INTRODUCTION
National spending on health care varies considerably among Organisation for Economic Co-operation and Development (OECD) countries. In 2012, health care costs in the United States accounted for 16.9% of its GDP, while all OECD countries spent a mean 9.3% of their GDP on healt care[1]. Technological progress has been proposed as the main driving force behind the growth of health care expenditures[2,3]. One of the medical fields that has intensively utilized the most advanced technologies is complex spinal surgery. A multitude of novel types of instrumentation, implants, navigation and biologics have recently become available for the use in complex spine surgery[4]. However, critics point out that technologically advanced treatments may offer little or no clinical benefit compared to traditional treatment strategies[5]. Thus, the use of expensive technology, combined with an increase in the number of complex spine procedures, has attracted public attention to the expenditures associated with spinal surgery.
One of the countervailing issues is that epidemiologically, spinal degenerative disease and particular low back pain are most prevalent amongst musculoskeletal disorders. Nearly 100% of all adults end up with spinal problems at least once during their lifetime, with a point prevalence of 4%-33%[6]. According to the Ontario Health Survey, 40% of all chronic disorders are caused by musculoskeletal conditions. Additionally, 54% of all cases of long-term disability are caused by to musculoskeletal diseases[7]. Based on surveys carried out in Canada, the United States, and Western Europe, physical disabilities caused by musculoskeletal conditions assume a 4%-5% prevalence in the adult population[8]. Symptomatic degenerative spinal disorders are treated with a myriad of treatment strategies ranging from medical management, physical therapy, chiropractic adjustments, injections, to complex spine surgery. In 2005, $85.9 billion was spent on the treatment of low back pain, representing a 65% increase from 1997[9,10]. The majority of those expenditures are related to non-surgical management of spinal pain. Nevertheless, data regarding clinical efficacy of spinal procedures are lacking in general. Indeed, less than 1% of studies on lumbar spine arthrodesis procedures published from 2004 to 2009 include cost effectiveness analysis[10]. There is an even more significant lack of data on the benefits of newer minimally invasive spinal (MIS) surgeries. It has been proposed that MIS procedures may have a lower complication rate and thus use fewer hospital resources compared to traditional open spine surgeries[11-13]. The current review attempts to shed light onto the current scientific evidence for cost effectiveness of MIS procedures compared to traditional open spine surgery.
VALUE-BASED HEALTH CARE
Recently, strategies in medical economics have changed: attempts have been made to move away from the traditional system of service-oriented payment, toward a value-based health care system[14]. With other words: the value of a health care intervention equals its quality divided by its cost measured over a certain period of time. Quality of care is evaluated by a variety of measures, including process measures, safety measures and outcome measures, with the latter being either disease specific or general health values[10,15]. Using the recent value-based model, each healthcare intervention can be assessed by the following simple formula:
Value = Quality/Cost
For value-based calculations, the direct and indirect costs of a particular medical intervention must be determined. Direct costs include the charges accumulated by the medical procedures, hospitalization, medications, imaging services and future direct costs that arise from the same medical condition. Indirect costs include decreased productivity caused by disability associated with the medical condition. The quality of a medical procedure is equivalent to the procedure’s effectiveness to improve the life of affected individuals[10]. For international comparability and reproducibility, it is crucial that outcome measures can be calculated easily in order to have consistent and valid data, which can be compared between different interventions, therapies and patient populations. Furthermore, outcome measures should focus on the patients’ wellbeing. The impact of lumbar disorders on quality of life (QoL) may be assessed with disease specific measures such as the oswestry disability index (ODI) or general measures such as Medical Outcomes Study 36-item Short-Form General Health Survey (SF-36), or the EuroQol (EQ)-5D. Patient’s functional impairment with activities of daily living caused by pain is assessed by the ODI, which is low-back-specific[16-18]. In contrast, the SF-36 is a general health measure, consisting of 8 scales, including a physical pain and bodily function scale, and two main groups based on physical and mental assessments[16-19]. Similar to the SF-36, the EQ-5D constitutes a general health measure. Utilizing the EQ-50, five health-dimensions of quality-of-life can be evaluated: mobility, self-care, daily activities, pain and discomfort and anxiety or depression. Typically, its fields of application are clinical investigation, cost-effectiveness analysis, and comparison of treatment effects across different disease states. Each of these 5 dimensions is evaluated utilizing a scale of 3 scores. In total, 243 different health states can be assessed using the EQ-5D. The quality-adjusted life year (QALY) is another outcome measure, which is used to assess the value of certain health outcomes, with value being defined as quality per cost over time. QALY measures both quality and duration of treatment. As Prieto et al[20] propose, QALY equals health as a measure of quantity and QoL. QALY is used to calculate a score based on these two components of health. After calculating the number of QALYs gained, which can be easily done with the formula: utility value of a treatment multiplied by the duration of effect caused by the particular treatment, the cost-effectiveness of different types of treatment can be evaluated[20]. Thus, with optimization of effectiveness and durability of a procedure, the benefits as measured in QALYs increase. To accurately analyze the durability of an intervention, long-term follow-up studies are the most useful[15,20]. QALY is intervention-specific[21].
Cost-effectiveness analysis is a very common application of QALY, which is cost divided by the QALYs gained from the treatment of a specific disease state. With this calculation, the benefit of a given procedure or medical intervention is evaluated, which is comparable with various fields of application. Mathematically, QALYs can be expressed by the formula:
QALY = 1×Q
Q stands for a value given to a health-related state and ranges from 0 and 1. The maximum Q value of 1.0 represents a state of perfect health. Q decreases when the QoL decreases. Death is assigned a Q value of 0. However, some have suggested that there can be QoL states worse than death, which are assigned a negative value[22-25]. QALY is most often used to assess the effect on QoL following a particular intervention[22]. In some developed countries, policy makers have proposed that the cost per QALY gained should not exceed $50000-$100000 in order to consider an intervention cost effective[14,26,27]. Once an intervention has been linked to a certain amount of QALY, it is possible to compare the cost-utility of two treatment approaches for a given disease. This may be accomplished with the cost-effectiveness ratio (ICER), which is expressed with the equation:
ICER = (C1 - C2)/(Q1 - Q2)
C1 and C2 represent the costs of the two different treatments. Q1 and Q2 are the QALY values associated with two different treatments[15]. Interventions that lead to a better clinical outcome or are less expensive will be favored by these types of analyses and are considered “dominant”[24,28,29]. Cost effective interventions are those whose costs per QALY gained are less than or equal to the societal or patient willingness to pay[30]. When this type of analysis is applied to surgical procedures, a reduced rate of postoperative complications enhances the cost-effectiveness as complications can affect both cost and outcome of the procedures.
LUMBAR MICRODISCECTOMY
Hansson et al[31] compared the cost effectiveness of surgical vs conservative treatment for lumbar disc herniations in a non-randomized 2-year study. Total costs favored surgery over non-operative management due to greater work productivity as well as lower indirect costs in the surgery group. The QALY gained was tenfold higher in the surgery group[31]. A 1-year Dutch study compared early surgery vs prolonged conservative care in patients with symptomatic lumbar disc herniations[32]. The QALY analysis favored early surgery over conservative therapy[32]. The direct costs of the surgery exceed that for conservative care, but significant differences in non-healthcare costs (paid domestic help and monetary loss from decreased work productivity) favored the surgical group. This results in similar total costs between the two interventions[32]. A cost utility analysis demonstrated an 87% probability that surgery is more cost effective than conservative care at a willingness-to-pay of 80000 Euros per QALY gained, suggesting that early surgical intervention for lumbar disc herniation is cost effective[32]. In conclusion, surgery was able to confer additional clinical benefits in contrast to conservative care at reasonable economic prices in part due to greater improvements in patient work productivity. Tosteson et al[33] evaluated the cost effectiveness of surgery vs conservative treatment for lumbar disc herniations. They analyzed 2-year follow-up data from the multicenter spine patient outcomes research trial (SPORT). Standard open discectomy resulted in greater QALYs gained but, on the other hand, was associated with higher total costs compared to conservative treatment. The ICER for surgery relative to non-operative care was calculated to be $69403 per QALY gained for the general population. It was lower at $34355 per QALY gained in Medicare patients. Because of this, surgical treatment for lumbar disc herniations seemed to be cost effective and to be even more cost effective in Medicare patients[33]. Using 4-year follow-up data of the SPORT patient cohort, both the QALY gained and total costs were higher in surgical patients. The difference in total costs between surgery and conservative treatment groups over 4 years was $7006, which was lower than the difference in total costs at the end of 2 years ($14142). The four-year cost per QUALY gained was estimated to be $20600, leading to the conclusion that surgical intervention for lumbar disc herniations becomes more cost effective with longer follow-up[34]. In conclusion, the current literature provides a solid body of evidence that traditional microdiscectomy constitutes a cost effective treatment for symptomatic lumbar disc herniations.
In an attempt to limit access related tissue disruption, transforaminal endoscopic techniques for patients with symptomatic lumbar disc herniations have gained popularity in recent years. Various papers have been published, describing these new minimal-invasive surgical techniques, which allow the surgeon to approach the pathological site via an endoscopic uniportal access. The endoscope is entered via a posterolateral access, passing through the intervertebral foramen between traversing and exiting spinal nerves[35-38]. Hermantin et al[39] conducted a prospective, randomized study on 60 patients to investigate the effectiveness endoscopic discectomy compared to traditional microdiscectomy. The clinical outcome of both procedures was comparable. Based on patient’s self-evaluation, clinical findings and ability to return to work, 93% of patients treated with a traditional microdiscectomy compared to 97% of patients who underwent an endoscopic discectomy had a satisfactory outcome. Postoperatively, patients who underwent the endoscopic procedure had a lower use of narcotics compared to the traditional group. While this study did not carry out a cost effectiveness analysis, they report that patients who underwent a traditional microdiscectomy stayed for an average of 49 d out of the work force compared to 27 d of patients who underwent an endoscopic procedure. Mayer et al[40] conducted a study to investigate the effectiveness of endoscopic discectomy compared to microsurgical discectomy in a total of 40 patients. Two years after the procedure, sciatic pain had disappeared in 80% of patients who underwent an endoscopic procedure compared to 65% in the traditional group. Sensory deficits disappeared in 92.3% in the endoscopic group and in 68.8% in the open group. Motor deficits resolved in all affected patients in both groups. Mean duration of postoperative disability following an endoscopic surgery was 7.7 wk; in the microdiscectomy group 22.9 wk of disability were reported. Return to their previous occupation was achieved in 95% in the percutaneous endoscopic cohort and in 72.2% in the microdiscectomy cohort. Ruetten et al[41] conducted a prospective study in patients undergoing either full-endoscopic or traditional microsurgical discectomy, with 178 patients completing 2-year follow-up. At two-year follow-up, a similar improvement of leg pain was measured in patients treated with endoscopic [visual analoge scale (VAS) for leg pain: preoperative 7.1; follow-up 0.9] or traditional (VAS for leg pain: preoperative 7.6; at 2-year follow-up 0.8) technique. Recurrent disc herniations occurred at a rate of 6.2% both treatment groups. Ruetten et al[41] report a significantly lower rate of complications in patients who underwent endoscopic discectomy compared to traditional microdiscectomy. Importantly, the average duration of disability was 25 d in patients with endoscopic discectomy compared to 49 d in patients who had undergone traditional microdiscectomy.
In summary, the current literature suggests that endoscopic lumbar discectomy achieves similar clinical outcomes and similar patient satisfaction compared to traditional microdiscectomy. While, there is some data that endoscopic discectomy is associated with less disc space and concomitant foraminal collapse[42] long-term data on long-term sequele, such as degenerative disc disease or segmental instability of Endoscopic vs Microsurgical lumbar discectomy is lacking. Shorter operative times, shorter hospital stays and earlier return to work following endoscopic discectomy are consistently reported in the current literature. While, no cost-effectiveness analysis comparing endoscopic with microscopic technique is available to date, shorter hospital stays and earlier return to work may contribute to potential cost-effectiveness of endoscopic discectomies if their good clinical outcomes are maintained.
DECOMPRESSIVE SURGERY
Degenerative lumbar spinal stenosis typically manifests with lumbar radiculopathy or neurogenic claudication, which is impairment of walking due to pain or discomfort in the lower extremities. Neurogenic claudication typically is relieved by anteflexion of the trunk. Usually, nonoperative therapy does not result in sustained improvement[43]. Thus, patients are commonly offered a surgical intervention, the most traditional procedure being a decompressive laminectomy. The goal of this procedure is decompression of the narrowed spinal canal. Posterior elements, including lamina and interspinous ligaments, are removed, leading to the exposure of the lateral recess and lumbar foramina. Tosteson et al[44] conducted an analysis of the costs of traditional lumbar laminectomy to treat spinal stenosis, utilizing the prospective SPORT cohort. Among 634 participants, 394 suffered from spinal stenosis without spondylolisthesis and of these patients 320 patients underwent traditional decompressive surgery without arthrodesis. At 2 years a QALY gain of 0.17 was reported at a cost of $77600 per QALY gained. Importantly, more recent 4-year follow-up data confirmed cost effectiveness of traditional lumbar laminectomy for spinal stenosis without spondylolisthesis[34]. At 4 years, decompressive surgery resulted in a 0.23 QALY gain at a cost of $42800 per QALY gained. Katz et al[45] reported similar findings. Lumbar laminectomy resulted in a significant relief of leg pain from preoperative values (3.4 out of maximum 5 points) to 0.9 at 2 years follow-up in patients with spinal stenosis. Direct costs for laminectomy were $12615 for lumbar decompressive procedures and similar to $17688 reported by Tosteson et al[44]. However, the report of Katz et al[45] lacked a more complete cost effectiveness analysis due to the lack of primary patient-reported data or a validated EuroQol instrument. Adogwa et al[46] investigated cost effectiveness of revision decompression, performed in 42 patients[46]. The cumulative gain in QALY relative to preoperative baseline was 0.84 over a two-year period. Estimated costs were as high as $49431. Costs per QALY gained were calculated to be around $60000, which makes revision decompressions a cost effective treatment for recurrent lumbar stenosis.
In traditional open laminectomy, posterior elements of the vertebral column are removed, including spinous processes and ligaments[30]. While the short-term outcome of traditional laminectomy is favorable[47,48] (Fu, 2008 #96), it has been proposed that 7-10 years after decompression, 33% of patients have severe back pain and 23% required reoperation[49]. Minimally invasive techniques for lumbar decompression aim to decrease the amount of tissue removal to minimize destabilization of the spine. Biomechanical studies propose that minimally invasive lumbar decompressive techniques cause significantly less hypermobility and less stiffness reduction compared to traditional laminectomy[50,51]. Fu et al[30] conducted a prospective study comparing two different decompression techniques (“Windows technique” laminoforaminotomy vs decompressive laminectomy) in patients with lumbar spinal stenosis. Results were good or outstanding in nearly 90% and acceptable in 11% 40 mo following less invasive laminoforaminotomy. There was no need for revision or secondary surgery in any case. In contrast, outcome in the open decompressive laminectomy group was good or excellent in 63% of cases, 30% of patients had acceptable outcomes, while outcome was poor in 7%. In this group, 6 cases of postoperative degenerative spinal instability with worsened back pain were found, which lead to secondary surgery in 4 patients due to recurrent stenosis and instability. Based on adequate long-term outcomes, few complications and low costs, the authors proposed the less invasive laminoforaminotomy as a possible standard method to treat degenerative spinal stenosis.
In a prospective, randomized study, Thomé et al[52] compared the clinical outcome of less invasive unilateral or bilateral laminotomy and traditional laminectomy. In all 120 patients adequate decompression was achieved and a massive decrease of pain was observed in each treatment group. The authors report that bilateral laminotomy resulted in significantly less pain compared to unilateral laminotomy or traditional laminectomy at the time of last-follow up 15.5 mo after the procedure. Similarly, SF-36 scores demonstrated marked improvement in all groups, but most pronounced in those patients undergoing bilateral laminotomy. Moreover, bilateral laminotomy exhibited a trend towards a lower rate of complications compared to the other groups. Based on these results, Thomé et al[52] propose that bilateral as well as unilateral laminotomy reliable and valid choices for decompressive surgery of lumbar stenosis, with the most crucial outcomes being relief of symptoms and improvement of QoL. While unilateral laminotomy and laminectomy seemed to be equal regarding clinical outcome, bilateral laminotomy enables more favorable results.
Parker et al[53] conducted cost-effectiveness study over a 2-year period, analyzing unilateral laminectomies using either a subperiosteal dissection via midline incision or tubular access via a paramedian incision. They especially focused on the utilization of medical resources provocated by back-related conditions, sickness absence rate and outcome measures like QALYs. The type of access did not affect direct, indirect or overall costs and both treatment arms presented with a cumulative gain of 0.72 QALYs at 2 years postoperative. Total costs averaged $23109 for a hemilaminotomy using a paramedian incision and $25420 for a hemilaminotomy using a midline incision. These results suggest that less invasive hemilaminotomies are a highly cost effective for the treatment of lumbar spinal stenosis. However, there is a paucity of literature comparing cost effectiveness of traditional open laminectomy to less invasive laminectomies. Knight et al[54] compared clinical outcome and cost in 104 patients with lumbar spinal stenosis following either traditional laminectomy or tubular decompression. Consistent with previous studies, the authors detected a similar degree of improvement of the ODI and VAS for back and leg pain following either open or tubular procedure. There was a trend towards more complications in patients treated with open laminectomy. While this study did no perform a complete cost effectiveness analysis it provides information regarding the direct cost of these procedures. Thus, the median cost for traditional laminectomy was $7305 compared to $4518 for tubular decompression.
In conclusion, several studies have demonstrated that traditional laminectomy is a highly cost efficient treatment for spinal stenosis. However, several controversies remain: While short-term outcome of laminectomy (without fusion) is satisfactory, concerns have been voiced regarding the long-term durability of clinical outcomes. Katz et al[49] reported that 7-10 years after decompression, 33% of patients have severe back pain and 23% already underwent reoperation. Moreover, it is not known if the use of less invasive decompression techniques with sparing of the posterior elements will decrease the occurrence of late symptomatic instability. The topic of increased instability is further complicated by co-existing spondylolisthesis. While spinal stenosis with concomitant spondylolisthesis is typically treated with decompression and arthrodesis, there is some emerging evidence that less invasive decompression techniques may be suitable even in the presence of mild spondylolisthesis. If less invasive decompression techniques achieve durable alleviation for spinal stenosis even in the setting of mild spondylolisthesis, abandoning the need for arthrodesis (in patients with only neurological symptoms) would certainly greatly enhance the cost effectiveness of less invasive decompression techniques. Further studies on the durability of clinical outcome and possible inclusion of spinal stenosis with mild spondylolisthesis as indication may dramatically increase the cost effectiveness of less invasive decompression techniques.
SHORT SEGMENT FIXATION
Chronic low back pain, defined as pain lasting for more than three months[55], is a common health issue causing a significant healthcare burden. The cost from back pain is mainly due to indirect cost from loss of work productivity. Direct costs associated with the disability were estimated to be around £1.6 billion in the United Kingdom in 1998[56], and the condition is estimated to be responsible for close to 120 million United Kingdom work days lost per year[57]. Back pain may be caused by spondylolisthesis and/or degenerative disc disease, both of which may surgically be treated with arthrodesis. Tosteson et al[44] analyzed the cost-effectiveness of traditional fusion utilizing the prospective SPORT cohort. Among 634 participants, 368 suffered from degenerative spondylosis. Of these patients 344 underwent surgical decompression with spinal fusion. At 2 years a QALY gain of 0.19 was reported at a cost of $120000 per QALY gained. Importantly, the cost effectiveness for spinal arthrodesis for degenerative spondylolisthesis improved markedly 4 years after the procedure. At 4 years, decompressive surgery resulted in a 0.29 QALY gain at a cost of $66300 per QALY gained spondylolisthesis[34]. Fritzell et al[58] performed a prospective randomized controlled study evaluating the cost-effectiveness of lumbar fusion compared to nonsurgical treatment for chronic low back pain. A total of 284 patients were randomized to either fusion surgery (n = 217) or best medical management (n = 67). At 2 years, clinical outcome was improved in 60% of patients who underwent lumbar fusion compared to 34% who received non-surgical treatment. During 2 years, the societal costs per patient, consisting of direct and indirect costs were higher in the surgical group [704000 Swedish kroner (SEK)] compared to the control group (636000 SEK). With a difference of 60200 SEK, average hospital care costs were significantly higher in the surgical group. The most probable explanation for this finding is the higher cost associated with the fusion procedure itself, as well as the postoperative hospitalization. When using the noninstrumented posterolateral fusion as a reference, there was a 66% increase in costs when instrumentation was performed and a 103% increase if an interbody procedure was performed as well. In contrast to hospital costs, primary and private care seemed to be more expensive in the non-surgical group, with a difference of 1000 SEK, as were drug-associated costs with a difference of 1400 SEK. Another important finding was the significant difference in return-to-work-incidence: patients who had undergone surgery were able to return to work in 33%, while the only 16% of nonsurgical treated patients returned to work. Costs for production losses per patient on sick leave in the control group were: 460200 SEK. To conclude, health care costs and societal costs were higher with surgical treatment. Nevertheless, treatment efficacy clearly favored surgical treatment.
Fritzell et al[59] performed a cost-effectiveness analysis based on data from a 2-year randomized controlled trial. They compared lumbar arthrodesis with arthroplasty in patients with discogenic low back pain. Both cohorts experienced similar improvements in QoL 2 years following the procedure (0.45 QALY). This study found that lumbar fusion was associated with significant greater hospital and total healthcare costs. This was due to a higher rate of reoperations following lumbar arthrodesis (36%) compared to arthroplasty (10%). However, the gross majority of re-operations (77%) in the arthrodesis group were performed for implant removal as the implant was determined by the surgeon to act as pain generator. The authors also included an analysis with costs for re-operation removed from both groups, which eliminated the cost difference form the perspective of both the hospital and healthcare sector. After 2 years there was a nonsignificant cost difference of combined indirect and direct costs of lumbar arthroplasty compared to lumbar arthrodesis surgery. Thus, the authors concluded that both procedures were equally cost effective for society within a 2-year time frame.
Adogwa et al[60] performed a cost-effectiveness analysis on open transforaminal lumbar interbody fusion (TLIF) surgery for the treatment of degenerative spondylolisthesis. The mean length of hospital stay was 4 d. There were no surgical site infections, CSF leaks or hardware failures, but 4 cases of incidental durotomy. One patient suffered from perioperative hematoma and thus had to be returned to the operating room. A significant improvement in back pain VAS score, leg pain VAS score and Oswestry Disability Index was observed 2 years after TLIF. There was a cumulative health utility value of 0.86 QALY gained over 2-years. The mean total 2-year cost of TLIF was $36836. Surgery costs were $21311. Outpatient resource utilization costs were $3940. Mean direct medical costs were $25251. Indirect costs were $11584, and the mean 2-year cost per QALY gained associated with TLIF was $42854. There was a median amount of 60 d (IQR 30-120 d) missed workdays. TLIF improved pain, disability, and QoL in patients with degenerative spondylolisthesis-associated back and leg pain.
In summary, traditional open spinal arthrodesis procedures are associated with high direct procedural costs and hospital fees. However, the current literature suggests that lumbar spinal arthrodesis procedures produce stable clinical improvements, which leads to an accumulation of QALYs while only few additional medical costs incur. Therefore, surgical interventions become more cost effective with time[61]. In the SPORT cohort cost effectiveness for spinal fusion surgery for spondylolisthesis was achieved 4 years following the procedure.
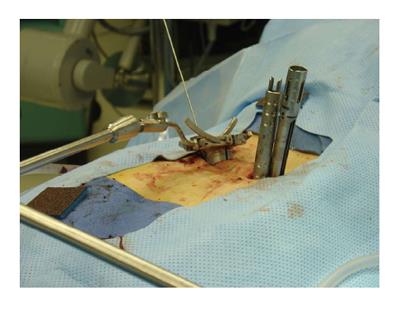
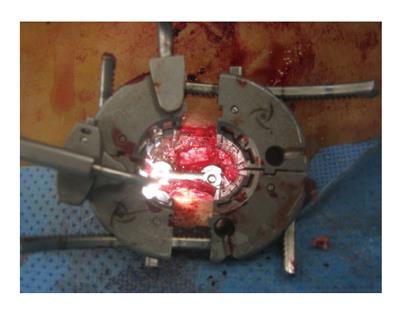
MIS arthrodesis procedures have been developed in order to achieve the same surgical goals compared to traditional surgery utilizing a smaller access corridor. The goal is to decrease intraoperative tissue trauma and disruption and hereby decrease perioperative morbidity and promote postoperative rehabilitation. In contrast to traditional TLIF which is performed via an open midline approach involving extensive soft tissue stripping and retraction of the paraspinous muscles[15], MIS TLIF utilizes a paramedian approach with muscle spreading (Figures 1 and 2). Parker et al[62] compared the clinical outcomes and conducted a cost-utility analysis of single-level MIS vs open TLIF procedures. While MIS TLIF’s required longer operative times, they were associated with reduced blood loss and decreased length of hospitalization. Return to work was quicker with the MIS group, but postoperative complications were similar between both techniques. No significant differences in outcomes were noted between the two groups in this study. This study also included a cost-utility analysis to compare the MIS and open procedures 2 years following the intervention. They found a significant difference in total costs: Whereas MIS TLIF resulted in total average cost of $38563, open TLIF was $47858 (P = 0.03). On the other hand, there were no statistically significant differences in the calculated QALYs (MIS = QALY gain of 0.77; open = QALY gain of 0.70). As the mean cost savings per MIS-TLIF procedure were $9295 with similar gain in QALYs, the MIS approach produced a higher value with a total cost per QALY of $122303[62].
Figure 1 Minimally invasive transforaminal interbody fusion.
An expandable tubular retractor is positioned via a paramedian approach. Note that the contralateral perdicle screws are in place.
Figure 2 Intraoperative view of a pedicle-rod construct during the final stages of a minimally invasive transforaminal lumbar interbody fusion.
Wang et al[63] conducted a retrospective analysis comparing acute hospitalization charges for 1- and 2-level MIS-TLIF vs open PLIF procedures. Patients having bilateral neurological symptoms were treated with open surgery; those with unilateral symptoms were treated with MIS. Blood loss was significantly reduced in MIS-TLIF procedures. Clinical outcomes did not differ significantly in single-level cases, but in two-level MIS-TLIF patients displayed a significant clinical improvement compared to 2-level open cases. Mean length of hospital stay was significantly lower in single-level MIS cases compared to open procedures. Those differences in length of hospital stay correlated with hospital charges: Single-level MIS-TLIF caused an average cost of $70159 compared to $78444 caused by the open approach. This results in average cost savings of $8285 per MIS-TLIF. For 2-level surgery, mean charges totaled $87454 for MIS vs $108843 for open surgery. Costs for implants and rhBMP-2 were nearly identical. The crucial factor for a cost-difference was the number of levels operated on[63].
Adogwa et al[64] conducted a study to compare narcotic use, return to work, disability, and QoL between MIS and open TLIF’s. The duration of narcotic use was significantly less in the MIS TLIF patients and return to work was shorter for the MIS TLIF cohort. However, significant improvements were observed in all clinical measures in both groups, without a significant difference between both groups at 2 years. Wu et al[65] conducted a quantitative meta-analysis on studies reporting fusion rates after single-level or multi-level open or minimally invasive/mini-open TLIF procedures[65]. Recombinant bone morphogenetic protein was used in 50% of MIS TLIFs and in 12.18% of open procedures. Mean fusion rate for open TLIF (16 studies, 716 patients) was 90.9%, compared to a mean fusion rate of MIS TLIF was 94.8% (8 studies, 312 patients). Complication rates differed between both treatment groups as well, with 12.6% for open TLIF vs 7.5% for minimally invasive TLIF. To conclude, fusion rates for both procedures are in similar. Complication rates are also similar, but there is a trend of a lower complication rate in minimally invasive TLIF procedures.
Overall, MIS fusion procedures are associated with a reduction in utilization of inpatient resources as well as increased cost-savings in the acute perioperative period, predominantly due to reduced complication rates[63]. Long-term cost-utility analysis of MIS vs open procedures is necessary to confirm the long-term clinical effectiveness of MIS procedures.
ADULT SPINAL DEFORMITY CORRECTION
Complex spine surgery is associated with high costs and performed with increasing frequency. It has therefore become a subject of intense economic scrutiny[66-68]. One of the fastest growing and most expensive areas of spine surgery is surgical correction of degenerative scoliosis and adult spinal deformity. Within 10 years (2000-2010) the total number of spine surgeries for the diagnosis of “curvature of the spine” (ICD-9), increased from 9400 to more than 20600[69]. In comparison, the number of all other ICD-9 spine primary diagnosis codes increased by 20% (from 675500 in 2000 to 813800 in 2010)[69]. Several publications have focused on this substantial topic, suggesting up to 32% of all adults being affected by scoliosis and a prevalence of up to 60% in the elderly[70-73]. Nationwide data from the healthcare cost and utilization project (HCUP) report that hospital costs for a principal diagnosis of curvature of the spine averaged $54000 in 2010, compared with $17000 for more common spine procedures (ICD-9 720-724)[69]. Costs for more complex multilevel fusions are estimated to be as high as $70000 (excluding overhead)[68]. Costs of readmissions subsequent to the initial surgery are estimated to range between $65000 and $80000 per readmission[74].
McCarthy et al[75] conducted a retrospective study to analyze the total per-patient hospital and operating room costs of adult spinal deformity surgery with minimum three levels fused through extended follow-up. Total hospital costs of surgical treatment averaged $120394. Primary surgery averaged $103143 and total readmission costs averaged $67262 per patient with a readmission (n = 130, 27% of all patients). Total costs in patients readmitted averaged $174629 compared to $100477 for patients without readmission. Average operating room costs were $70514 per patient, constituting 59% of total hospital costs. Due to prevalent readmissions, the average cost of spinal deformity surgery in adults rose by at least 70%, which illustrates the financial burden of revisions/reoperations. In another study, McCarthy et al[75] compared observed postoperative QALYs with predicted QALYs using observed preoperative health-related theoretical QALYs lacking operative treatment. One cohort consisted of surgical patients who completed 3-year follow-up and the other group of crossover patients with two preoperatively completed HRQOL (health related QoL) assessments. Average total hospital costs, with discounting and including readmissions, were $125407. At the 3-year follow-up assessment, there was an average QALY of 1.93. Average nonsurgical QALYs were supposed to be 1.6 at this same point of time. Three-years postoperative, the ICER was estimated to be $375000 and 5-year follow-up it was $198000. At the 10-year follow-up, the ICER was $80000. Based upon these calculations, McCarthy et al[75] proposed a cost-effectiveness of surgical spinal deformity treatment in adults after 10-years. This emphasizes the need for the durability of surgical treatment to assess the value of surgery.
Surgery for ASD definitely remains challenging. While marked clinical improvement may be seen following successful surgical correction of ASD, intra- and postoperative complications and morbidity remain probable. In a recently published report using data of the Spinal Deformity Study Group, the rate of minor complications was estimated to be 26.2% and the rate of major complications 15.5% in a cohort of 206 patients[76]. This has led spine surgeons all over the world to develop minimally invasive surgical techniques to address spinal deformities. During the last decade a multitude of minimally invasive techniques has evolved to correct coronal spinal deformities. Wang et al[77] presented their early experience with minimally invasive thoracolumbar surgery in 2010. They achieved internal fixation with an extreme lateral interbody fusion. RhBMP-2 was routinely used in all fusion sites and levels. Radiographic fusion was recorded in 84 of 86 operated levels. There was no pseudarthrosis. The patient cohort experienced significant improvement in leg and axial back pain (P < 0.01). Only 3 patients had minimal or no improvement of symptoms. Two patients had to be returned to the OR. One patient required extension of the construct and the other patient had a CSF leak. The majority of complications were associated with the transpsoas access. Thus, 30.4% of all patients experienced new postoperative neurological complications like thigh numbness or painful sensations, which lateralized to the side of the approach. Two of these patients had to be admitted to inpatient rehabilitation. In one patient neurological complications were severe, making the permanent use of assistive ambulatory devices mandatory.
Uribe et al[78] analyzed complications associated with 3 types of surgery: minimally invasive, hybrid and open. Blood loss was the least in the MIS group, while the open group had the shortest operating time. Length of hospital stay was similar among the groups. Oswestry disability index and visual analog scale scores improved significantly in all groups, except for leg pain, which was not significantly improved in the MIS group. Open surgery achieved significantly better correction of the pelvic incidence-lumbar lordosis mismatch compared to the MIS group postoperatively (P < 0.03). Complication rates were as follows: 30% in the MIS group, 47% in the hybrid group and 63% in the open group.
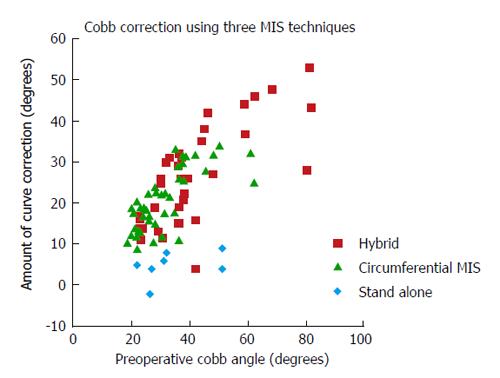
Wang et al[79] surveyed 3 different surgical methods to treat adult spinal deformity: stand-alone, 360° MIS and hybrid. The stand-alone procedure was least invasive; the hybrid group underwent the most substantial procedure. The circumferential MIS group was intermediate. Surgical time was lowest in the stand-alone group and highest in the hybrid group. Clinical improvement was seen in all 3 groups, without significant differences. The hybrid construct allowed for the highest degree of coronal curve correction (Figure 3), and was the only procedure that led to substantial correction of lumbar lordosis (16.6°). Major complications occurred in 29% of stand-alone procedures, 14% of circumferential procedures and in 40% of hybrid group patients. Wang et al[77] concluded that less invasive approaches are associated with specific limitations to coronal and sagittal plane deformity. More invasive procedures have the potential to result in comparable outcomes as open surgery, but are also associated with a higher morbidity.
Figure 3 Scatterplot depicting scoliotic curve correction achieved at last follow-up as a function of peroperative curve severity.
In conclusion, MIS ASD surgery constitutes a rapidly evolving field which shows promise for certain types of deformities. Several studies have demonstrated that MIS deformity surgery leads to improvement of clinical outcomes and may reduce the rate of perioperative morbidity[80]. Current research is determining particular limitations for scoliotic curve correction utilizing specific less invasive surgical techniques. To date there is no data on the impact of minimally invasive surrey on the cost effectiveness of deformity surgery.
CONCLUSION
In the lumbar spine, MIS techniques are available for treating a variety of clinical indications. In general, clinical outcomes following MIS procedures compare favorably to traditional open surgery. MIS procedures appear to improve the cost effectiveness of lumbar spine procedures by decreasing hospital stay and rehabilitation time. Less invasive decompressive techniques such as endoscopic foraminal decompression and tubular spinal decompression also hold great promise to greatly reduce cost by replacing arthrodesis procedures in strictly selected indications. MIS ASD surgery is currently evolving and will potentially play an important role to make adult deformity surgery economically feasible in our aging society. Our current study identifies a great need for high quality cost-effectiveness studies comparing standard open lumbar spine surgeries with MIS techniques.