Published online Jul 18, 2013. doi: 10.5312/wjo.v4.i3.124
Revised: March 22, 2013
Accepted: May 9, 2013
Published online: July 18, 2013
Processing time: 214 Days and 2.8 Hours
AIM: To investigate the in vivo effects of type I diabetes on the mechanical strength of tibial bone in a rodent model.
METHODS: The biomechanical effect of diabetes on the structural integrity of the tibia in streptozotocin induced diabetic Wistar rats was analysed. Induction of diabetes was achieved by an intra-peritoneal injection and confirmed by measuring serial blood glucose levels (> 150 mg/dL). After 8 wk the tibiae were harvested and compared to a control group. Biomechanical analysis of harvested tibiae was performed using a three-point bending technique on a servo hydraulic MTS 858 MiniBionix frame. Maximum force applied to failure (N), stiffness (N × mm) and energy absorbed (N/mm) were recorded and plotted on load displacement curves. A displacement control loading mode of 1 mm/min was selected to simulate quasi-static loading conditions. Measurements from load-displacement curves were directly compared between groups.
RESULTS: Fourteen streptozotocin induced diabetic Wistar rats were compared against nineteen non-diabetic controls. An average increase of 155.2 g in body weight was observed in the control group compared with only 5 g in the diabetic group during the experimental study period. Levels of blood glucose increased to 440.25 mg/dL in the diabetic group compared to 116.62 mg/dL in the control group.The biomechanical results demonstrate a highly significant reduction in the maximum load to failure from 69.5 N to 58 N in diabetic group compared to control (P = 0.011). Energy absorption to fracture was reduced from 28.2 N in the control group to 23.5 N in the diabetic group (P = 0.082). No significant differences were observed between the groups for bending stiffness.
CONCLUSION: Streptozotocin-induced diabetes in rodents reduces the maximum force and energy absorption to failure of bone, suggesting a predisposition for fracture risk.
Core tip: The bones of streptozotocin-induced diabetic Wistar rats are more fragile with reduced toughness, characterized by a reduction in the capacity to absorb energy and with lower forces required to induce fracture in comparison to those in the control group. Our findings confirm previous studies and lend weight to the literature describing the detrimental relationship between the mechanical properties of bone subjected to diabetes mellitus. Further research needs to be conducted to ascertain whether uncontrolled diabetes in a human population affects the structural and biomechanical properties of bone.
-
Citation: Korres N, Tsiridis E, Pavlou G, Mitsoudis A, Perrea DN, Zoumbos AB. Biomechanical characteristics of bone in streptozotocin-induced diabetic rats: An
in-vivo randomized controlled experimental study. World J Orthop 2013; 4(3): 124-129 - URL: https://www.wjgnet.com/2218-5836/full/v4/i3/124.htm
- DOI: https://dx.doi.org/10.5312/wjo.v4.i3.124
Bone is a composite of organic collagen and inorganic crystalline hydroxyapatite. Bone loss in diabetes mellitus (DM) has been attributed to metabolic abnormalities, abnormal calcium concentration within cells, and high blood glucose levels[1]. DM interferes with the formation of the collagen network, therefore affecting the biomechanical integrity of bone. Any basis for reduction in bone integrity and material strength has yet to be accurately defined. A lack of insulin in in vitro experiments results in a reduction in ossification and calcification and a reduction in cartilage formation[2]. Furthermore, in rat studies the proliferation of osteoblasts and nucleotide synthesis[3,4] in vitro are associated with insulin binding through expression of insulin receptors[5]. Production of advanced glycation end products has also been implicated in the reduction of bone material strength in diabetes, through the non-enzymatic cross-linking of collagen[6,7]. Despite molecular evidence for this theory, mechanical data on the effect of diabetes on bone remain conflicting and sparse.
Clinical and experimental studies demonstrate that diabetes is associated with molecular and cellular changes with resultant alterations to bone physiology[8]. Patients with type 1 DM have been observed to exhibit a disproportionately high risk of fracture with reduced bone mass, leading to speculation that diabetic bone has reduced strength[9,10]. Furthermore studies indicate that diabetes exerts a similar effect on bone to that observed in the normal ageing process, with a predisposition to fracture susceptibility, delayed union and osteoporosis[11]. The biomechanical properties of bone in diabetes have been poorly addressed in the literature with conflicting results. Fleischli et al[12] demonstrated no differences in the material properties of human metatarsal bones when comparing younger diabetics to older non-diabetic donors. In a subsequent study on cadaveric human tibiae no significant differences were demonstrated between diabetic and non-diabetic specimens[13]. Animal studies suggest a reduction in bone mineral density as a direct consequence of DM. This is exhibited by bone loss in trabecular bone and failure to accrue cortical bone due to premature cessation of growth[14]. Further biomechanical experimental animal studies have demonstrated either increased stiffness[9,15] or reduced stiffness[16-18]. These variances are confusing but may be accounted for due to a number of factors. Stiffness as an indicator of overall bone strength alone is not the only significant biomechanical factor that can be affected by diabetes. Taken in isolation, changes in stiffness may be a consequence of a reduction in total whole bone strength. Any decoupling of stiffness or strength as a ratio may account for the reported differences observed in studies. Changes to strength may not only be an effect of the material strength of the tissues but also a consequence of differences in the size and shape of the bone being tested. Furthermore, the length of time that the rats were exposed to a diabetic state may also account for differences in results.
The aim of the current study is to examine and quantify the mechanical behavior of bone in streptozotocin-induced diabetic rats compared to normal controls.
The experimental protocol was ethically approved by the General Directorate of Veterinary Services (license No: K/7559/29-10-09) and by the Bioethics Committee of University of Athens Medical School, Hellas. The study was conducted in accordance with Hellenic legislation for experimental animal studies (P.D.160/91) and in compliance with European Union law (86/609/EEN.2015/92) and the Convention on Vertebrate Animals Protection for experimental or other scientific purposes (123/1986). Forty male Wistar rats aged 3 mo, weighing between 200-300 g were supplied by the Institute Pasteur. Wistar rats represent a close homology to the human type 1 DM phenotype, demonstrating comparable genetic and physiological characteristics. All animals had free access to food and water. Animals were randomly assigned to a control (C) or diabetic (D) group. Those assigned to the D group were induced to a diabetic state by an intra-peritoneal injection of streptozotocin at a dose of 55 mg/kg body weight. Streptozotocin is an agent known to be specifically toxic to the beta cells in the islets of Langerhans in the pancreas. The mechanism of action is thought to be mediated by alkylation of DNA bases, resulting in reduction of nicotinamide adenine dinucleotide. This, therefore, eliminates production of insulin and induces a hyperglycaemic state. After 1 wk, body weight estimation, and glucose blood sampling was conducted to determine animals in a hyperglycemic state, defined as blood sugar > 150 mg/dL. Twenty-six animals were originally induced with streptozotocin to a diabetic state. Seven out of those 26 animals were excluded from the study (3 died and 4 did not respond) leaving 19 animals in the D group. The diabetic state was defined as polyuria and minimal weight gain post streptozotocin injection. Fourteen rats remained in the C group and compared with the 19 rats in the D group. Eight weeks after induction of diabetes, the animals were euthanized according to the Convention on Vertebrate Animals Protection for experimental scientific purposes (123/1986) using isoflurane gas and sodium-pentobarbital. Tibial bones were carefully dissected of soft tissue from each animal in each group, isolated and harvested for mechanical testing.
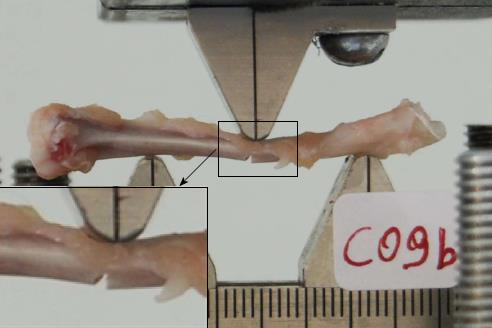
Biomechanical analysis was performed by three-point bending mechanical tests. The experiments were conducted using a servo hydraulic MTS 858 Mini Bionix frame (MTS Systems, Eden Prairie, MN, United States). Tibiae were placed horizontally on the frame on rounded edges at a distance of 24 mm. Attention was paid to ensure all the specimens were placed in exactly the same manner with regards to position and orientation in an effort to minimize variability. The load was applied at the mid-shaft of the diaphysis using a punch with a rounded notch (Figure 1). The displacement control loading mode was selected. The rate of the imposed displacement was selected as 1 mm/min in an effort to simulate quasi-static loading conditions. The displacement was imposed continuously until fracture. The load-displacement curves and the maximal load at fracture in Newtons (N) were recorded. Failure was defined and observed by a propagation of an almost vertical fracture starting almost universally at the lower cortical bone surface. This is expected in bending tests of a brittle material because of the relatively lower tensile strengths compared to the respective opposite compressive strength.
Maximum force applied to failure (N), stiffness (N × mm) and energy absorbed (N/mm) were recorded and plotted on load-displacement curves. The maximum load is represented by the maximum compressive force applied until fracture. Deformation (strain) was defined as the degree of transverse displacement at the loading point. The initial non-linear curve corresponds to the adaptation of the specimens on the rounded edges of the loading device. The almost perfectly linear portion represents the linear elastic behavior of the tissue and the slope is equal to the stiffness. The nonlinear portion corresponds to the non-elastic (plastic) behavior of the tissue.
Statistical analysis on the groups was conducted using analysis of variance. An overall P value of < 0.05 was considered to be statistically significant.
Table 1 demonstrates weight measurements and glucose measurements of both groups over the experimental study period. An average increase of 155.2 g in body weight was observed in the C group compared with only 5 g in the D group during the experimental study period. Levels of blood glucose increased to 440.25 mg/dL in the D group compared to 116.62 mg/dL in the C group.
| Prior to induction | One weekpost-induction | Ateuthanasia | ||||
| C group | D group | C group | D group | C group | D group | |
| Weight (g) | 263.5 | 272.5 | 367 | 284.7 | 418.7 | 277.5 |
| Glucose (mg/dL) | 135.5 | 128.5 | 121.37 | 359.37 | 116.62 | 440.25 |
Table 2 summarizes the differences in maximum force to failure, stiffness and energy-absorbed values recorded between the two groups. Maximal load to failure in C was 69.5 ± 10.3 N (mean ± SD) compared with a reduction to 58 ± 13.2 N (mean ± SD) in the D group. This demonstrated a statistically significant difference (P = 0.011). Stiffness measurements demonstrated no significant differences between the two groups. A statistically significant reduction (P = 0.019) was observed in measurements for energy absorption from 28.2 ± 5 N in the control group to 23.5 ± 5.6 N in the D group.
| Max force (N) | Stiffness (N × mm) | Energy (N/mm) | |
| Diabetic group | 58.0 ± 13.2 | 101.1 ± 30.2 | 23.5 ± 5.6 |
| Control group | 69.5 ± 10.3 | 118.4 ± 23.0 | 28.2 ± 5.0 |
| P value | 0.011 | 0.082 | 0.019 |
Our experimental randomized controlled study demonstrated the effect of diabetes on bone strength.
The literature on the association between bone strength and fracture risk in DM remains weak. The bone changes directly observed in DM can be attributed to a multitude of interrelated factors. Both the material and geometric properties of bone are implicated in influencing mechanical strength. Macroscopic structure (size and shape), architecture (cortical and cancellous components) and the bone substance (organic and inorganic components) are all influenced by DM. Furthermore, changes in collagen, elastin and proteoglycan concentrations, formation of advanced glycation end products and the orientation of collagen fibers are all important determinants of mechanical integrity, which are also affected.
In our study, we aimed to directly quantify whether an uncontrolled diabetic state directly alters the mechanical properties of appendicular long bone of tibiae, to add to the quality of published evidence that DM adversely affects bone quality. Immature rodents were used in an attempt to mimic the presentation of DM in humans, which typically occurs prior to skeletal maturity. Wistar rats were selected for the animal model as this genotype represents a close homology to DM in humans. We observed statistically significant changes in the D group, with reductions in maximum force and energy absorbed to failure demonstrating that the DM alters mechanical properties after as little as 8 wk. Interestingly, in our study we found no statistically significant differences in stiffness between C and D groups. Reported information on stiffness is conflicting in the literature, with some demonstrating increased[9] and other decreased values[16,18]. These variances are confusing but may be accounted to a number of factors. Stiffness as an indicator of overall bone strength alone is not the only significant biomechanical factor that can be affected by diabetes. Taken in isolation, changes in stiffness may be a consequence of a reduction in total bone strength. Any decoupling of stiffness or strength as a ratio may account for the reported differences observed in studies. The changes to strength therefore may not only be an effect of material strength of the tissues but also as a consequence of differences in the size and shape of the bone being tested. When values for stiffness are normalized against the geometry and structural shape of the bone, these differences in stiffness can be accounted for.
A number of experimental rodent studies exist documenting the biomechanical effects of bone in streptozotocin induced DM. These studies exhibit various experimental protocols. To our knowledge, eight studies exist evaluating bone mechanics using a type 1 diabetic model in rodent studies[9,14-16,19-22]. All these studies exhibit differences in their methodology, specifically duration of induced DM, species of rodent and diabetogen used to initiate DM. However, despite their differences these studies consistently demonstrate reductions in ultimate force to failure in the D groups, in keeping with the result of our study. The three studies which report on values for energy to failure[15,16,22] all demonstrate a reduction in energy to failure in the diabetic groups, in keeping with our analysis.
Our findings lend weight to the argument that DM (in a type 1 DM rodent model) reduces the mechanical behavior of bone. However, the changes that occur are probably not only to be result of changes in mechanical properties but are probably also due to inherent detrimental changes which occur in the structural material properties. This theory has been confirmed in a mouse model where significant differences were observed in the strength-structure relationship, with reductions to the tissue mineral density of bone in DM, which became apparent after only 10 wk[23].
Our experimental model subjected the D group of rats to uncontrolled levels of hyperglycaemia. This experimental protocol represents a scenario, which would only be representative of the small proportion of the human DM population who poorly control their blood glucose levels. It is likely be that our experimental model represents a worse case scenario. Conversely the duration that the rats were exposed to an uncontrolled hyperglycaemic state was only 8 wk. This is unlikely to represent the chronic human diabetic state. It is possible that subjecting the animals to a longer period of hyperglycaemia would cause further deterioration in the material properties of bone. This has been confirmed by Nyman et al[23] in a mouse model exposed to DM for up to 18 wk, in which further deterioration in mechanical properties was observed with longer exposure.
There are several limitations to our study. Firstly the duration of induced DM was only 8 wk, which may not be representative of the changes that occur chronically. It could be postulated that any effects on mechanical properties could be under-estimated and the further detrimental changes could have occurred if DM was allowed to continue. The experimental model also represents a scenario where DM remains unchecked with hyperglycemia allowed to develop without control. This model is unlikely to be representative of a clinical scenario where DM is treated and therefore represents a worse case presentation. Furthermore, this study did not investigate the effects of DM on bone structure and architecture. No histomorphometric analysis was conducted to investigate whether the mechanical changes to the material observed, exhibited correlation to bone quality.
Although the pathogenesis of osteopenia in diabetes is a poorly understood phenomenon, decreased bone formation, mineralization and absorption seem to be associated with inferior mechanical properties of bone turnover in diabetes. These changes may be not so frequently observed in humans, as most people do not allow serum glucose levels to go unchecked and manage DM with strict administration of insulin therapy. Significant changes to bone biomechanics may, therefore, only be representative of a small cohort of human diabetics who have long-standing prolonged disease which is resistant to or poorly controlled by insulin therapy.
The bones of streptozotocin-induced diabetic Wistar rats are more fragile with reduced toughness characterized by a reduction in the capacity to absorb energy and with lower force required to induce fracture, in comparison to those in the control group. Our findings confirm previous studies and add weight to the literature investigating the detrimental relationship between the mechanical properties of bone subjected to DM.
Clinical and experimental studies demonstrate that diabetes is associated with molecular and cellular changes with resultant alterations to bone physiology. Patients with type 1 diabetes mellitus (DM) have been observed to exhibit a disproportionately high risk of fracture with reduced bone mass, leading to speculation that diabetic bone has reduced strength. The resultant biomechanical changes and properties of bone in DM have been poorly addressed in the literature with conflicting results.
Experimental studies demonstrating alterations to physiology and structural changes to bone in DM is sparse and conflicting. Uncontrolled DM has been suggested to result in detrimental biomechanical properties of bone. Furthermore, studies indicate that diabetes exerts a similar effect on bone to that observed in the normal ageing process, with a predisposition to fracture susceptibility, delayed union and osteoporosis.
This study lends weight to the literature that an uncontrolled DM state in a rodent population induced by streptozotocin results in reduction in the biomechanical properties of bone, specifically with reduction in ultimate strength to failure and capacity to absorb energy.
By understanding how an uncontrolled diabetic state detrimentally alters bone biomechanics, future strategies to target diabetic patients may result in better bone health and reduction in osteoporosis and fracture risk.
Wistar rats represent a close homology to the human type 1 DM phenotype, demonstrating comparable genetic and physiological characteristics. Thus, they likely to be the closest animal model representative of an uncontrolled diabetic state in humans.
The authors examined the effects of an induced DM state and its relationship to the biomechanical properties of bone. Results revealed decreased bone quality and reduction in energy absorbing capacity. The results are interesting and may represent a close homology to the effects seen in the bone health of patients who have DM.
P- Reviewer Laborde M S- Editor Zhai HH L- Editor Hughes D E- Editor Ma S
| 1. | Gevins AS, Zeitlin GM, Yingling CD, Doyle JC, Dedon MF, Schaffer RE, Roumasset JT, Yeager CL. EEG patterns during ‘cognitive’ tasks. I. Methodology and analysis of complex behaviors. Electroencephalogr Clin Neurophysiol. 1979;47:693-703. [PubMed] |
| 2. | Fiorelli G, Orlando C, Benvenuti S, Franceschelli F, Bianchi S, Pioli P, Tanini A, Serio M, Bartucci F, Brandi ML. Characterization, regulation, and function of specific cell membrane receptors for insulin-like growth factor I on bone endothelial cells. J Bone Miner Res. 1994;9:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Hickman J, McElduff A. Insulin promotes growth of the cultured rat osteosarcoma cell line UMR-106-01: an osteoblast-like cell. Endocrinology. 1989;124:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Peck WA, Messinger K. Nucleoside and ribonucleic acid metabolism in isolated bone cells. Effects of insulin and cortisol in vitro. J Biol Chem. 1970;245:2722-2729. [PubMed] |
| 5. | Levy JR, Murray E, Manolagas S, Olefsky JM. Demonstration of insulin receptors and modulation of alkaline phosphatase activity by insulin in rat osteoblastic cells. Endocrinology. 1986;119:1786-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Garnero P, Borel O, Gineyts E, Duboeuf F, Solberg H, Bouxsein ML, Christiansen C, Delmas PD. Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone. 2006;38:300-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 362] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 8. | Armas LA, Akhter MP, Drincic A, Recker RR. Trabecular bone histomorphometry in humans with Type 1 Diabetes Mellitus. Bone. 2012;50:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Funk JR, Hale JE, Carmines D, Gooch HL, Hurwitz SR. Biomechanical evaluation of early fracture healing in normal and diabetic rats. J Orthop Res. 2000;18:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Schwartz AV. Diabetes Mellitus: Does it Affect Bone. Calcif Tissue Int. 2003;73:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Räkel A, Sheehy O, Rahme E, LeLorier J. Osteoporosis among patients with type 1 and type 2 diabetes. Diabetes Metab. 2008;34:193-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Fleischli JG, Laughlin TJ, Lavery LA, Shah B, Lanctot D, Agrawal CM, Athanasiou K. The effects of diabetes mellitus on the material properties of human metatarsal bones. J Foot Ankle Surg. 1998;37:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Fleischli JG, Laughlin TJ, Athanasiou K, Lanctot DR, Lavery L, Wang X, Agrawal CM. Effect of diabetes mellitus on the material properties of the distal tibia. J Am Podiatr Med Assoc. 2006;96:91-95. [PubMed] |
| 14. | Silva MJ, Brodt MD, Lynch MA, McKenzie JA, Tanouye KM, Nyman JS, Wang X. Type 1 diabetes in young rats leads to progressive trabecular bone loss, cessation of cortical bone growth, and diminished whole bone strength and fatigue life. J Bone Miner Res. 2009;24:1618-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Reddy GK, Stehno-Bittel L, Hamade S, Enwemeka CS. The biomechanical integrity of bone in experimental diabetes. Diabetes Res Clin Pract. 2001;54:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Einhorn TA, Boskey AL, Gundberg CM, Vigorita VJ, Devlin VJ, Beyer MM. The mineral and mechanical properties of bone in chronic experimental diabetes. J Orthop Res. 1988;6:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Erdal N, Gürgül S, Kavak S, Yildiz A, Emre M. Deterioration of bone quality by streptozotocin (STZ)-induced type 2 diabetes mellitus in rats. Biol Trace Elem Res. 2011;140:342-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Verhaeghe J, Suiker AM, Einhorn TA, Geusens P, Visser WJ, Van Herck E, Van Bree R, Magitsky S, Bouillon R. Brittle bones in spontaneously diabetic female rats cannot be predicted by bone mineral measurements: studies in diabetic and ovariectomized rats. J Bone Miner Res. 1994;9:1657-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Beam HA, Parsons JR, Lin SS. The effects of blood glucose control upon fracture healing in the BB Wistar rat with diabetes mellitus. J Orthop Res. 2002;20:1210-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Facchini DM, Yuen VG, Battell ML, McNeill JH, Grynpas MD. The effects of vanadium treatment on bone in diabetic and non-diabetic rats. Bone. 2006;38:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Macey LR, Kana SM, Jingushi S, Terek RM, Borretos J, Bolander ME. Defects of early fracture-healing in experimental diabetes. J Bone Joint Surg Am. 1989;71:722-733. [PubMed] |
| 22. | Zhang SQ, Chen GH, Lu WL, Zhang Q. Effects on the bones of vanadyl acetylacetonate by oral administration: a comparison study in diabetic rats. J Bone Miner Metab. 2007;25:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Nyman JS, Even JL, Jo CH, Herbert EG, Murry MR, Cockrell GE, Wahl EC, Bunn RC, Lumpkin CK, Fowlkes JL. Increasing duration of type 1 diabetes perturbs the strength-structure relationship and increases brittleness of bone. Bone. 2011;48:733-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |