Published online Apr 18, 2015. doi: 10.5312/wjo.v6.i3.340
Peer-review started: September 29, 2014
First decision: October 14, 2014
Revised: December 14, 2014
Accepted: January 15, 2015
Article in press: January 19, 2015
Published online: April 18, 2015
Processing time: 191 Days and 8 Hours
The overall incidence of osteochondral defect in the general population is estimated to be 15 to 30 per 100000 people. These lesions can become symptomatic causing pain, swelling and decreased function of the knee, and may eventually progress to osteoarthritis. In the young and active population, partial or total knee arthroplasty (TKA) is rarely the treatment of choice due to risk of early failure. Osteochondral allograft transplantation has been demonstrated to be a safe and effective treatment of large osteochondral and chondral defects of the knee in appropriately selected patients. The treatment reduces pain, improves function and is a viable limb salvage procedure for patients, especially young and active patients for whom TKA is not recommended. Either large dowels generated with commercially available equipment or free hand shell allografts can be implanted in more posterior lesions. Current recommendations for fresh allografts stored at 4C advise implantation within 21-28 d of procurement for optimum chondrocyte viability, following screening and testing protocols. Higher rates of successful allograft transplantation are observed in younger patients, unipolar lesions, normal or corrected malalignment, and defects that are treated within 12 mo of symptom onset. Patients with bipolar lesions, uncorrectable malalignment, advanced osteoarthritis, and those over 40 tend to have less favourable outcomes.
Core tip: Osteochondral allograft transplantation has been demonstrated to be a safe and effective treatment of large osteochondral and chondral defects of the knee in appropriately selected patients. The treatment reduces pain, improves function and is a viable limb salvage procedure for patients, especially young and active patients for whom total knee arthroplasty is not recommended. Current recommendations for fresh allografts stored at 4C advise implantation within 21-28 d of procurement for optimum chondrocyte viability, following screening and testing protocols. Higher rates of successful allograft transplantation are observed in younger patients, unipolar lesions, normal or corrected malalignment, and defects that are treated within 12 mo of symptom onset.
- Citation: Chui K, Jeys L, Snow M. Knee salvage procedures: The indications, techniques and outcomes of large osteochondral allografts. World J Orthop 2015; 6(3): 340-350
- URL: https://www.wjgnet.com/2218-5836/full/v6/i3/340.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i3.340
The overall incidence of osteochondral defect in the general population is estimated to be 15 to 30 per 100000 people. These lesions can become symptomatic causing pain, swelling and decreased function of the knee, and may eventually progress to osteoarthritis. In the young and active population, partial or total knee arthroplasty (TKA) is rarely the treatment of choice due to risk of early failure. The need for alternate options has propelled the development and use of biologic interventions to repair damaged osteochondral lesions in the past few decades. Such treatments include microfracture, chondroplasty, autologous chondrocyte implantation, osteochondral autograft transplant and osteochondral allograft transplant. Amongst the numerous biologic interventions available for osteochondral and chondral defects, osteochondral allograft transplantation emerges to be the most suitable option for large lesions (> 4 cm2) with associated bony defects. Osteochondral allograft transplantation can restore the entire osteochondral unit with both viable hyaline cartilage and bone in a single procedure[1]. Improvements in allograft procurement protocols and surgical techniques over time have led to increase use of osteochondral allografts for knee salvage procedures.
In addition to transplantation of architecturally sound bone and viable articular cartilage capable of maintaining metabolic activity after implantation, osteochondral allografts offer many other advantages. Size and surface contours of the lesion may be matched with that of the donor graft, which also eliminates donor site morbidity associated with autograft transfers. Articular cartilage is aneural and relatively avascular, obtaining its nutrition from surrounding synovial fluid through diffusion, optimizing it for transplantation[2,3]. Allogenic cartilage is immunologically privileged tissue as the intact cartilage matrix acts as a barrier between donor chondrocytes and host antibodies, protecting chondrocytes from host immune surveillance[4]. This permits long-term survival of transplanted donor chondrocytes and makes tissue matching or therapeutic immunosuppression unnecessary. Retrieval studies at 25 years following transplantation have demonstrated survival of the allograft chondral tissue[5].
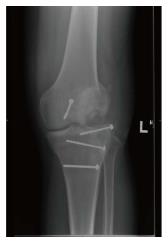
Osteochondral allograft transplantation in the knee is typically indicated for patients with large focal full-thickness chondral or osteochondral defects (> 2 cm2), for which other techniques such as microfracture, osteochondral autograft transplantation and autologous chondrocyte implantation are inadequate due to the size, location or depth of the lesion. It is also indicated as a salvage procedure for previously failed restoration treatments of the knee. Osteochondral allograft transplantation is indicated for treatment of osteoneocrosis, post-traumatic osteochondral defects (Figure 1), osteochondritis dissecans (OCD), patellofemoral arthrosis and uni-compartmental degenerative tibiofemoral arthrosis.
Contraindications for osteochondral allograft transplantation include advanced or diffuse degenerative changes as seen on weight-bearing radiographs and advanced multi-compartmental arthrosis. It is also relatively contraindicated in patients with uncorrectable malalignment, ligamentous instability, meniscal insufficiency, and inflammatory arthropathies. Osteochondral allografting should be avoided in patients who are obese or may have altered bone metabolism, as seen in smoking, chronic steroid use and alcohol abuse. Low success rate is observed in patients over 40[6], who may be considered for arthroplasty if criteria for joint replacement is met.
Previously, grafts were implanted within 24 h of procurement. Today, grafts undergo strict protocols of screening and testing to reduce the risk of disease transmission[7], leading to a minimum of 14 d before fresh grafts are implanted. Following harvest and 24 h of treatment in antibiotic solution, fresh allografts are refrigerated at 4 °C in either lactated Ringer’s solution or a physiologic culture medium to maintain chondrocyte viability. An inverse relationship has been demonstrated between storage time and chondrocyte viability and density: at 4 d of storage nearly all chondrocytes are viable, at 7 d 98% remain viable and at 28 d chondrocyte viability significantly declines to 70%[3]. Graft storage in physiologic culture medium has produced higher chondrocyte viability percentage compared to Ringer’s solution[8]. Chondrocyte function is paramount in achieving clinical success. However, the exact association between cell viability and clinical outcomes has yet to be determined[8]. Current recommendations advise implantation of grafts within 21-28 d of procurement[9], with a maximum storage period of 42 d[10] of fresh allografts. Residual donor cells within the allograft are a potential source of an immune reaction. Disease transmission in fresh allograft tissue remains an issue of concern despite extensive tissue bank screening guidelines.
Fresh-frozen ostechondral allografts undergo deep freezing to -80 °C and have the advantages of indefinite storage period, decreased immunogenicity and reduced disease transmission relative to other graft types. But this is at the expense of 95% chondrocyte death, lost of mature articular cartilage cells and damage to the extracellular matrix within the graft[11-13]. Studies on retrieved large allografts have also demonstrated deterioration of cells and matrix over time[14].
Cryopreserved allografts undergo rate-controlled freezing to -70 °C in a cryoprotectant storage medium of either glycerol or dimethyl sulfoxide to preserve cellular viability. In an animal study chondrocyte viability of allografts preserved with glycerol was 77% in weight-bearing joints[15]. Another study investigating a cryopreserved allograft using dimethyl sulfoxide demonstrated that chondrocyte viability was limited only to the superficial layer of the articular cartilage[16] due to failure of the cryopreservent to penetrate the deeper zones. In an ovine model, cryopreserved allografts produced intermediate results when compared to fresh autografts, and it was observed that the membrane integrity of the allograft chondrocytes were the most reliable predictor of long-term outcomes of the graft[17].
A detailed history, examination and imaging must be conducted to assess the mechanical and biologic condition of the cartilage and subchondral bone of the knee. Pre-operative evaluation includes comprehensive use of radiographs and magnetic resonance imaging (MRI), as well as careful planning of necessary concurrent procedures, such as ligament reconstruction and meniscus transplantation. From the images, the femoral condyle or tibial plateau size is measured with correction for magnification. An appropriate allograft is identified with donor-recipient size matched within 2 mm.
Diagnostic arthroscopy is utilized to assess the size and location of the lesion and identify any concomitant pathology that necessitates treatment. Radiographs usually required include: lower extremity alignment views; weight-bearing anteroposterior and flexion posteroanterior views; lateral and patellar views. The images will determine the degree of joint space narrowing and allow measurement of the mechanical axis through the knee. Corrective osteotomy may be considered for any observed varus or valgus malalignment to return mechanical axis to neutral. Normal joint alignment is crucial for successful allograft transplantation[6] and it is essential to correct malalignment prior to implantation of allograft[18]. MRI is used to determine size, depth and condition of the lesion, as well as any associated meniscal or ligament deficiencies. Sizing of the allograft is also established from MRI evaluation.
The two main surgical techniques are cylindrical dowel grafts or free-hand shell grafts. Allografts should be size-matched and be of the same compartment.
The patient is positioned supine and the lower limb placed in a leg holder to position the knee at an angle of flexion that facilitates appropriate access to the lesion. A limited lateral or medial arthrotomy over the involved compartment without subluxation of the patella is usually sufficient. If greater exposure is needed, the incision can be extended using a quadriceps-sparing sub-vastus approach or mid-vastus approach.
Once adequate exposure is achieved and the lesion identified, a cylindrical dowel is used to outline and determine the size of the proposed graft. A guide wire is placed in the center of the sizing dowel, perpendicular to the articular surface. A core reamer is then used to remove a total of 5-8 mm of cartilage and subchondral bone to form a base of healthy cancellous bone. For lesions of fibrous and necrotic bone from osteochondritis dissecans or osteonecrosis, deeper reaming may be necessary until healthy, bleeding osseous bone is reached. For even deeper lesions, morselized autologous bone graft may be required to pack any defects, which can be collected from the reaming. The guide wire is removed and depth measurements of the prepared recipient site in all four quadrants are made to harvest a matching allograft. A reference mark is made on the recipient site for allograft plug orientation.
A plug is retrieved from the corresponding anatomic location on the donor allograft that is held in a workstation. The same reference mark is made on the allograft before a size-matched coring reamer is used to harvest the plug. Following extraction of the plug, corresponding depth measurements of the four quadrants from the recipient site are marked on the plug and then cut to the appropriate thickness. Multiple attempts of trimming of the plug may be necessary in order to achieve precise thickness. When the graft is prepared and ready, high-pressure lavage is used to remove all marrow elements to reduce any potential immunological reaction.
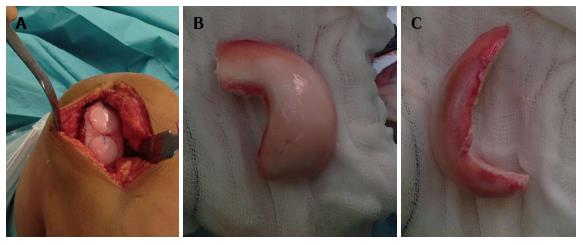
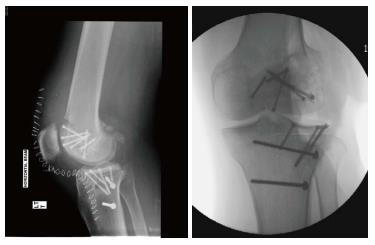
The graft is inserted into the recipient site by hand, matching the reference marks to allow proper orientation and then gently press-fitted into place until flush with the surrounding articular surface (Figure 2A). This step should be carried out with care to minimize insult to the articular surface of both the recipient and donor tissue in order to protect chondrocyte viability. The knee is then taken through a full range of motion to access graft stability and identify any catching or soft tissue obstruction. Additional fixation is usually not required, however if it is determined necessary or if the graft is large, absorbable pins or screws are used to provide stability (Figure 3).
The shell technique is typically used for defects that are located posteriorly, which are difficult to access perpendicularly with a dowel, or defects of the tibial plateau. After identification of the lesion, the edges are defined with a surgical pen, with attempts to create a geometric shape recipient site that allows hand crafting of a matching shell graft. The recipient site is cut to a depth of 4-5 mm with burr and osteotomes to remove all tissue in the marked area. A slightly larger matching graft is cut freehand from donor tissue, and then excess cartilage and bone is gradually removed as the graft is shaped to fit the recipient site (Figure 2B). A foil or paper template of the recipient site may be used to shape the graft. The shell allograft is then inserted until flush with the articular surface and fixed with bioabsorbable pins and screws, if necessary.
The femoral condyles are the most common site to implant osteochondral allografts in the knee. Bakay et al[6] reported the results of 18 patients following cryopreserved femoral condyle plug-shaped allografts and reported 4 failures, 13 excellent or good and 5 fair or poor clinical results. The average success rate for femoral allograft transplantation was 72%. In 3 patients, allograft transplantation was performed upon moderate varus knees without corrective realignment and each graft disintegrated within 6 mo. In 3 other patients where corrective realignment procedures were carried out, the grafts healed with good results.
Davidson et al[19] analysed 10 knees in 8 patients using second-look arthroscopic evaluation and biopsy at a mean of 40 mo post fresh allograft transplantation in the distal femur. The mean International Knee Documentation Committee (IKDC) score significantly improved from 27 to 79 (P = 0.002). The mean Lysholm score significantly improved from 37 to 78 (P = 0.002). The mean Tegner activity level improved from 4.3 to 5.3 (P = 0.16). The mean Short Form 36 (SF-36) physical score significantly improved from 38 to 51 (P = 0.002). The mean SF-36 mental score showed minimal improvement. Histological analysis post-operatively showed no significant difference between native and graft cartilage biopsy specimens for mean thickness of articular surface (P = 0.625), chondrocyte cellular viability (P = 0.555) and cell density (P = 0.129). MRI and plain radiographs demonstrated complete incorporation of the bony compartment of the graft in all patients, and mean modified Outerbridge MRI scores improved from 4.3 pre-operatively to 0.6 post-operatively (P = 0.002).
Pearsall et al[20] evaluated the results of 12 fresh allograft, 12 frozen allograft and 24 autograft transplantations on 48 patients with an average follow up of 37.1 mo. Femoral condyles constituted more than half of the treated lesions. The mean Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score significantly improved for pain from 10.9 pre-operatively to 14.5 post-operatively (P = 0.0001); for stiffness from 4.1 pre-operatively to 5.6 post-operatively (P = 0.00001); and for function from 38.3 pre-operatively to 49.7 post-operatively (P = 0.0002). The mean Knee Society Score (KSS) improved significantly from 112.8 pre-operatively to 154.2 post-operatively (P = 0.0001). Eight allografts did not improve after transplantation and underwent knee arthroplasty. No significant difference was observed in outcome improvement scores between autografts and allografts for WOMAC (P = 0.1), KSS (P = 0.8), knee range of motion (P = 0.2) and pain (P = 0.7).
McCulloch et al[21] prospectively assessed 25 patients who underwent fresh osteochondral allograft transplantation for femoral condyle defects for an average follow up of 35 mo. Statistically significant improvements from pre-operative to post-operative evaluations were reported. Patients reported 84% overall satisfaction with transplantation results and 79% satisfaction with functionality compared to the unaffected knee. Radiographic assessment revealed 22 grafts (88%) had incorporated into host bone.
Williams et al[22] prospectively analyzed 19 patients treated with fresh osteochondral allografts for defects of the knee with an average follow up of 48 mo. MRI evaluation at a mean interval of 25 mo post-operatively demonstrated that, in general, thickness of the implanted allograft articular cartilage was maintained. Activities of Daily Living score significantly increased from 56 pre-operatively to 70 pre-operatively (P < 0.05) and SF-36 score significantly increased from 51 pre-operatively to 66 post-operatively (P < 0.005). Osseous trabecular incorporation was complete in 3, partial in 11 and poor in 4 allografts. Four grafts failed clinically.
In a prospective non-randomized study, Gross et al[23] performed fresh femoral condyle allograft reconstruction surgery on 60 patients with an average follow up of 10 years. 20% of grafts failed and required graft removal alone or with TKA. Survivorship analysis demonstrated 95% survival at 5 years and 85% survival at 10 years. Of the 48 remaining intact grafts, the average modified Hospital for Special Surgery (HSS) score was 83 out of 100, with excellent or good results achieved in 40 patients.
Emmerson et al[24] reported on the use of fresh osteochondral allografts in the surgical management of type 3 and 4 osteochondritis dissecans of the femoral condyles. Sixty-six knees in 64 patients were assessed pre-operatively and post-operatively using an 18-point modified D’Aubigne and Postel scale[25,26]. Forty-seven of 65 knees reported excellent or good results; mean D’Aubigne and Postel scale significantly increased from 13.0 pre-operatively to 16.4 post-operatively (P < 0.01). Survival analysis revealed 91% survivorship at 2 years and 76% survivorship at 10 and 15 years. 92% of the 59 patients who completed a patient questionnaire were satisfied with their treatment and 90% reported less pain.
Murphy et al[27] reported on osteochondral allograft transplantation of the knee in the pediatric and adolescent population in a case series of 43 knees in 39 patients with an average follow up of 8.4 years. The most common site of lesions was at the femoral condyles (Table 1). OCD (61%), avascular necrosis (16%) and traumatic chondral injury (14%) were the most common causes of the lesions. 5 knees experienced failure of the allograft at a median of 2.7 years; 4 knees were consequently successfully salvaged with an additional allograft transplant and 1 knee underwent prosthetic arthroplasty 8.6 years after revision allograft. Allograft survivorship of the entire patient cohort was 90% at 10 years. Pre-operative and post-operative comparison of patients with allografts in situ at final follow-up showed improvement in all outcome measures (Table 2).
| Location | Number |
| Femur | |
| Medial condyle | 18 |
| Lateral condyle | 15 |
| Trochlea | 2 |
| Patella | 3 |
| Tibial plateau | 1 |
| Multiple sites | 4 |
| Outcome measure | Pre-operative | Post-operative |
| IKDC scores | ||
| Pain | 5.7 ± 2.7 (0-10) | 2.5 ± 2.4 (0-8) |
| Function | 3.7 ± 1.9 (0-9) | 7.8 ± 1.7 (4-10) |
| Total | 42.0 ± 16.6 (14-98) | 75.2 ± 20.2 (33-100) |
| Modified D'Aubigne Postel 18-point score | 13.1 ± 2.1 (9-18) | 16.6 ± 1.6 (12-18) |
| Knee Society Function score | 69.3 ± 18.8 (45-100) | 89.4 ± 16.3 (40-100) |
In a prospective study of 43 athletes treated with fresh-stored osteochondral allograft transplantation, Krych et al[28] evaluated the clinical outcomes and rate of return to athletic activity with an average 2.5 year follow up. 38 of 43 (88%) athletes achieved limited return to sport, with full return to pre-injury level possible in 34 of 43 (79%) athletes at 9.6 ± 3.0 mo after the procedure. Statistically significant increases from baseline to the most recent follow up was reported in Activities of Daily Living Score from 62.0 to 82.8 (P < 0.01), IKDC score from 46.2 to 79.2 (P < 0.01) and Marx Activity Rating Scale from 5.5 to 8.4(P = 0.01). The study also showed that risk factors affecting the ability to return to sport were age more than 25 years (P = 0.04) and pre-operative duration of symptoms more than 12 mo (P = 0.003).
Raz et al[29] reviewed 58 cases of osteochondral allograft transplantation to the distal femur with an average follow up of 21.8 years. 13 of 58 cases required further surgery of graft removal or TKA, and 1 case underwent multiple debridements before above-the-knee amputation. Kaplan-Meier analysis revealed graft survivorship of 91%, 84%, 69% and 59% at 10, 15, 20 and 25 years, respectively. A mean modified HSS score of 86 was reported for patients with a surviving allograft at 15 years or more post-operatively. Radiographic evidence was available for 55 of 58 patients at 10 or more years following surgery. Graft sclerosis and fragmentation was seen in 10 patients, non-union was seen in 5 patients and successful graft incorporation to host was observed in 40 patients.
Sixty-five patients, all with post-traumatic tibial plateau fractures, received fresh osteochondral allografts for reconstruction of the tibial plateau with an average follow up of 11.8 years in a prospective non-randomized study by Gross et al[23]. Survivorship analysis revealed 95% survival at 5 years and 80% survival at 10 years and 65% at 15 years. Twenty-one patients required knee arthroplasty after graft failure. At the end of the study period, the mean modified HSS score was 85.3 for the intact grafts.
In a study by Bakay et al[6] post-traumatic defects of the tibial condyle were treated with cryopreserved allografts with additional metal fixation screws in 5 patients with an average 2 years follow up. Three cases were successful and reported excellent or good clinical results. Two cases of failures were reported in patients both aged over 40 years; 1 graft disintegrated and 1 graft was implanted technically incorrectly, producing a poor clinical result.
Ghazavi et al[30] reported on the use of fresh osteochondral allografts to reconstruct post-traumatic defects in 126 knees of 123 patients with a mean follow up of 7.5 years. The locations of the lesions were: tibial plateau (63), femoral condyles (50), bipolar tibial and femoral (8) and patellofemoral (2). One hundred and eight of 126 knees (86%) were successfully reconstructed, whilst 18 of 126 grafts (14%) were reported as failures (4 of 8 bipolar grafts and 14 of 118 unipolar grafts). Survivorship analysis revealed 95% survival at 5 years, 71% at 10 years and 66% at 20 years. The mean modified HSS Knee score of successful cases increased from 66 pre-operatively to 83 post-operatively.
Colangeli et al[31] compared results of 10 patients who underwent total knee modular megaprosthesis and 8 patients who underwent osteochondral allograft transplantation for reconstruction of the knee with proximal tibia bone tumours. When compared to total knee replacement, patients treated with osteochondral allografts had a lower incidence of knee extension lag; higher rate of normal knee pattern during gait; and superior knee extensor strength. Abnormal knee kinematics and knee motion during gait observed in 5 patients treated with allograft were attributed to a shortened patellar tendon and an oversize mismatch of the femur. Osteochondral allograft transplantation, when optimally reconstructed, gave superior functional results compared to total knee replacement.
Muscolo et al[32] retrospectively reviewed 58 osteochondral allograft transplantations after resection of proximal tibial bone tumours in 52 patients, with an average follow up of 10.3 years. Six patients died from tumour-related causes without allograft failure before 5 years follow up. At the most recent follow up, from 32 of the 52 remaining allografts 20 had failed, Kaplan-Meier survival analysis revealed 65% graft survivorship at 5 and 10 years. Of the 32 surviving allografts, the average musculoskeletal tumour society functional score was 26 out of 30 points and the average International Society of Limb Salvage radiographic score was 87%, an excellent radiographic result. Similarly, Hornicek et al[33] and Shi et al[34] both reported on limb salvage procedures with osteochondral allograft after resection of proximal tibia tumours, with success rates of 66% and 80%, respectively. Infection and allograft fractures are the main causes of failures in these studies.
Jamali et al[35] retrospectively analyzed 20 knees in 18 patients treated with fresh osteochondral allograft transplantation of the patellofemoral joint with an average clinical follow up of 94 mo. Procedures were performed on the patella in all 20 knees and on the trochlea in 12 knees. Indications for surgery included secondary arthrosis from patellar subluxation (7 knees); post-traumatic arthrosis (6 knees); primary patellofemoral arthrosis (4 knees); and primary chondromalacia patellae (3 knees). Five patients experienced clinical failures, defined as revision allograft surgery, TKA or arthrodesis. The remaining 13 patients were classified as having successful clinical outcomes. For the knees with successful results, the average modified 18-point D’Aubigne-Postel score significantly improved from 11.7 pre-operatively to 16.3 at the most recent follow up (P = 0.001). Sixty percent of all patients reported excellent or good results and Kaplan-Meier survivorship analysis revealed at 10 years allograft survival was 67%.
Bakay et al[6] performed whole patellar surface replacement with mushroom-shaped cryopreserved osteochondral allografts in 8 patients. Six excellent or good and 2 fair clinical results were reported, producing an average success rate of 75%. One graft fragmentation failure occurred due to hyper-pressure of the patellofemoral joint in a patient aged over 45 years. The study also found that patellar allografts produced superior results to femoral allografts, showing more rapid revascularization and better integration of the mushroom-shaped allografts.
Bipolar lesions are considered a contraindication for treatment with osteochondral allograft transplantations and studies have reported poor results in reconstructed bipolar cases. Bakay et al[6] reported 2 cases of medial compartment bipolar allograft transplantations. Both grafts disintegrated within 6 mo with very poor clinical results. Beaver et al[18] performed 19 bipolar allograft reconstructions with an average follow up of 68 mo. Bipolar graft survivorship analysis was 60% at 5 years and 40% at 10 years. In this study, when compared to unipolar grafts, bipolar grafts show a lower success rate (P = 0.09). However, the proportion of bipolar grafts was greater in patients aged over 60. An example of such a case is shown in Figure 2.
The demographic details of all studies are summarized in Table 3; survivorship analysis results of all studies are summarized in Table 4; outcome scores and results of all studies are summarized in Tables 5-7.
| Ref. | Population | Indication | Location | Patientno. | No. ofknees | Meanage (yr) | Meanfollow up | Failurerate |
| Bakay et al[6] | - | Osteoarthritis; post-traumatic; OCD; chondromalacia | MFC, LFC, T, P, bipolar | 18 | 33 | 48 | 19 mo | - |
| Ghazavi et al[30] | Young, active patients | Post-traumatic | MFC, LFC, T, bipolar, P | 123 | 126 | 35 | 7.5 yr | 15% |
| Langer et al[4] | Young, active patients | Post-traumatic; OCD; osteonecrosis; osteoarthritis | MFC, LFC | 60 | 60 | 27 | 10 yr | 20% |
| Gross et al[5] | Young, active patients | Tibial plateaus fractures | T | 65 | 65 | 42.8 | 11.8 yr | 32% |
| Jamali et al[35] | - | Post-traumatic; patella subluxation; primary patellofemoral arthrosis; primary chondromalacia patellae | P, FT | 18 | 20 | 94 mo | 28% | |
| Colangeli et al[31] | - | Proximal tibia bone tumour | T | 8 | 8 | 23.1 | 37 mo | - |
| Davidson et al[19] | - | OCD; post-traumatic | MFC, FT | 8 | 10 | 32.6 | 40 mo | - |
| Emmerson et al[24] | - | OCD | MFC, LFC | 64 | 66 | 28.6 | 7.7 yr | 15% |
| McCulloch et al[21] | - | Degenerative; post-traumatic; OCD; osteonecrosis | MFC, LFC | 25 | 25 | 35 | 35 mo | 4% |
| Williams et al[8] | - | Full-thickness cartilage defect; OCD; osteonecrosis | MFC, LFC | 19 | 19 | 34 | 48 mo | 21% |
| Pearsall et al[3] | - | Full-thickness cartilage defect | MFC, LFC, FT, P | 48 | 24 allografts | 46 | 37.1 mo | 19% |
| Muscolo et al[32] | - | Proximal tibia bone tumour; previous allograft failure | T | 52 | 58 | 24 | 10.3 yr | 39% |
| Krych et al[28] | Athletes | Post-traumatic; non-traumatic focal chondral and osteochondral lesion; OCD | MFC, LFC, FT, multiple sites | 43 | 43 | 32.9 | 2.5 yr | - |
| Murphy et al[27] | Pediatric and adolescent population | OCD; avascular necrosis; post-traumatic; osteochondral fracture; degenerative lesion | MFC, LFC, FT, P, T, multiple sites | 39 | 43 | 16.4 | 8.4 yr | 12% |
| Raz et al[29] | Young, active patients | Post-traumatic; OCD | MFC, LFC | 58 | 58 | 28 | 21.8 yr | 22% |
| Ref. | Location | No. of knees | Survivorship | ||||
| 5 yr (%) | 10 yr (%) | 15 yr (%) | 20 yr (%) | 25 yr (%) | |||
| Bakay et al[6] | MFC, LFC, T, P, bipolar | 33 | - | - | - | - | - |
| Ghazavi et al[30] | MFC, LFC, T, bipolar, P | 126 | 95 | 71 | 66 | - | - |
| Langer et al[4] | MFC, LFC | 60 | 95 | 85 | - | - | - |
| Gross et al[5] | T | 65 | 95 | 80 | - | - | - |
| Jamali et al[35] | P, FT | 20 | - | 67 | - | - | - |
| Colangeli et al[31] | T | 8 | - | - | - | - | - |
| Davidson et al[19] | MFC, FT | 10 | - | - | - | - | - |
| Emmerson et al[24] | MFC, LFC | 66 | 91 | 76 | 76 | - | - |
| McCulloch et al[21] | MFC, LFC | 25 | - | - | - | - | - |
| Williams et al[8] | MFC, LFC | 19 | - | - | - | - | - |
| Pearsall et al[3] | MFC, LFC, FT, P | 24 | - | - | - | - | - |
| Muscolo et al[32] | T | 58 | 65 | 65 | - | - | - |
| Krych et al[28] | MFC, LFC, FT, multiple sites | 43 | - | - | - | - | - |
| Murphy et al[27] | MFC, LFC, FT, P, T, multiple sites | 43 | - | - | - | - | - |
| Raz et al[29] | MFC, LFC | 58 | - | 91 | 84 | 69 | 59 |
| Ref. | Location | No. ofknees | D'Aubigneand Postel | HSS | IKDC | Lysholm | KSS | KSF | ADL | MTS | ISLS | SF-12 | SF-36 | Tegner | Outerbridge | Marx | ||||||||||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |||
| Bakay et al[6] | MFC, LFC, T, P, bipolar | 33 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Ghazavi et al[30] | MFC, LFC, T, bipolar, P | 126 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Gross et al[5] | MFC, LFC | 60 | - | - | - | 83/100 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Gross et al[23] | T | 65 | - | - | - | 85.3/100 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Jamali et al[35] | P, FT | 20 | 11.7 | 16.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Colangeli et al[31] | T | 8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Davidson et al[19] | MFC, FT | 10 | - | - | - | - | 27 | 79 | 37 | 78 | - | - | - | - | - | - | - | - | - | - | - | - | 38 | 51 | 4.3 | 5.3 | 4.3 | 0.6 | - | - |
| Emmerson et al[24] | MFC, LFC | 66 | 13 | 16.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| McCulloch et al[21] | MFC, LFC | 25 | - | - | - | - | 29 | 58 | 39 | 67 | - | - | - | - | - | - | - | - | - | - | 36 | 40 | - | - | - | - | - | - | - | - |
| Williams et al[8] | MFC, LFC | 19 | - | - | - | - | - | - | - | - | - | - | - | - | 56 | 70 | - | - | - | - | - | - | 51 | 66 | - | - | - | - | - | - |
| Pearsall et al[20] | MFC, LFC, FT, P | 24 | - | - | - | - | - | - | - | - | 110 | 119 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Muscolo et al[32] | T | 58 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 26/30 | - | 87% | - | - | - | - | - | - | - | - | - | - |
| Krych et al[28] | MFC, LFC, FT, multiple sites | 43 | - | - | - | - | 46 | 79 | - | - | - | - | - | - | 62 | 82 | - | - | - | - | - | - | - | - | - | - | - | - | 5.5 | 8.4 |
| Murphy et al[27] | MFC, LFC, FT, P, T, multiple sites | 43 | 13.1 | 16.6 | - | - | 42 | 75 | - | - | - | - | 69.3 | 89.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Raz et al[29] | MFC, LFC | 58 | - | - | - | 86/100 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Year | Ref. | Location | No. of knees | KOOS Pain | KOOS Symptoms | KOOS ADL | KOOS SRF | KOOS QOL | |||||
| Pre-operative | Post-operative | Pre-operative | Post-operative | Pre-operative | Post-operative | Pre-operative | Post-operative | Pre-operative | Post-operative | ||||
| 2007 | McCulloch et al[21] | MFC, LFC | 25 | 43 | 73 | 46 | 64 | 56 | 83 | 18 | 46 | 22 | 50 |
| Year | Ref. | Location | No. of knees | WOMAC Pain | WOMAC Stiffness | WOMAC Function | |||
| Pre-operative | Post-operative | Pre-operative | Post-operative | Pre-operative | Post-operative | ||||
| 2008 | Pearsall et al[20] | MFC, LFC, FT, P | 24 | 10.9 | 14.5 | 4.1 | 5.6 | 38.3 | 49.7 |
Immediate continuous passive motion (CPM) is traditionally used after osteochondral allografting and any chondral resurfacing procedures. Patients are generally permitted unrestricted, full-range of motion unless a concomitant reconstructive procedure dictates knee motion restrictions. Braces are typically not necessary except maybe for patellofemoral joint allografts, where flexion is limited to 45° for 4 to 6 wk, and for bipolar tibial and femoral allografts, in which an unloader brace can prevent excessive stress on the reconstructed sites. CPM is used for 6 to 8 h per day for the initial 6 wk to avoid adhesions, promote the healing process and encourage graft nutrition.
Supervised physical therapy commences after initial post-operative visit and the patient is kept non-weight bearing or toe-touch weight bearing for first 6-12 wk, depending on the size of the graft, type of fixation and radiographic signs of incorporation. By 3 mo patients are expected to have full range of motion and regain normal quadriceps strength. At 4-6 mo functional rehabilitation should be complete and patients can begin light recreational activities but avoid excessive impact of allografts. By 12 mo return to higher impact activities can be considered.
Risk of disease transmission is a potential disadvantage of fresh osteochondral allograft transplantations. Although exceedingly rare due to careful donor screening protocols, allograft-associated infections can still potentially be fatal. Safety guidelines established by the American Association of Tissue Banks require extensive donor screening, with a detailed medical history and social history; serologic testing; viral and bacteriologic testing; procurement and storage requirements; and graft quarantine until negative testing results are confirmed[36].
Based on an observational study of 11391 donors to United States tissue banks between 2000 and 2002, the estimated risk of viral transmission at time of donation was 1 in 34000 for Hepatitis B, 1 in 42000 for Hepatitis C, 1 in 55000 for human immunodeficiency virus (HIV) and 1 in 129000 for human T-lymphotropic virus[37]. Despite these high risks of donor viremia observed in the study, the estimated actual risk of disease transmission with allograft tissue transplantation is low. Risk of HIV transmission is 1 in 1.6 million[38], with the only reported case of disease transmission from allogeneic graft before screening standards were set up in 1985[39].
Deep infection should be distinguished from superficial infection, by physical examination and joint aspiration if necessary, and be treated with irrigation, surgical debridement and graft removal. Graft fragmentation and collapse are the main causes of failure, commonly presenting as new onset of pain, joint effusion, and mechanical symptoms. Failure of allograft transplantation most often occurs in the osseous portion of the graft due to subchondral collapse, delayed union or non-union. Larger grafts are at higher risk of these complications. MRI can assess causes of symptoms and host-graft incorporation, however caution must be taken when interpreting MRI images as well-functioning grafts can demonstrate signaling abnormalities that may resolve over time as creeping substitution occurs.
Allograft subsidence may also occur as a milder complication. Some patients may have persistent pain following fresh osteochondral allograft transplantation due to low-grade chronic inflammatory reaction to the graft.
Osteochondral allograft transplantation has been demonstrated to be a safe and effective treatment of large osteochondral and chondral defects of the knee in appropriately selected patients. The treatment reduces pain, improves function and is a viable limb salvage procedure for patients, especially young and active patients for whom TKA is not recommended. Current recommendations for fresh allografts stored at 4 °C advise implantation within 21-28 d of procurement for optimum chondrocyte viability, following screening and testing protocols. Higher rates of successful allograft transplantation are observed in younger patients, unipolar lesions, normal or corrected malalignment, and defects that are treated within 12 mo of symptom onset. Patients with bipolar lesions, uncorrectable malalignment, advanced osteoarthritis, and those over 40 tend to have less favourable outcomes.
Future research should explore the effects of the duration of prolonged storage of allografts on clinical outcomes. Investigation should be carried out on techniques to maximize storage time, whilst maintaining viability of chondrocytes during storage and implantation. The influence of impaction at graft insertion remains to be established, as well as the role of post-operative protected weight bearing. Further research may work to produce a valid radiographic criterion for outcome assessment; functional MRI techniques can be applied to non-invasively assess biochemical health of cartilage after allograft transplantation. Improvement in allograft-host fixation and graft incorporation will likely further advance patient short- and long-term outcomes. Modulating the healing response by donor-recipient matching or the use of bioactive growth factors, may further improve outcomes of osteochondral allograft transplantations.
P- Reviewer: Fenichel I, Fisher DA, Knutsen G S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Meyers MH, Akeson W, Convery FR. Resurfacing of the knee with fresh osteochondral allograft. J Bone Joint Surg Am. 1989;71:704-713. [PubMed] |
| 2. | Czitrom AA, Keating S, Gross AE. The viability of articular cartilage in fresh osteochondral allografts after clinical transplantation. J Bone Joint Surg Am. 1990;72:574-581. [PubMed] |
| 3. | Pearsall AW, Tucker JA, Hester RB, Heitman RJ. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Langer F, Gross AE. Immunogenicity of allograft articular cartilage. J Bone Joint Surg Am. 1974;56:297-304. [PubMed] |
| 5. | Gross AE, Kim W, Heras FL, Backstein D, Safir O, Pritzker KPH. Fresh osteochondral allografts for posttraumatic knee defects: Long-term followup. Clin Orthop Relat Res. 2008;466:1863-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Bakay A, Csönge L, Papp G, Fekete L. Osteochondral resurfacing of the knee joint with allograft. Clinical analysis of 33 cases. Int Orthop. 1998;22:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Görtz S, Bugbee WD. Fresh osteochondral allografts: graft processing and clinical applications. J Knee Surg. 2006;19:231-240. [PubMed] |
| 8. | Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, Sah RL, Bugbee WD. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85A:2111-2120. [PubMed] |
| 9. | Strauss EJ, Sershon R, Barker JU, Kercher J, Salata M, Verma NN. The basic science and clinical applications of osteochondral allografts. Bull NYU Hosp Jt Dis. 2012;70:217-223. [PubMed] |
| 10. | Ranawat AS, Vidal AF, Chen CT, Zelken JA, Turner AS, Williams RJ. Material properties of fresh cold-stored allografts for osteochondral defects at 1 year. Clin Orthop Relat Res. 2008;466:1826-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Enneking WF, Campanacci DA. Retrieved human allografts a clinicopathological study. J Bone Joint Surg Am. 2001;83:971-986. [PubMed] |
| 12. | LaPrade RE, Botker J, Herzog M, Agel J. Refrigerated Osteoarticular Allografts to Treat Articular Cartilage Defects of the Femoral Condyles A Prospective. Outcomes Study. J Bone Joint Surg Am. 2009;91A:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Judas F, Rosa S, Teixeira L, Lopes C, Ferreira Mendes A, editors . Chondrocyte viability in fresh and frozen large human osteochondral allografts: effect of cryoprotective agents. Transplant Proc. 2007;39:2531-2534. [PubMed] |
| 14. | Enneking WF, Mindell ER. Observations on massive retrieved human allografts. J Bone Joint Surg Am. 1991;73:1123-1142. [PubMed] |
| 15. | Gole MD, Poulsen D, Marzo JM, Ko SH, Ziv I. Chondrocyte viability in press‐fit cryopreserved osteochondral allografts. J orthopaedic Res. 2004;22:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Ohlendorf C, Tomford WW, Mankin HJ. Chondrocyte survival in cryopreserved osteochondral articular cartilage. J Orthop Res. 1996;14:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 122] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Schachar NS, Novak K, Hurtig M, Muldrew K, McPherson R, Wohl G, Zernicke RF, McGann LE. Transplantation of cryopreserved osteochondral Dowel allografts for repair of focal articular defects in an ovine model. J Orthop Res. 1999;17:909-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Beaver RJ, Mahomed M, Backstein D, Davis A, Zukor DJ, Gross AE. Fresh osteochondral allografts for post-traumatic defects in the knee. A survivorship analysis. J Bone Joint Surg Br. 1992;74:105-110. [PubMed] |
| 19. | Davidson PA, Rivenburgh DW, Dawson PE, Rozin R. Clinical, histologic, and radiographic outcomes of distal femoral resurfacing with hypothermically stored Osteoarticular allografts. Am J Sports Med. 2007;35:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Pearsall AT, Madanagopal SG, Hughey JT. Osteoarticular autograft and allograft transplantation of the knee: 3 year follow-up. Orthopedics. 2008;31:73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle - Minimum 2-year follow-up. Am J Sports Med. 2007;35:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Williams RJ, III , Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. Journal of Bone and Joint Surgery-American Volume. 2007;89A:718-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Gross AE, Shasha N, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 200] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 24. | Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 25. | Chu CR, Convery FR, Akeson WH, Meyers M, Amiel D. Articular cartilage transplantation. Clinical results in the knee. Clin Orthop Relat Res. 1999;159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 189] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | d’Aubigné RM, Postel M. The classic: functional results of hip arthroplasty with acrylic prosthesis. 1954. Clin Orthop Relat Res. 2009;467:7-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Murphy RT, Pennock AT, Bugbee WD. Osteochondral Allograft Transplantation of the Knee in the Pediatric and Adolescent Population. Am J Sports Med. 2014;42:635-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Krych AJ, Robertson CM, Williams RJ, III , Cartilage Study G. Return to Athletic Activity After Osteochondral Allograft Transplantation in the Knee. Am J Sports Med. 2012;40:1053-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 29. | Raz G, Safir OA, Backstein DJ, Lee PT, Gross AE. Distal Femoral Fresh Osteochondral Allografts: Follow-up at a Mean of Twenty-two Years. J Bone Joint Surg Am. 2014;96:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Ghazavi MT, Pritzker KP, Davis AM, Gross AE. Fresh osteochondral allografts for post-traumatic osteochondral defects of the knee. J Bone Joint Surg Br. 1997;79:1008-1013. [PubMed] |
| 31. | Colangeli M, Donati D, Benedetti MG, Catani F, Gozzi E, Montanari E, Giannini S. Total knee replacement versus osteochondral allograft in proximal tibia bone tumours. Int Orthop. 2007;31:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Muscolo DL, Ayerza MA, Farfalli G, Aponte-Tinao LA. Proximal tibia osteoarticular allografts in tumor limb salvage surgery. Clin Orthop Relat Res. 2010;468:1396-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Hornicek Jr E, Mnaymneh W, Lackman R, Exner G, Malinin T. Limb salvage with osteoarticular allografts after resection of proximal tibia bone tumors. Clinical orthop related Res. 1998;352:179-186. [DOI] [Full Text] |
| 34. | Shi X, Wu S, Zhao J. [Limb salvage with osteoarticular allografts after resection of proximal tibia bone]. Zhongguo Xiufuchongjian Waike Zazhi. 2006;20:966-969. [PubMed] |
| 35. | Jamali AA, Emmerson BC, Chung C, Convery FR, Bugbee WD. Fresh osteochondral allografts: results in the patellofemoral joint. Clin Orthop Relat Res. 2005;176-185. [PubMed] |
| 36. | Mroz TE, Joyce MJ, Steinmetz MP, Lieberman IH, Wang JC. Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg. 2008;16:559-565. [PubMed] |
| 37. | Zou S, Dodd RY, Stramer SL, Strong DM. Probability of viremia with HBV, HCV, HIV, and HTLV among tissue donors in the United States. N Engl J Med. 2004;351:751-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Buck BE, Malinin TI, Brown MD. Bone transplantation and human immunodeficiency virus. An estimate of risk of acquired immunodeficiency syndrome (AIDS). Clin Orthop Relat Res. 1989;129-136. [PubMed] |
| 39. | Simonds RJ, Holmberg SD, Hurwitz RL, Coleman TR, Bottenfield S, Conley LJ, Kohlenberg SH, Castro KG, Dahan BA, Schable CA. Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med. 1992;326:726-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 412] [Article Influence: 12.5] [Reference Citation Analysis (0)] |