Published online Jan 18, 2015. doi: 10.5312/wjo.v6.i1.127
Peer-review started: April 15, 2014
First decision: June 18, 2014
Revised: June 19, 2014
Accepted: July 25, 2014
Article in press: July 29, 2014
Published online: January 18, 2015
Processing time: 280 Days and 16.5 Hours
Recent advancements in the field of musculoskeletal tissue engineering have raised an increasing interest in the regeneration of the anterior cruciate ligament (ACL). It is the aim of this article to review the current research efforts and highlight promising tissue engineering strategies. The four main components of tissue engineering also apply in several ACL regeneration research efforts. Scaffolds from biological materials, biodegradable polymers and composite materials are used. The main cell sources are mesenchymal stem cells and ACL fibroblasts. In addition, growth factors and mechanical stimuli are applied. So far, the regenerated ACL constructs have been tested in a few animal studies and the results are encouraging. The different strategies, from in vitro ACL regeneration in bioreactor systems to bio-enhanced repair and regeneration, are under constant development. We expect considerable progress in the near future that will result in a realistic option for ACL surgery soon.
Core tip: This article reviews the current research strategies in anterior cruciate ligament tissue engineering and highlights the most promising strategies in this field.
- Citation: Nau T, Teuschl A. Regeneration of the anterior cruciate ligament: Current strategies in tissue engineering. World J Orthop 2015; 6(1): 127-136
- URL: https://www.wjgnet.com/2218-5836/full/v6/i1/127.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i1.127
Knee injuries frequently result in ruptured ligaments, typically through high-pivoting sporting activities such as skiing, football and basketball. In 2005, around 400000 physician office visits in the United States were related to knee injuries[1]. The worldwide estimation of young sports players that require surgery following a knee injury lies between 17%-61%[2]. The anterior cruciate ligament (ACL), a main stabilizing structure of the knee, is one of the most commonly injured ligaments. In the United States alone, around 350000 reconstructive surgeries of the ACL are performed annually. According to the National Center for Health Statistics, the annual costs for the acute care of these injuries are around $6 billion[3].
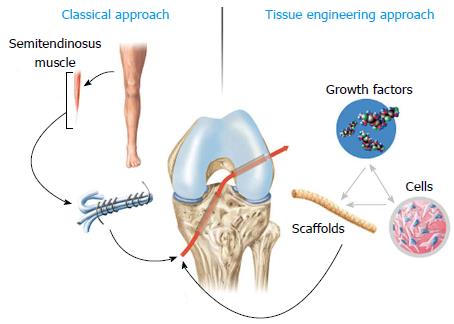
Historically, the treatment of ACL injuries involved different strategies, from non-operative care to several surgical procedures[4]. Simple primary suturing in the 1970s was abandoned due to bad clinical results. Augmented ACL repair using natural as well as synthetic grafts leads to somewhat improved results. Synthetic grafts were popular in the 1980s but resulted in serious complications and bad clinical results. From the early 1990s onwards, ACL reconstruction with autograft or allograft material has become the method of choice for most surgeons (Figure 1). Despite the ongoing success of autografts, problems mostly associated with donor site morbidity remain, such as anterior knee pain, infrapatellar contracture, tendonitis, patellar fracture, muscle weakness and limited graft availability[5]. In terms of allograft material, the risk for transmissions of blood-borne diseases and the delayed biological incorporation were mentioned as the main disadvantages[6]. In addition, relatively high failure rates of ACL reconstruction, especially in young and active patients, have been reported for allografts[7]. An incidence of osteoarthritis as high as 50% within 7-14 years after injury and reconstruction of the ACL is still the main drawback of this surgical strategy. The development of osteoarthritis following ACL injury is not fully understood and may be caused not only by the limitation of the current grafts, but also by the initial joint trauma and the trauma caused by the surgeon. However, this has resulted in enormous ongoing research interest in that topic[8,9].
The regeneration of musculoskeletal tissues has become increasingly popular in the field of orthopedic research. Typically, structures that are injured or lost due to trauma and disease are the ideal candidates to be engineered. Tissue engineering as a multidisciplinary field includes strategies of engineering, material science and biology, with the aim of regenerating tissues that not only recreate the morphology, but also restore the normal function. In the late 1980s, Langer and Vacanti[10] first described the classic four basic components that are needed in tissue engineering: a structural scaffold, a cell source, biological modulators and mechanical modulators[10].
The ACL, with its limited healing capacity and the consequent need for reconstructive surgery, certainly is an appealing but also challenging structure for tissue engineering. In contrast to extra-articular ligaments, such as the medial collateral ligament, the intra-articular location of the ACL apparently prevents its primary healing. The disruption of the synovial sheath does not allow local hematoma formation crucial for the onset of the inflammatory response that would stimulate primary healing[11]. In addition, the complex three dimensional structure of the ACL, with different tensioning patterns throughout the knee path of motion, contributes to the difficulty of regenerating this ligament in terms of form and function.
It is the purpose of this article to review the current approaches in tissue-engineering of the ACL, to provide an overview of the current problems and limitations, and to present future directions of this evolving research technology.
Many different biomaterials have been introduced as a potential scaffold for ACL tissue engineering. Ideally, the scaffold has to be biocompatible and its mechanical properties should mimic the natural ACL as closely as possible. It also needs to be biodegradable to enable tissue ingrowth, which is crucial for the new ligament to form. Biological materials, biodegradable polymers and composite materials have all been or still are under evaluation for ligament regeneration[11].
Dunn et al[12] and Bellincampi et al[13] developed scaffolds made of collagen fibrils. They showed that ACL fibroblasts adhered to these scaffolds and remained viable, in vitro as well as in vivo. Unfortunately, after 6 wk the constructs were completely resorbed. Goulet et al[14] reported on the decreasing mechanical strength of collagen scaffolds seeded with ACL fibroblasts. Murray et al[15] demonstrated that a collagen-glycosaminoglycan composite scaffold supported cell growth and the expression of fibroblast markers. Several techniques have been explored to improve the mechanical properties of collagen-based scaffolds, including cross linking the collagen or a special braid-twist design[16-18]. However, despite considerable improvements of the mechanical properties, collagen-based scaffolds thus far have not been able to mimic the strength of the natural ACL.
Similar challenges regarding the mechanical strength have also been reported for other biological materials, such as alginate, chitosan and hyaluronic acid[19-25]. Many different composites of these materials have been explored and it has been shown that some of them may be an interesting option in terms of cell attachment and cell proliferation. However, the mechanical insufficiency of these biological materials remains a considerable problem for their routine practical use in ligament regeneration. To overcome the mechanical weakness, Panas-Perez et al[26] developed a collagen-silk composite and concluded that a scaffold with > 25% silk provides sufficient mechanical support very close to the properties of the native ACL.
The use of silk in ligament scaffolds is not restricted to combinations with other biomaterials. In various studies, its functionality in diverse tissue engineering approaches, especially in the musculoskeletal field, has been proven. The properties that make silk an attractive candidate as biomaterial are its remarkable strength and toughness compared to other natural as well as synthetic biomaterials[27-34]. The majority of studies dealing with silk as raw material for scaffold production use fibers from cocoons of the mulberry silkworm Bombyx mori. Due to biocompatibility issues, silkworm silk requires removal of the surface protein layer sericin, which can elicit adverse immune responses[35,36]. Once sericin is removed, the remaining silk fibroin fibers are non-immunogenic, biocompatible and capable of promoting cell adhesion, growth and, in the case of progenitor cells such as mesenchymal stromal cells (MSCs), differentiation. The classical way to remove this protein layer is to boil raw silk fibers in alkaline solutions such as sodium carbonate. Recently, Teuschl et al[36] successfully removed sericin from a compact and highly-ordered raw Bombyx mori silk fiber scaffold using borate buffer based solutions. The possibility of removing sericin after the textile engineering process eases the production of complex 3D structures in TE applications because the gliding properties of the silk fiber due to the gum-like sericin assist during textile engineering steps (e.g., braiding and weaving). The pioneers in using silk fibers as raw material for ACL scaffolds are Altman and Kaplan, who demonstrated that the mechanical properties of their twisted fiber scaffolds match that of the native human ACL[37]. Moreover, Horan et al[38] demonstrated the processability of silk fibers with a huge number of different textile engineering techniques, enabling the generation of complex hierarchical structures with defined properties. Another characteristic that makes silk an attractive candidate for ACL tissue engineering is its slow rate of biodegradation (proteolytic degradation). Thus, ACL scaffolds made out of silk fibroin can provide the primary stability over an extended period of time, allowing ingrowing cells to rebuild neoligamentous tissue without exposing the knee joint to periods of instability. Moreover, the gradual transfer of stabilizing properties from the silk scaffold to the new forming tissue should allow a neotissue formation similar to the initial native tissue regarding collagen alignment, vascularization, etc.
In the literature, silk-based ligament grafts have been tested in animal models in only a few studies[26,39-41]. Historically, former ACL studies with synthetic materials have shown that the extrapolation of findings from animal data to humans needs large animal studies, like goat, sheep or pig models. To the best of our knowledge, only two studies have already tested silk-based ACL grafts in large animal studies with encouraging results[42,43]. In a pig model, Fan et al[43] demonstrated that their woven silk ligament scaffold in conjunction with seeded MSCs supported ligament regeneration after the 24 wk post implantation period. In conclusion, these very promising in vivo studies suggest that ACL scaffolds fabricated from silk fibroin have great potential for the translation into clinical applications. Moreover, clinical trials of silk-based ACL grafts proving functionality and safety in human knees have already been documented[44].
Apart from biological materials, synthetic biodegradable polymers have been introduced in ligament tissue engineering. Petrigliano et al[45] mentioned the advantages of synthetic polymers as proper selection and different manufacturing techniques allow for exact adaptation of the mechanical properties, cellular response and degradation rate[45]. Lin et al[46] used a scaffold composed of polyglycolic acid coated with polycaprolactone. Buma et al[47] worked with a braided polydioxanone scaffold in an in vivo animal study but reported an early loss of mechanical properties. Lu et al[48] compared different synthetic braided materials and concluded that poly L-lactic acid (PLLA) scaffolds had the best results in terms of mechanical properties as well as fibroblast proliferation. Laurencin et al[49] also developed a PLLA scaffold in a 3 dimensional braided fashion with distinct regions for the bony portions and the intra-articular portion of the construct. The same group consequently compared a different PLLA scaffold with different manufacturing techniques and demonstrated that a braid-twist scaffold had the most favorable viscoelastic properties[50,51]. In another study, a polyethylene glycol hydrogel was added to the PLLA scaffold which resulted in even better viscoelastic performance of the construct, but on the other hand, this also led to a decreased pore size of the scaffold which may negatively influence cell proliferation[52].
More recently, electrospinning has been used for the development of scaffolds for ligament tissue engineering[53]. This technique can be used to produce very thin fibers in the nanometer to micron range. This allows for a more exact adaptation of the mechanical properties. Some of the studies using this technique reported better cell proliferation and extracellular matrix production[53,54]. However, these techniques are under constant investigation and while early in vitro studies show interesting results, the overall biological and mechanical performance still has to be examined further to draw any conclusions for a later clinical use of these materials.
Two different types of cells are mainly regarded as the primary choice for ACL regeneration: mesenchymal stem cells (MSC) and ACL fibroblasts[55].
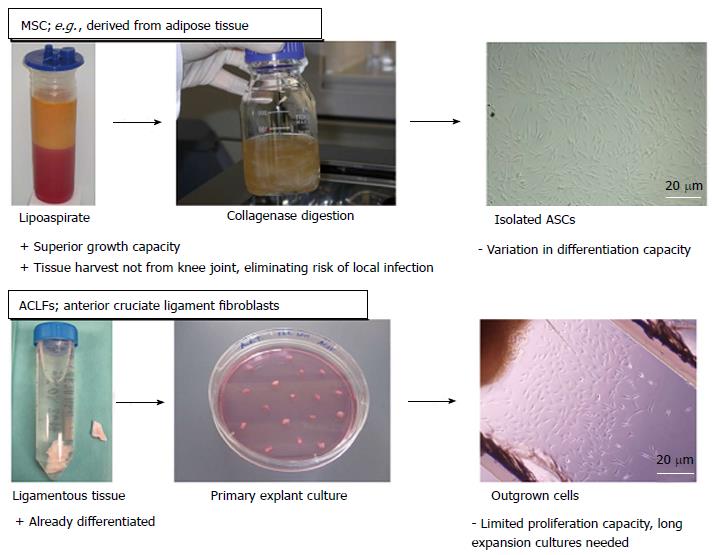
MSCs are present in almost all tissue types of the body[56,57]. However, for cell therapeutic purposes, bone marrow and adipose tissue are regarded as the main feasible sources to isolate MSCs[58,59]. The potential of MSCs to differentiate into various mesenchymal lineages, including fibroblastic, osteogenic, chondrogenic and myogenic, was proven in numerous studies. Furthermore, MSCs have already been effectively applied to enhance repair in different musculoskeletal tissues, in particular in bone and ligaments (Figure 2)[40,60-62].
The use of ACL fibroblasts involves the risk of local infection in the knee during biopsy harvesting. From the view that the seeded cells should rebuild the ligament tissue by deposition of extracellular matrix, the appropriate cell type would be ACL fibroblasts since they are the native cell type in intact ligament tissue. Therefore, they are used as control cells for cell behavior such as protein expression, especially in in vitro studies. Interestingly, different studies have demonstrated that the ACL tissue contains populations of cells sharing MSC characteristics, such as clusters of differentiation markers or multipotency[63,64]. Although stem cells are present in the ACL tissue, their regenerative capacity is too restricted to be capable of healing ruptured ligaments. As ACL tissue can only be harvested reasonably in diagnostic arthroscopic procedures after ACL rupture, other ligament fibroblast sources have been discussed, such as the medial collateral ligament[65]. Nevertheless, the majority of studies involving cell therapy approaches in ACL tissue engineering uses mesenchymal stromal cells as a cell source since they can be obtained much more easily in higher numbers and, moreover, MSCs show higher proliferation and collagen productions rates compared to ligament fibroblasts[66,67].
From a cellular view, the knee joint comprises different sources of cells[68] (ligament tissue, synovium, etc.) that have been shown to participate during the ligament regeneration process, such as the above described ACL fibroblasts or MSCs that are natively recruited after ligament ruptures or tears. The activation and recruitment of regenerating cells can be augmented mechanically, for instance by the surgical procedure (e.g., drilling of bone holes for the graft which gives access to the vasculature of bone tissue) or biochemically, by the use of growth factors or gene-based therapeutic approaches.
Growth factors can either be directly applied via inserted cells (producing these biochemical signal molecules in situ), via local delivery of growth factors or via gene-based therapeutic approaches where vehicles are encoding the chosen growth factor.
The most frequently used factors belong to proteins that directly affect the deposition of extracellular matrix proteins, such as the bone morphogenetic proteins (BMPs) or the degradation of ECM components assisting in remodeling impaired tissue. BMPs belong to the TGF-β superfamily. Their most prominent characteristic is to induce the differentiation of MSCs into the chondrogenic and osteogenic lineage. A special class of the BMPs, the growth and differentiation factors (GDF) 5/6 and 7, has been shown to be able to ectopically induce neotendon/ligament formation in vivo[69]. Furthermore, Aspenberg et al[70] (1999) demonstrated the enhanced regenerative effect of GDF 5 and 6 in an Achilles tendon rat model[70]. Interestingly, from a mechanistic point of view, the effects of GDFs depend on the mechanical loading of the injection site. Forslund et al[71] (2002) showed that the injection of GDF 6 in unloaded Achilles tendon defects led to the induction of bone formation[71], which in contrast was not observable in control groups of loaded tendons. This clearly indicates the interaction of the effect of growth factors and mechanical stimulation.
Other factors that have also been used to enhance the repair of tendon/ligament structures but are not directly associated with ECM turnover are insulin-like growth factor 1 (IGF1)[72,73], vascular endothelial growth factor (VEGF)[74], epidermal growth factor (EGF)[75] and platelet derived growth factor (PDGF)[76-79]. For instance, VEGF is well known to be a powerful stimulator of angiogenesis and the main function of IGF1 is mainly attributed to an anti-inflammatory effect[80] since functional analysis revealed a decreased recovery time but no biomechanical improvement in an Achilles tendon injury model.
An autologous and already clinically applied approach to augment tendon and ligament healing with growth factors is the use of platelet-rich plasma (PRP). PRP is obtained by plasma separation and constituents of platelets, blood proteins such as fibrin and a mixture of diverse growth factors (PDGF, VEGF, TGF-β, IGF, etc.) involved in general healing processes. Beside its autologous nature, another advantage generally attributed to PRP is its combination of growth factors in native proportions[81,82]. This feature of PRP is noteworthy as various studies have proven the synergistic effects of different growth factor combinations. Although beneficial effects of PRP have been demonstrated in cell culture studies as well as in in vivo models on tendon/ligament regeneration, the effectiveness of PRP in clinical use is still debated due to varying outcomes[81,83-87]. In a review by Yuan et al[87], these variances were mainly attributed to non-optimized treatment protocols.
Another strategy to trigger the healing capacity is to deliver therapeutic genes, either in vivo with vehicles or ex vivo in cells which are subsequently implanted. Wei et al[74] demonstrated that autologous graft remodeling in an ACL rabbit model can be enhanced by local administration of TGFβ-1/VEGF165 gene-transduced bone MSCs, leading to superior mechanical properties compared to solely TGFβ-1 gene transduced cells. In another very promising study by Hoffmann et al[88], MSCs were genetically modified to coexpress Smad8 and BMP2. These genetically modified MSCs enhanced the regeneration of the Achilles tendon in a mouse model. Taken together, the co-expression of growth factors is more efficient and potent than single gene therapeutic approaches.
Mechanical stimuli and dynamic loading are necessary for ligaments to maintain their strength. In a number of studies, Woo et al[89] demonstrated that immobilization leads to weakened mechanical properties of ligaments[89-91]. From a mechanistic point of view, it is known that cells react to mechanical stimuli via integrin-mediated focal adhesions and cytoskeleton deformation[92-94]. Altman et al[95,96] demonstrated that mechanical stimuli are able to influence stem cell differentiation as well as the production of extracellular matrix (ECM). Mechanical strain resulted in the differentiation of MSCs into fibroblast-like cells, as seen by the upregulation of ligament markers tenascin-C, collagen types I and III, and the formation of collagen fibers[95,97]. Petrigliano et al[98] showed that uniaxial cyclic strain of a three-dimensional polymer scaffold seeded with MSCs resulted in upregulated tenascin-C, collagen type I and III. Berry et al[99] reported on the proliferative effect of uniaxial strain on young fibroblasts. Park et al[100] demonstrated that 8% cyclical strain in ligament fibroblasts leads to higher cell proliferation and collagen production compared to a 4% strain and unloaded controls. In their review, Leong et al[11] mentioned that despite the known fact that mechanical stimuli play an important role in ligament tissue engineering, the timing, direction and magnitude of the stimuli as well as the cell type can all be of significant influence on the cellular response. As an example, they discussed a study by Moreau et al[101] in which MSCs were stimulated immediately after seeding and showed an inhibited expression of collagen I and II. In contrast, the opposite effect was observed when the mechanical loading was applied at the peak of MSC proliferation. Leong et al[11] mentioned that in case of ACL tissue engineering, additional investigation is required to elucidate the mechanotransduction pathways that are necessary for tissue formation and maintenance. They also stated that, to date, it is not known if any mechanical stimulation is required prior to implantation of tissue engineered ACL constructs.
In a recent questionnaire study by Rathbone et al[102], 300 orthopedic surgeons were asked if they would consider a tissue engineered ACL if it were an available option. Eight-six percent answered positively if the construct demonstrates biological and mechanical success. For 63%, improved patient satisfaction was important and 76% of the participants mentioned that a tissue engineered ACL would be superior to any of the currently used autograft materials. It was also clearly stated that a fully load-bearing construct for implantation is needed and that several ACL tissue engineering strategies should address this need for mechanical integrity. This seems to be of crucial importance as the presently used ACL reconstruction techniques with autograft or allograft material provide an immediate load bearing environment. It seems obvious that, until the results of any regenerated ACL can compare with the current relatively successful autograft methods, patients are likely to prefer the autograft. As most surgeons do not require immobilization after reconstructive surgery, immobilization is likely to be unacceptable.
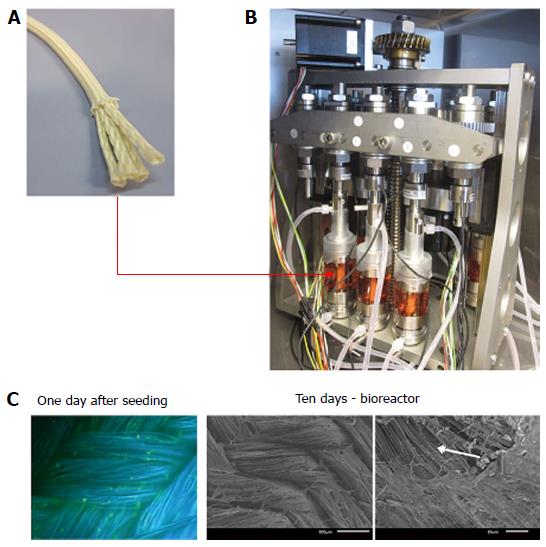
Another important aspect that will need consideration is the timing of the tissue engineering process and consequent implantation. In recent studies, our group focused on the mechanical stimulation of silk grafts with a custom-made bioreactor system[103] in order to increase the maturity of cell-loaded grafts prior to implantation (Figure 3). In accordance with a study by Altman et al[95], we triggered MSCs to produce layers of ECM on silk-based grafts. Our hypothesis is that the applied mechanical stimulation triggers the MSCs into ligamentous cells which in conjunction with the cells’ own secreted ECM leads to more functionality of the cell/scaffold construct and therefore will superiorly fulfil its tasks once it is implanted.
Future studies using a combination of in vitro bioreactor engineering with consequent in vivo implantation are certainly needed to get a clearer picture of this complex topic. On the other hand, engineering mechanically appropriate scaffolds that are implantable at any time also seems to be a good option. Future research efforts may also demonstrate which cell type seeded on these scaffolds is the ideal candidate for direct in vivo implantation in this case. Furthermore, there is also some interest in exploring the regenerative potential of solely implanted scaffolds that would recruit in vivo cells, provided there is the appropriate mechanical and physiological environment. Just recently, Murray et al[104] proposed the strategy of repair and regeneration. Here, tissue engineering efforts are undertaken to overcome the obstacles to native ACL healing. This group proposed a bio-enhanced ACL repair technique that uses a collagen scaffold saturated with platelet-rich plasma. In a number of animal studies, they demonstrated improved mechanical and biological healing of the ACL[84,105-107]. In a recent randomized large animal trial, bio-enhanced ACL repair had equal results compared with ACL reconstruction. It was also shown that the knees treated with enhanced ACL repair had a lower rate of osteoarthritis in contrast to those treated with ACL reconstruction which developed osteoarthritis in 80% after one year[108]. Despite these interesting findings, it may be problematic to draw direct conclusions as osteoarthritis is not common a year after ACL injury in humans.
There is a growing research interest in the tissue engineering of the ACL and the clinical need seems obvious. Different strategies from in vitro engineering of ACL grafts to bio-enhanced repair and regeneration are followed. For the surgical community, any type of engineered ACL may be a future option provided that it is easy to implant, does allow for at least the same aggressive rehabilitation protocol as currently used and will lead to better patient satisfaction and outcome.
The authors want to thank Johannes Zipperle for his outstanding artwork.
P- Reviewer: Finestone AS, Pappas E, Zheng N S- Editor: Ji FF L- Editor: Roemmele A E- Editor: Wu HL
| 1. | Goodwin E. The American Orthopaedic Society for Sports Medicine Conference on Allografts in Orthopaedic, Sports Medicine. 2005;. |
| 2. | Louw QA, Manilall J, Grimmer KA. Epidemiology of knee injuries among adolescents: a systematic review. Br J Sports Med. 2008;42:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Hing E, Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2004 summary. Adv Data. 2006;1-33. [PubMed] |
| 4. | Seitz H, Pichl W, Matzi V, Nau T. Biomechanical evaluation of augmented and nonaugmented primary repair of the anterior cruciate ligament: an in vivo animal study. Int Orthop. 2013;37:2305-2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Ma J, Smietana MJ, Kostrominova TY, Wojtys EM, Larkin LM, Arruda EM. Three-dimensional engineered bone-ligament-bone constructs for anterior cruciate ligament replacement. Tissue Eng Part A. 2012;18:103-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Jackson DW, Grood ES, Goldstein JD, Rosen MA, Kurzweil PR, Cummings JF, Simon TM. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1977;21:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 345] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Kaeding CC, Aros B, Pedroza A, Pifel E, Amendola A, Andrish JT, Dunn WR, Marx RG, McCarty EC, Parker RD. Allograft Versus Autograft Anterior Cruciate Ligament Reconstruction: Predictors of Failure From a MOON Prospective Longitudinal Cohort. Sports Health. 2011;3:73-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 363] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 8. | Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 287] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 9. | Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145-3152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1022] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 10. | Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8352] [Cited by in RCA: 6646] [Article Influence: 207.7] [Reference Citation Analysis (0)] |
| 11. | Leong NL, Petrigliano FA, McAllister DR. Current tissue engineering strategies in anterior cruciate ligament reconstruction. J Biomed Mater Res A. 2014;102:1614-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Dunn MG, Liesch JB, Tiku ML, Zawadsky JP. Development of fibroblast-seeded ligament analogs for ACL reconstruction. J Biomed Mater Res. 1995;29:1363-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 118] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Bellincampi LD, Closkey RF, Prasad R, Zawadsky JP, Dunn MG. Viability of fibroblast-seeded ligament analogs after autogenous implantation. J Orthop Res. 1998;16:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Goulet F, Rancourt D, Cloutier R, Germain L, Poole AR, Auger FA. Tendons and ligaments. Principles of Tissue Engineering. London: Elsevier Academic Press 2011; 911–914. |
| 15. | Murray MM, Spector M. The migration of cells from the ruptured human anterior cruciate ligament into collagen-glycosaminoglycan regeneration templates in vitro. Biomaterials. 2001;22:2393-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Koob TJ, Willis TA, Qiu YS, Hernandez DJ. Biocompatibility of NDGA-polymerized collagen fibers. II. Attachment, proliferation, and migration of tendon fibroblasts in vitro. J Biomed Mater Res. 2001;56:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Caruso AB, Dunn MG. Changes in mechanical properties and cellularity during long-term culture of collagen fiber ACL reconstruction scaffolds. J Biomed Mater Res A. 2005;73:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Walters VI, Kwansa AL, Freeman JW. Design and analysis of braid-twist collagen scaffolds. Connect Tissue Res. 2012;53:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Cristino S, Grassi F, Toneguzzi S, Piacentini A, Grigolo B, Santi S, Riccio M, Tognana E, Facchini A, Lisignoli G. Analysis of mesenchymal stem cells grown on a three-dimensional HYAFF 11-based prototype ligament scaffold. J Biomed Mater Res A. 2005;73:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 20. | Hansson A, Hashom N, Falson F, Rousselle P, Jordan O, Borchard G. In vitro evaluation of an RGD-functionalized chitosan derivative for enhanced cell adhesion. Carbohydr Polym. 2012;90:1494-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Shao HJ, Lee YT, Chen CS, Wang JH, Young TH. Modulation of gene expression and collagen production of anterior cruciate ligament cells through cell shape changes on polycaprolactone/chitosan blends. Biomaterials. 2010;31:4695-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Shao HJ, Chen CS, Lee YT, Wang JH, Young TH. The phenotypic responses of human anterior cruciate ligament cells cultured on poly(epsilon-caprolactone) and chitosan. J Biomed Mater Res A. 2010;93:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Masuko T, Iwasaki N, Yamane S, Funakoshi T, Majima T, Minami A, Ohsuga N, Ohta T, Nishimura S. Chitosan-RGDSGGC conjugate as a scaffold material for musculoskeletal tissue engineering. Biomaterials. 2005;26:5339-5347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Yamane S, Iwasaki N, Majima T, Funakoshi T, Masuko T, Harada K, Minami A, Monde K, Nishimura S. Feasibility of chitosan-based hyaluronic acid hybrid biomaterial for a novel scaffold in cartilage tissue engineering. Biomaterials. 2005;26:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Majima T, Funakosi T, Iwasaki N, Yamane ST, Harada K, Nonaka S, Minami A, Nishimura S. Alginate and chitosan polyion complex hybrid fibers for scaffolds in ligament and tendon tissue engineering. J Orthop Sci. 2005;10:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Panas-Perez E, Gatt CJ, Dunn MG. Development of a silk and collagen fiber scaffold for anterior cruciate ligament reconstruction. J Mater Sci Mater Med. 2013;24:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Blasioli DJ, Kim HJ, Kim HS, Kaplan DL. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials. 2006;27:4434-4442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 285] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 28. | Hofmann S, Knecht S, Langer R, Kaplan DL, Vunjak-Novakovic G, Merkle HP, Meinel L. Cartilage-like tissue engineering using silk scaffolds and mesenchymal stem cells. Tissue Eng. 2006;12:2729-2738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Meinel L, Betz O, Fajardo R, Hofmann S, Nazarian A, Cory E, Hilbe M, McCool J, Langer R, Vunjak-Novakovic G. Silk based biomaterials to heal critical sized femur defects. Bone. 2006;39:922-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Park SY, Ki CS, Park YH, Jung HM, Woo KM, Kim HJ. Electrospun silk fibroin scaffolds with macropores for bone regeneration: an in vitro and in vivo study. Tissue Eng Part A. 2010;16:1271-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | MacIntosh AC, Kearns VR, Crawford A, Hatton PV. Skeletal tissue engineering using silk biomaterials. J Tissue Eng Regen Med. 2008;2:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Wang Y, Kim HJ, Vunjak-Novakovic G, Kaplan DL. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27:6064-6082. [PubMed] |
| 33. | Jin HJ, Kaplan DL. Mechanism of silk processing in insects and spiders. Nature. 2003;424:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 873] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 34. | Rockwood DN, Preda RC, Yücel T, Wang X, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6:1612-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1837] [Article Influence: 131.2] [Reference Citation Analysis (0)] |
| 35. | Vepari C, Kaplan DL. Silk as a Biomaterial. Prog Polym Sci. 2007;32:991-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2256] [Cited by in RCA: 1607] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 36. | Teuschl AH, van Griensven M, Redl H. Sericin removal from raw Bombyx mori silk scaffolds of high hierarchical order. Tissue Eng Part C Methods. 2014;20:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, Kaplan DL. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131-4141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 540] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 38. | Horan RL, Toponarski I, Boepple HE, Weitzel PP, Richmond JC, Altman GH. Design and characterization of a scaffold for anterior cruciate ligament engineering. J Knee Surg. 2009;22:82-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Chen X, Qi YY, Wang LL, Yin Z, Yin GL, Zou XH, Ouyang HW. Ligament regeneration using a knitted silk scaffold combined with collagen matrix. Biomaterials. 2008;29:3683-3692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. | Fan H, Liu H, Wong EJ, Toh SL, Goh JC. In vivo study of anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold. Biomaterials. 2008;29:3324-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 41. | Liu H, Fan H, Toh SL, Goh JC. A comparison of rabbit mesenchymal stem cells and anterior cruciate ligament fibroblasts responses on combined silk scaffolds. Biomaterials. 2008;29:1443-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Altman GH, Horan RL, Weitzel P, Richmond JC. The use of long-term bioresorbable scaffolds for anterior cruciate ligament repair. J Am Acad Orthop Surg. 2008;16:177-187. [PubMed] |
| 43. | Fan H, Liu H, Toh SL, Goh JC. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials. 2009;30:4967-4977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Serica Technologies I. SeriACLTM Device (Gen IB) Trial for Anterior Cruciate Ligament (ACL) Repair (NCT00775892) 2008. Available from: http://clinicaltrials.gov/ct2/show/NCT00490594. |
| 45. | Petrigliano FA, McAllister DR, Wu BM. Tissue engineering for anterior cruciate ligament reconstruction: a review of current strategies. Arthroscopy. 2006;22:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Lin VS, Lee MC, O’Neal S, McKean J, Sung KL. Ligament tissue engineering using synthetic biodegradable fiber scaffolds. Tissue Eng. 1999;5:443-452. [PubMed] |
| 47. | Buma P, Kok HJ, Blankevoort L, Kuijpers W, Huiskes R, Van Kampen A. Augmentation in anterior cruciate ligament reconstruction-a histological and biomechanical study on goats. Int Orthop. 2004;28:91-96. [PubMed] |
| 48. | Lu HH, Cooper JA, Manuel S, Freeman JW, Attawia MA, Ko FK, Laurencin CT. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials. 2005;26:4805-4816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 49. | Laurencin CT, Freeman JW. Ligament tissue engineering: an evolutionary materials science approach. Biomaterials. 2005;26:7530-7536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 50. | Freeman JW, Woods MD, Laurencin CT. Tissue engineering of the anterior cruciate ligament using a braid-twist scaffold design. J Biomech. 2007;40:2029-2036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 51. | Freeman JW, Woods MD, Cromer DA, Wright LD, Laurencin CT. Tissue engineering of the anterior cruciate ligament: the viscoelastic behavior and cell viability of a novel braid-twist scaffold. J Biomater Sci Polym Ed. 2009;20:1709-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Freeman JW, Woods MD, Cromer DA, Ekwueme EC, Andric T, Atiemo EA, Bijoux CH, Laurencin CT. Evaluation of a hydrogel-fiber composite for ACL tissue engineering. J Biomech. 2011;44:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Cardwell RD, Dahlgren LA, Goldstein AS. Electrospun fibre diameter, not alignment, affects mesenchymal stem cell differentiation into the tendon/ligament lineage. J Tissue Eng Regen Med. 2014;8:937-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 54. | James R, Toti US, Laurencin CT, Kumbar SG. Electrospun nanofibrous scaffolds for engineering soft connective tissues. Methods Mol Biol. 2011;726:243-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Chen J, Altman GH, Karageorgiou V, Horan R, Collette A, Volloch V, Colabro T, Kaplan DL. Human bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. J Biomed Mater Res A. 2003;67:559-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 56. | Sinclair K, Yerkovich ST, Chambers DC. Mesenchymal stem cells and the lung. Respirology. 2013;18:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 57. | Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, Megges M, Prigione A, Adjaye J, Kassem M, Aldahmash A. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. 2013;9:32-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 271] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 58. | Pendleton C, Li Q, Chesler DA, Yuan K, Guerrero-Cazares H, Quinones-Hinojosa A. Mesenchymal stem cells derived from adipose tissue vs bone marrow: in vitro comparison of their tropism towards gliomas. PLoS One. 2013;8:e58198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 59. | Ong WK, Sugii S. Adipose-derived stem cells: fatty potentials for therapy. Int J Biochem Cell Biol. 2013;45:1083-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 60. | Keibl C, Fügl A, Zanoni G, Tangl S, Wolbank S, Redl H, van Griensven M. Human adipose derived stem cells reduce callus volume upon BMP-2 administration in bone regeneration. Injury. 2011;42:814-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Peterbauer-Scherb A, van Griensven M, Meinl A, Gabriel C, Redl H, Wolbank S. Isolation of pig bone marrow mesenchymal stem cells suitable for one-step procedures in chondrogenic regeneration. J Tissue Eng Regen Med. 2010;4:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Butler DL, Gooch C, Kinneberg KR, Boivin GP, Galloway MT, Nirmalanandhan VS, Shearn JT, Dyment NA, Juncosa-Melvin N. The use of mesenchymal stem cells in collagen-based scaffolds for tissue-engineered repair of tendons. Nat Protoc. 2010;5:849-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Cheng MT, Yang HW, Chen TH, Lee OK. Isolation and characterization of multipotent stem cells from human cruciate ligaments. Cell Prolif. 2009;42:448-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Steinert AF, Kunz M, Prager P, Barthel T, Jakob F, Nöth U, Murray MM, Evans CH, Porter RM. Mesenchymal stem cell characteristics of human anterior cruciate ligament outgrowth cells. Tissue Eng Part A. 2011;17:1375-1388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 65. | Nagineni CN, Amiel D, Green MH, Berchuck M, Akeson WH. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 146] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Huang TF, Chen YT, Yang TH, Chen LL, Chiou SH, Tsai TH, Tsai CC, Chen MH, Ma HL, Hung SC. Isolation and characterization of mesenchymal stromal cells from human anterior cruciate ligament. Cytotherapy. 2008;10:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Ge Z, Goh JC, Lee EH. Selection of cell source for ligament tissue engineering. Cell Transplant. 2005;14:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 68. | Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, Makino H, Umezawa A, Sekiya I. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology (Oxford). 2008;47:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 69. | Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, Dube JL, DiBlasio-Smith E, Nove J, Song JJ. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 407] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 70. | Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand. 1999;70:51-54. [PubMed] |
| 71. | Forslund C, Aspenberg P. CDMP-2 induces bone or tendon-like tissue depending on mechanical stimulation. J Orthop Res. 2002;20:1170-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Letson AK, Dahners LE. The effect of combinations of growth factors on ligament healing. Clin Orthop Relat Res. 1994;207-212. [PubMed] |
| 73. | Lyras DN, Kazakos K, Verettas D, Chronopoulos E, Folaranmi S, Agrogiannis G. Effect of combined administration of transforming growth factor-b1 and insulin-like growth factor I on the mechanical properties of a patellar tendon defect model in rabbits. Acta Orthop Belg. 2010;76:380-386. [PubMed] |
| 74. | Wei X, Mao Z, Hou Y, Lin L, Xue T, Chen L, Wang H, Yu C. Local administration of TGFβ-1/VEGF165 gene-transduced bone mesenchymal stem cells for Achilles allograft replacement of the anterior cruciate ligament in rabbits. Biochem Biophys Res Commun. 2011;406:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 75. | Yasuda K, Tomita F, Yamazaki S, Minami A, Tohyama H. The effect of growth factors on biomechanical properties of the bone-patellar tendon-bone graft after anterior cruciate ligament reconstruction: a canine model study. Am J Sports Med. 2004;32:870-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Li F, Jia H, Yu C. ACL reconstruction in a rabbit model using irradiated Achilles allograft seeded with mesenchymal stem cells or PDGF-B gene-transfected mesenchymal stem cells. Knee Surg Sports Traumatol Arthrosc. 2007;15:1219-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Nakamura N, Shino K, Natsuume T, Horibe S, Matsumoto N, Kaneda Y, Ochi T. Early biological effect of in vivo gene transfer of platelet-derived growth factor (PDGF)-B into healing patellar ligament. Gene Ther. 1998;5:1165-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 117] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | Thomopoulos S, Das R, Silva MJ, Sakiyama-Elbert S, Harwood FL, Zampiakis E, Kim HM, Amiel D, Gelberman RH. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res. 2009;27:1209-1215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 79. | Hildebrand KA, Woo SL, Smith DW, Allen CR, Deie M, Taylor BJ, Schmidt CC. The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study. Am J Sports Med. 1998;26:549-554. [PubMed] |
| 80. | Kurtz CA, Loebig TG, Anderson DD, DeMeo PJ, Campbell PG. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am J Sports Med. 1999;27:363-369. [PubMed] |
| 81. | de Mos M, van der Windt AE, Jahr H, van Schie HT, Weinans H, Verhaar JA, van Osch GJ. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 345] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 82. | Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1223] [Cited by in RCA: 1225] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 83. | Chen L, Dong SW, Tao X, Liu JP, Tang KL, Xu JZ. Autologous platelet-rich clot releasate stimulates proliferation and inhibits differentiation of adult rat tendon stem cells towards nontenocyte lineages. J Int Med Res. 2012;40:1399-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Mastrangelo AN, Vavken P, Fleming BC, Harrison SL, Murray MM. Reduced platelet concentration does not harm PRP effectiveness for ACL repair in a porcine in vivo model. J Orthop Res. 2011;29:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 85. | Fernández-Sarmiento JA, Domínguez JM, Granados MM, Morgaz J, Navarrete R, Carrillo JM, Gómez-Villamandos RJ, Muñoz-Rascón P, Martín de Las Mulas J, Millán Y. Histological study of the influence of plasma rich in growth factors (PRGF) on the healing of divided Achilles tendons in sheep. J Bone Joint Surg Am. 2013;95:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 86. | Figueroa P D, Figueroa B F, Ahumada P X, Calvo R R, Vaisman B A. Use of platelet rich plasma in knee ligament surgery. Rev Med Chil. 2013;141:1315-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 87. | Yuan T, Zhang CQ, Wang JH. Augmenting tendon and ligament repair with platelet-rich plasma (PRP). Muscles Ligaments Tendons J. 2013;3:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 88. | Hoffmann A, Pelled G, Turgeman G, Eberle P, Zilberman Y, Shinar H, Keinan-Adamsky K, Winkel A, Shahab S, Navon G. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest. 2006;116:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 89. | Woo SL, Gomez MA, Sites TJ, Newton PO, Orlando CA, Akeson WH. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J Bone Joint Surg Am. 1987;69:1200-1211. [PubMed] |
| 90. | Woo SL, Debski RE, Withrow JD, Janaushek MA. Biomechanics of knee ligaments. Am J Sports Med. 1999;27:533-543. [PubMed] |
| 91. | Woo SL, Gomez MA, Woo YK, Akeson WH. Mechanical properties of tendons and ligaments. II. The relationships of immobilization and exercise on tissue remodeling. Biorheology. 1982;19:397-408. [PubMed] |
| 92. | Tetsunaga T, Furumatsu T, Abe N, Nishida K, Naruse K, Ozaki T. Mechanical stretch stimulates integrin alphaVbeta3-mediated collagen expression in human anterior cruciate ligament cells. J Biomech. 2009;42:2097-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Henshaw DR, Attia E, Bhargava M, Hannafin JA. Canine ACL fibroblast integrin expression and cell alignment in response to cyclic tensile strain in three-dimensional collagen gels. J Orthop Res. 2006;24:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 94. | Berry CC, Shelton JC, Bader DL, Lee DA. Influence of external uniaxial cyclic strain on oriented fibroblast-seeded collagen gels. Tissue Eng. 2003;9:613-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 95. | Altman GH, Lu HH, Horan RL, Calabro T, Ryder D, Kaplan DL, Stark P, Martin I, Richmond JC, Vunjak-Novakovic G. Advanced bioreactor with controlled application of multi-dimensional strain for tissue engineering. J Biomech Eng. 2002;124:742-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 96. | Altman GH, Horan RL, Martin I, Farhadi J, Stark PR, Volloch V, Richmond JC, Vunjak-Novakovic G, Kaplan DL. Cell differentiation by mechanical stress. FASEB J. 2002;16:270-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 418] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 97. | Vunjak-Novakovic G, Altman G, Horan R, Kaplan DL. Tissue engineering of ligaments. Annu Rev Biomed Eng. 2004;6:131-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 98. | Petrigliano FA, English CS, Barba D, Esmende S, Wu BM, McAllister DR. The effects of local bFGF release and uniaxial strain on cellular adaptation and gene expression in a 3D environment: implications for ligament tissue engineering. Tissue Eng. 2007;13:2721-2731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | Berry CC, Cacou C, Lee DA, Bader DL, Shelton JC. Dermal fibroblasts respond to mechanical conditioning in a strain profile dependent manner. Biorheology. 2003;40:337-345. [PubMed] |
| 100. | Park SA, Kim IA, Lee YJ, Shin JW, Kim CR, Kim JK, Yang YI, Shin JW. Biological responses of ligament fibroblasts and gene expression profiling on micropatterned silicone substrates subjected to mechanical stimuli. J Biosci Bioeng. 2006;102:402-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 101. | Moreau JE, Bramono DS, Horan RL, Kaplan DL, Altman GH. Sequential biochemical and mechanical stimulation in the development of tissue-engineered ligaments. Tissue Eng Part A. 2008;14:1161-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 102. | Rathbone S, Maffulli N, Cartmell SH. Most british surgeons would consider using a tissue-engineered anterior cruciate ligament: a questionnaire study. Stem Cells Int. 2012;2012:303724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Hohlrieder M, Teuschl AH, Cicha K, van Griensven M, Redl H, Stampfl J. Bioreactor and scaffold design for the mechanical stimulation of anterior cruciate ligament grafts. Biomed Mater Eng. 2013;23:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 104. | Murray MM, Fleming BC. Biology of anterior cruciate ligament injury and repair: Kappa delta ann doner vaughn award paper 2013. J Orthop Res. 2013;31:1501-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 105. | Vavken P, Fleming BC, Mastrangelo AN, Machan JT, Murray MM. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy. 2012;28:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 106. | Murray MM, Magarian E, Zurakowski D, Fleming BC. Bone-to-bone fixation enhances functional healing of the porcine anterior cruciate ligament using a collagen-platelet composite. Arthroscopy. 2010;26:S49-S57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 107. | Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37:2401-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 108. | Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41:1762-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |