limits the increase in intraocular pressure. Adequate resuscitation, blood pressure maintenance, invasive monitoring, and staged procedures can limit blood loss and duration of anesthesia, thus reducing the severity and total time of decreased perfusion. Still, informed consent for POVL should be obtained in high-risk patients, including obese patients, patients with diabetes or vascular disease, or patients for whom a large estimated blood loss or long procedure is expected.
| Complication | Avoidance strategy |
| Ophthalmological complications | |
| ION[14] | Reverse trendelenburg positioning, colloid administration by anesthesia, limit prolonged intraoperative hypotension |
| Posterior ION[13] | Limit prolonged intraoperative hypotension |
| Anterior ION[16] | None |
| Central retinal artery occlusion[5,13] | Avoid compression of the globe |
| Cortical blindness[13] | Limit prolonged intraoperative hypotension |
| Neurologic complications | |
| Acute cervical myelopathy[20] | Thorough history and preoperative imaging, careful neck positioning during patient transfers and surgical procedure |
| Brachial plexopathy[21] | Careful anatomic positioning of the arm, limiting extension and external rotation of shoulder |
| Ulnar nerve palsy[30] | Avoid compression and pressure at the elbow, maintain arm position during procedure (avoid arm falling off of arm board) |
| Myocutaneous complications | |
| Compartment syndrome[34-36] | Avoid pressure on anterior thigh and leg, avoid extremely long surgical procedures. Extra care with obese patients |
| Pressure ulcers[37,38] | Pad bony prominences. Consider Garner-Wells tongs to eliminate pressure on the face during lengthy procedures |
| Femoral head avascular necrosis[42] | Avoid pressure directly over the groin |
The risk of neurological injury is inherent to nearly all spine surgery procedures. Certain neurological complications (e.g., acute cervical myelopathy, spinal cord infarction and brachial plexopathy) may be directly attributed to prone positioning[19-22] (Table 1).
Acute cervical disc herniation resulting in neurologic deficits can occur in patients with pre-existing cervical spine disease during spinal surgery, though it is very rare. It may result from a combination of neck extension during endotracheal intubation and prone positioning, loss of muscle support when anesthetized, and agitation during surgery[20]. Chen et al[20] described a case of a patient who underwent an uneventful lumbar laminectomy for spinal stenosis but was found to have paralysis of his lower extremities postoperatively. An MRI demonstrated an acute C6-C7 disc herniation. Despite urgent discectomy, the patient remained paralyzed[20]. The patient later recalled a history of episodic paresthesia in his right hand, but his cervical spine was never a primary complaint. Though only a handful of case reports document this devastating complication, it is important to be cognizant of coexisting cervical spine disease and to carefully handle the cervical spine during intubation, patient transfers, and patient positioning[20,23,24].
Spinal cord infarction following prone positioning is also rare, but has been documented in patients with skeletal dysplasia and chest wall deformity[19]. Several case reports have suggested that the decrease in cardiac output observed with prone positioning is even greater in patients with chest wall deformity, such as those with pectus excavatum[19,25,26]. These patients can experience severe intraoperative hypotension, particularly when hypovolemic. Tong et al[19] reported on a young patient with Morquio syndrome who sustained an ischemic injury to her upper thoracic spinal cord during an occipitocervical decompression for cervical myelomalacia secondary to atlantoaxial instability[19]. Upon awakening from anesthesia, she was found to have complete sensory and motor deficits below T4, which did not improve. The absence of motor evoked potentials in the lower limbs during the case was incorrectly assumed to be due to the patient’s Morquio syndrome, which emphasizes the need to obtain recordings prior to positioning[19].
Postoperative brachial plexopathy and other peripheral nerve injuries are considerably more common than spinal cord dysfunction. The neural lesion generally takes the form of a neuropraxia or axonotmesis and may occur as a result of patient positioning[21]. The brachial plexus innervates all upper limb musculature (except for the trapezius and levator scapulae) and supplies sensation to the upper limb with the exception of the axillary, superior shoulder, and dorsal scapular-innervated regions. The plexus is fixed at the cervical vertebrae and the axillary fascia[21], increasing the risk of traction injury. Additionally, the plexus passes three mobile bony structures: the clavicle, first rib, and humeral head. The position of these osseous structures relative to the plexus may compress or stretch the neural elements resulting in ischemia to the vasa nervorum. Hypovolemia, hypothermia, diabetes mellitus, and alcoholism increase the risk of nerve injury[21].
A recent review of postoperative brachial plexus injuries identified 17 out of 517 patients who sustained brachial plexopathies during spine surgery in the prone position[21]. Abduction of the arm greater than 90 degrees placed patients at highest risk[21]. Extension, external rotation and abduction of the arm, rotation and lateral flexion of neck in the same direction, and application of shoulder braces were also associated with an increased risk of brachial plexus injury[21]. Evidence of injury was detected with intraoperative electrophysiological monitoring (SSEPs and MEPs) in 15 patients, preventing the development of symptoms. The remaining two patients, who experienced no monitoring abnormalities, reported upper extremity weakness postoperatively that resolved within two weeks[21]. A review of 22 patients with postoperative brachial plexus dysfunction found that surgery in the prone position was more likely to result in motor deficits[27]. As expected with neuropraxias, recovery from brachial plexus injury often occurs, but it is not always complete[28].
Ulnar nerve palsy has also been reported following prone positioning for spine surgery[29]. Ulnar nerve injury can occur due to direct pressure over the cubital tunnel at the elbow, excessive flexion of the elbow (> 90 degrees), malposition of a blood pressure cuff, or with accidental change in position of the arm during surgery, such as the arm falling off of the armboard. Chung et al[30] noted in a recent study that the ulnar nerve is more vulnerable to ischemia from brachial artery compression than are the median and radial nerves, with immediate loss of SSEPs seen in that case. They also noted that obesity was a risk factor for the development of ulnar nerve palsy following prone positioning. Intraoperative neuromonitoring can help detect and prevent the development of symptoms[30]. A preoperative physical exam is also valuable for identifying pre-existing cubital tunnel syndrome, which increases the likelihood of post-operative palsy.
Lateral femoral cutaneous nerve neuropathy, also called meralgia paresthetica, has been reported from direct compression with an incidence as high as 24% after posterior spinal surgery[31]. Compression most often occurs from placement of pelvic bolsters near the anterior superior iliac spine. Additionally, if the bolsters are too close together the likelihood of bilateral meralgia paresthetica increases[31]. Risk factors include duration of surgery greater than 3.5 h and degenerative spinal disease with pre-existing damage to the second or third lumbar nerve roots. Interestingly, some authors have noted an increased risk in obese patients, while Gupta et al[32] reported an increased incidence in thinner patients[32,33]. Post-operatively, patients may complain of sensitivity or paresthesia in the thigh, though complete resolution within 6 mo is the rule. Steroid injections or decompression can be considered in severe cases. The condition may in some cases be prevented with adequate placement and additional padding.
Compartment syndrome and cutaneous complications such as pressure ulcers are well-known complications of prone surgical positioning (Table 1). Compartment syndrome after spine surgery is rare, although anterior thigh and anterior tibial compartment syndromes have both been reported[34-36]. The diagnosis of postoperative compartment syndrome is often delayed or missed because the symptoms are similar to other postoperative conditions including nerve root injury, persistent nerve root compression, traction neuropraxia, or peripheral nerve palsy.
Dahab et al[34] report a case of a patient who developed severe right thigh pain and swelling after an L4-S1 posterolateral instrumented fusion for degenerative spondylolisthesis. The procedure took five hours but was otherwise uncomplicated. Computed tomography angiogram demonstrated vessel patency with low density changes in rectus femoris and vastus intermedius, and anterior compartment pressure measured 150 mmHg. Fasciotomy was performed, and the patient improved. The patient was positioned on a standard padded Wilson frame with attention to pressure areas, but the patient was obese (BMI 34), and the authors believe that this may have increased his risk[34].
Ahmad et al[35] reported two additional cases of anterior thigh compartment syndrome after lumbar spine surgery. Both patients were positioned on a Jackson table with well-padded bony prominences, but the thigh and iliac crest pads were switched in order to increase lumbar lordosis[35]. Both patients were also obese. Postoperatively, both patients complained of pain and stiffness in their bilateral thighs and mild quadriceps weakness. One patient steadily improved, and he remained undiagnosed until MRI of the thighs performed two weeks postoperatively revealed local muscle necrosis. The other patient developed intense swelling and pain and was taken to the operating room for bilateral fasciotomies on postoperative day two. He also developed rhabdomyolosis and acute kidney injury[35].
Geisler et al[36] reported two cases of anterior tibial compartment syndrome after lower lumbar surgery performed in the prone-sitting position on an Andrews frame. Both patients complained of shin pain, foot drop, and decreased sensation between the first and second toes. After intracompartmental pressures were found to be significantly elevated, both underwent urgent fasciotomy[36].
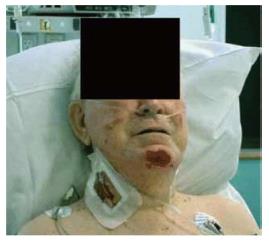
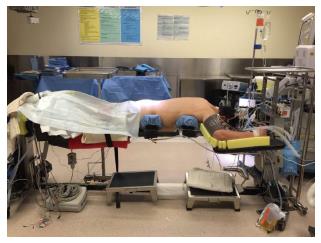
Pressure ulcers can also occur as a result of prone positioning, though investigation has shown that the prone position results in lower pressure on bony prominences than the supine or lateral positions[37]. Prone positioning places pressure on the forehead, chin, shoulders, thorax, pelvis, knees, and ankles. Tissue ischemia may occur after two to six hours of pressure; necrosis occurs after six hours. The duration of the surgical procedure is the largest risk factor for the development of ulcers, although advanced age, obesity, and administration of steroids can increase the risk[38]. Bilateral iliac crest ulcers have been reported in a patient who was positioned prone in the intensive care unit, though no spine surgery was performed[37]. Facial pressure ulcers on the chin or forehead may occur in patients positioned on an Andrews frame for prolonged spine procedures (Figure 2). Use of a Mayfield clamp or Gardner-Wells tongs with 10-15lbs of traction to free the face from pressure may reduce the risk of facial pressure ulcer[38] (Figure 3). Appropriate padding and attention to bony prominences is critical to avoiding cutaneous complications (Table 2).
Prone positioning for spine surgery has also been associated with rare airway and vascular complications. Massive tongue swelling resulting in airway obstruction has been reported in patients in a flexed thoracic-cervical position; corticosteroid administration improved these symptoms[39]. Airway obstruction has also been reported in a patient with Duchenne muscular dystrophy and lordoscoliosis[40] with concomitant airway malacia who underwent spine surgery in the prone position. Shifting the patient to the semi-lateral position resolved his symptoms[40].
Vascular complications including femoral artery ischemia and avascular necrosis of the femoral head are other rarely-described complications of prone positioning (Table 1). Tseng et al[41] reported a case of bilateral lower extremity hypoperfusion in a pediatric patient with idiopathic scoliosis, which was detected with intraoperative neuromonitoring. Loss of SSEPs and MEPs prompted examination of the patient, who was found to have no pulses in his distal lower extremities. The thigh pads were moved proximally and pulses then returned[41].
Pressure on the arteries and inguinal area combined with reduced venous outflow and hypotension from anesthesia has been implicated in cases of avascular necrosis of the femoral head[42]. Orpen et al[42] report two cases of bilateral avascular necrosis and one case of unilateral avascular necrosis after lumbar spine surgery in patients with no other risk factors for avascular necrosis. All three spine surgeries were uncomplicated. Preoperative radiographs showed early osteoarthritis in all five affected joints, which may have increased their susceptibility to ischemic insult[42].
Prone positioning complications associated with spine surgery can cause serious patient morbidity. Awareness of these complications, careful patient positioning, efficient use of anesthesia time, and avoidance of intraoperative hypotension may help reduce the incidence of prone positioning related complications.
P- Reviewer: Angelini A, Erkan S, Mica L, Sakamoto A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Tabara Y, Tachibana-Iimori R, Yamamoto M, Abe M, Kondo I, Miki T, Kohara K. Hypotension associated with prone body position: a possible overlooked postural hypotension. Hypertens Res. 2005;28:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (2)] |
| 2. | Dharmavaram S, Jellish WS, Nockels RP, Shea J, Mehmood R, Ghanayem A, Kleinman B, Jacobs W. Effect of prone positioning systems on hemodynamic and cardiac function during lumbar spine surgery: an echocardiographic study. Spine (Phila Pa 1976). 2006;31:1388-1393; discussion 1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Schonauer C, Bocchetti A, Barbagallo G, Albanese V, Moraci A. Positioning on surgical table. Eur Spine J. 2004;13 Suppl 1:S50-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Slocum HC, O’neal KC, Allen CR. Neurovascular complications from malposition on the operating table. Surg Gynecol Obstet. 1948;86:729-734. [PubMed] |
| 5. | Nickels TJ, Manlapaz MR, Farag E. Perioperative visual loss after spine surgery. World J Orthop. 2014;5:100-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 6. | Nandyala SV, Marquez-Lara A, Fineberg SJ, Singh R, Singh K. Incidence and risk factors for perioperative visual loss after spinal fusion. Spine J. 2014;14:1866-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22:1319-1324. [PubMed] |
| 8. | Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33:1491-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Zimmerer S, Koehler M, Turtschi S, Palmowski-Wolfe A, Girard T. Amaurosis after spine surgery: survey of the literature and discussion of one case. Eur Spine J. 2011;20:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Singer MS, Salim S. Bilateral acute angle-closure glaucoma as a complication of facedown spine surgery. Spine J. 2010;10:e7-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 11. | Lee LA, Roth S, Posner KL, Cheney FW, Caplan RA, Newman NJ, Domino KB. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105:652-659; quiz 867-868. [PubMed] |
| 12. | Carey TW, Shaw KA, Weber ML, DeVine JG. Effect of the degree of reverse Trendelenburg position on intraocular pressure during prone spine surgery: a randomized controlled trial. Spine J. 2014;14:2118-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Stambough JL, Dolan D, Werner R, Godfrey E. Ophthalmologic complications associated with prone positioning in spine surgery. J Am Acad Orthop Surg. 2007;15:156-165. [PubMed] |
| 14. | Postoperative Visual Loss Study G. Risk factors associated with ischemic optic neuropathy after spinal fusion surgery. Anesthesiology. 2012;116:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Holy SE, Tsai JH, McAllister RK, Smith KH. Perioperative ischemic optic neuropathy: a case control analysis of 126,666 surgical procedures at a single institution. Anesthesiology. 2009;110:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251:1873-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Hollenhorst RW, Svien HJ, Benoit CF. Unilateral blindness occurring during anesthesia for neurosurgical operations. AMA Arch Ophthalmol. 1954;52:819-830. [PubMed] |
| 18. | Akhaddar A, Boucetta M. Subconjunctival hemorrhage as a complication of intraoperative positioning for lumbar spinal surgery. Spine J. 2012;12:274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Tong CK, Chen JC, Cochrane DD. Spinal cord infarction remote from maximal compression in a patient with Morquio syndrome. J Neurosurg Pediatr. 2012;9:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Chen SH, Hui YL, Yu CM, Niu CC, Lui PW. Paraplegia by acute cervical disc protrusion after lumbar spine surgery. Chang Gung Med J. 2005;28:254-257. [PubMed] |
| 21. | Uribe JS, Kolla J, Omar H, Dakwar E, Abel N, Mangar D, Camporesi E. Brachial plexus injury following spinal surgery. J Neurosurg Spine. 2010;13:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Schwartz DM, Sestokas AK, Hilibrand AS, Vaccaro AR, Bose B, Li M, Albert TJ. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Suzuki T, Abe E, Murai H, Kobayashi T. Nontraumatic acute complete paraplegia resulting from cervical disc herniation: a case report. Spine (Phila Pa 1976). 2003;28:E125-E128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Ueyama T, Tamaki N, Kondoh T, Miyamoto H, Akiyama H, Nagashima T. Non-traumatic acute paraplegia associated with cervical disc herniation: a case report. Surg Neurol. 1999;52:204-206; discussion 206-207. [PubMed] |
| 25. | Alexianu D, Skolnick ET, Pinto AC, Ohkawa S, Roye DP, Solowiejczyk DE, Hyman JE, Sun LS. Severe hypotension in the prone position in a child with neurofibromatosis, scoliosis and pectus excavatum presenting for posterior spinal fusion. Anesth Analg. 2004;98:334-335, table of contents. [PubMed] |
| 26. | Bafus BT, Chiravuri D, van der Velde ME, Chu BI, Hirshl R, Farley FA. Severe hypotension associated with the prone position in a child with scoliosis and pectus excavatum undergoing posterior spinal fusion. J Spinal Disord Tech. 2008;21:451-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Ben-David B, Stahl S. Prognosis of intraoperative brachial plexus injury: a review of 22 cases. Br J Anaesth. 1997;79:440-445. [PubMed] |
| 28. | Daniels AH, Deodhar AA, Hart RA. Traumatic spondyloptosis resulting from high-energy trauma concurrent with a tonic-clonic seizure. Spine J. 2009;9:e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Sherman CE, Rose PS, Pierce LL, Yaszemski MJ, Sim FH. Prospective assessment of patient morbidity from prone sacral positioning. J Neurosurg Spine. 2012;16:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Chung I, Glow JA, Dimopoulos V, Walid MS, Smisson HF, Johnston KW, Robinson JS, Grigorian AA. Upper-limb somatosensory evoked potential monitoring in lumbosacral spine surgery: a prognostic marker for position-related ulnar nerve injury. Spine J. 2009;9:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 31. | Cho KT, Lee HJ. Prone position-related meralgia paresthetica after lumbar spinal surgery: a case report and review of the literature. J Korean Neurosurg Soc. 2008;44:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Gupta A, Muzumdar D, Ramani PS. Meralgia paraesthetica following lumbar spine surgery: a study in 110 consecutive surgically treated cases. Neurol India. 2004;52:64-66. [PubMed] |
| 33. | Yang SH, Wu CC, Chen PQ. Postoperative meralgia paresthetica after posterior spine surgery: incidence, risk factors, and clinical outcomes. Spine (Phila Pa 1976). 2005;30:E547-E550. [PubMed] |
| 34. | Dahab R, Barrett C, Pillay R, De Matas M. Anterior thigh compartment syndrome after prone positioning for lumbosacral fixation. Eur Spine J. 2012;21 Suppl 4:S554-S556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Ahmad FU, Madhavan K, Trombly R, Levi AD. Anterior thigh compartment syndrome and local myonecrosis after posterior spine surgery on a Jackson table. World Neurosurg. 2012;78:553.e5-553.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Geisler FH, Laich DT, Goldflies M, Shepard A. Anterior tibial compartment syndrome as a positioning complication of the prone-sitting position for lumbar surgery. Neurosurgery. 1993;33:1117. [PubMed] |
| 37. | Dudek NL, Buenger UR, Trudel G. Bilateral anterior superior iliac spine pressure ulcers: a case report. Arch Phys Med Rehabil. 2002;83:1459-1461. [PubMed] |
| 38. | Goodwin CR, Recinos PF, Omeis I, Momin EN, Witham TF, Bydon A, Gokaslan ZL, Wolinsky JP. Prevention of facial pressure ulcers using the Mayfield clamp for sacral tumor resection. J Neurosurg Spine. 2011;14:85-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Miura Y, Mimatsu K, Iwata H. Massive tongue swelling as a complication after spinal surgery. J Spinal Disord. 1996;9:339-341. [PubMed] |
| 40. | Yang JH, Bhandarkar AW, Lim BG, Modi HN, Suh SW. Intraoperative airway obstruction in a Duchenne muscular dystrophy patient. Eur Spine J. 2013;22 Suppl 3:S491-S496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Tseng MD, Cappetto L, Majid K, Sternberg D, Settecerri JJ. Bilateral femoral artery ischemia detected by multimodality neuromonitoring during posterior scoliosis surgery: a case report. Spine (Phila Pa 1976). 2010;35:E799-E803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Orpen N, Walker G, Fairlie N, Coghill S, Birch N. Avascular necrosis of the femoral head after surgery for lumbar spinal stenosis. Spine (Phila Pa 1976). 2003;28:E364-E367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |