Revised: August 25, 2011
Accepted: August 31, 2011
Published online: September 18, 2011
Massive segmental bone loss due to chronic osteomyelitis represents a considerable challenge to orthopedic surgeons and is a limb threatening condition. The only option available in such a clinical situation is segment transport using the Ilizarov technique of distraction osteogenesis; yet the most common problem in cases of bone transport with the Ilizarov technique in massive bone loss, is the long duration of the fixator. In addition to autologous bone grafting, several mechanical, biologic, and external physical treatment modalities may be employed to promote bone formation and maturation during segment transport in osteomyelitis patients. Mechanical approaches include compressive loading of the distraction regenerate, increased frequency of small increments of distraction, and compression-distraction. Intramedullary nailing and hemicorticotomy can reduce the time in external fixation; however, these techniques are associated with technical difficulties and complications. Exogenous application of low-intensity pulsed ultrasound or pulsed electromagnetic fields may shorten the duration of external fixation. Other promising modalities include diphosphonates, physician-directed use (off-label use) of bone morphogenetic proteins, and local injection of bone marrow aspirate and platelet gel at the osteotomy site. Well-designed clinical studies are needed to establish safe and effective guidelines for various modalities to enhance new bone formation during distraction osteogenesis after segment transfer.
- Citation: Emara KM, Ghafar KAA, Kersh MAA. Methods to shorten the duration of an external fixator in the management of tibial infections. World J Orthop 2011; 2(9): 85-92
- URL: https://www.wjgnet.com/2218-5836/full/v2/i9/85.htm
- DOI: https://dx.doi.org/10.5312/wjo.v2.i9.85
Musculoskeletal infections remain a common problem. The treatment strategy for chronic osteomyelitis has changed to a great extent over the past twenty years[1] because of better staging systems, developments in surgical techniques, antibiotics and adjuvant treatment modalities such as hyperbaric oxygen. Chronic osteomyelitis leads to necrosis of bone and soft tissues to a variable extent. The dead bone forms a nidus for hosting pathogens. Moreover, host defense mechanisms are often not in an optimal condition to deal with microorganisms, and antibiotic delivery to the infection site may be impaired because of poor circulation[2]. Cierny et al[3] classified chronic osteomyelitis into four anatomical types, a system that is known as the Cierny-Mader classification, and further staged the pathology according to the extent of the local and systemic compromise in the patient. They developed guidelines for management according to this system.
Appropriate radical debridement requires excision of all necrotic bone and soft tissue, often resulting in limb instability. The unstable situation requires some type of fixation and reconstruction of the resultant bone and soft-tissue defects. Since the introduction of distraction osteogenesis by Ilizarov, the technique has been employed successfully to achieve union, correct deformity, reestablish limb-length equality, and reconstruct segmental defects[4]. The time spent in an external fixator (the external fixation index) depends on the length of distraction required and is not free of complications. When the distraction phase is over, the consolidation phase, which is more than double the distraction time, becomes difficult for the patient to tolerate. It is associated with many complications such as pin-track infection, angulation; post operative scarring, loosening of the frame, malalignment and stiffness of the knee and ankle joints. Removal of the external fixator before satisfactory consolidation has occurred is associated with fracture, deformity, and shortening occurring through the distracted callus[5]. To shorten the duration of an Ilizarov external fixator in management of tibial bone loss, there are different modalities to enhance bone regeneration during the distraction osteogenesis. These modalities include mechanical stimulation of the regenerate, new surgical techniques, exogenous application of low-intensity pulsed ultrasound (LIPU) or pulsed electromagnetic fields (PEMFs), local injection of bone morphogenetic protein (BMP), pluripotent stem cells, and platelet-rich plasma (PRP). Anticatabolic agents, such as diphosphonates, may also be used.
Distraction osteogenesis (DO) consists of three sequential phases that can be controlled by the surgeon. Latency phase is that period immediately following the osteotomy and application of the distractor; it ranges from one to seven days. After the latency phase is the distraction phase. During this phase, the distraction device is activated by turning some type of axial screw, usually at 1 mm/d in four equal increments of 0.25 mm each, to form the regenerate. Once distraction is complete, the third and final phase is the consolidation phase. Typically, the consolidation phase, in which the regenerate will consolidate to form a solid bone, is twice as long as the time required for distraction. The regeneration of new bone formation is increased with low-energy osteotomy or corticotomy, preferably during the latency phase, and is dependent on patient age as well as the quality of the underlying bone and surrounding soft-tissue envelope. Duration of three to ten days is recommended following osteotomy with apposed bone ends[6]. The local inflammatory response, which consists of migration of pluripotential cells with a rich milieu of cytokines and growth factors, facilitates new bone formation during the distraction phase. In this phase, the osteotomized fragments are gradually pulled apart, typically at a rate of 1 mm/d in two to four evenly spaced time increments. This specific rate and frequency of distraction is crucial for reliable generation of new bone in the distraction gap and for adaptive changes and growth in the surrounding soft tissues, including skin, muscles, nerves, blood vessels and the lymphatic system. In the consolidation phase, the regenerate in the distraction gap matures into normal bone that can withstand physiologic loads and remodels over time. In general, the consolidation phase is twice as long as the distraction phase.
The external fixation index denotes the number of days the external fixator is attached to the bone per centimeter of length gained. Using conventional Ilizarov fixation, this index is typically thirty days per centimeter of length gained; however, the rate differs based on variables such as patient age, osteotomy site and amount of lengthening[7,20].
The bone healing index is the time to bony union in months, divided by the amount of lengthening in centimeters. Attempts to accelerate the distraction phase can negatively affect the quality of bone formation, as well as limb function, secondary to the effect of acceleration on neurovascular and musculotendinous structures.
However, several modalities have been used to shorten the consolidation phase of distraction osteogenesis thereby improving these indices.
The regenerate that fills the distraction gap is formed via intramembranous ossification. In the distraction phase, the regenerate between the two osteotomized fragments has a distinct histologic appearance, with five recognizable zones. The central fibrous inter-zone measures 4 to 8 mm in length, with immature collagen bundles and fibroblast-like cells arranged parallel to the distraction force. The fibrous inter-zone is bordered on either side by the primary mineralization front, in which clusters of osteoblast-like cells produce an osteoid-like matrix that consolidates the collagen into longitudinal microcolumns surrounded by capillary buds. The zone of microcolumn formation rests between the native bone of the osteotomized fragment and the primary mineralization front. The primary bone units begin to mineralize in this zone, expanding to a maximum diameter of 150 to 200 μm[6]. The primary bone units cross-link with other units that are surrounded by vascular sinusoids and span the cross-section of the distraction gap.
During the consolidation phase, the primary mineralization front traverses the fibrous inter-zone, followed by the zone of microcolumn formation. In this way, the distraction gap is replaced by mature bone that remodels with formation of the medullary canal and distinct cortices, in accordance with Wolff’s law. Endochondral bone formation has been noted in experimental models of DO, in which new bone formation is delayed secondary to poor blood supply or lack of stable external fixation[6,20].
A valid and reliable means of assessing the quality of new bone formation during DO is essential. Imaging techniques such as ultrasonography[8] and quantitative bone scintigraphy[9] have been investigated. However, plain radiography is used mainly in assessing the quality and quantity of distraction regenerate.
Pattern recognition of the radiographic appearance of new bone formation has typically been used to make clinical decisions regarding the rate of distraction, advancement of weight-bearing status and scheduling the removal of external fixation. Poor healing is suggested by delayed radiographic appearance of bone formation (i.e. > 30 d postosteotomy), multiple radiolucencies in the distraction regenerate, axial deviation of the regenerate, which is suggestive of suboptimal mechanical stability; and hourglass configuration of the regenerate[10,11]. Attempts have been made based on plain radiographic appearance to classify the shape, consistency and polarity of bone regenerate during limb lengthening. Surgeon’s experience with limb lengthening and use of digital image enhancement techniques can improve the reliability and accuracy of radiographic assessment of bone formation during DO[12,20].
Poor bone formation with prolonged external fixation and bone healing indices can take a physical, emotional, and financial toll on patients. A weak regenerate may not withstand physiologic loads and is prone to deformation and fracture following removal of the external fixator[13]. Several factors can affect the volume and quality of bone formation negatively during distraction osteogenesis, including advanced age and a diaphyseal lengthening site. Adequate blood supply and mechanical stability are crucial to the formation of a healthy regenerate. Two clinical studies indicated fracture rates of 8.1% and 9.4% in adult and pediatric patients respectively who underwent limb lengthening with external fixation for a variety of diagnoses[14].
Predisposition to poor bone formation can be attributed to host-related factors as well as local and iatrogenic causes. Host-related factors include use of non-steroidal anti-inflammatory drugs, smoking, congenital etiology, and systemic illness (e.g. diabetes).
Local predisposing factors include a scarred soft-tissue envelope, overlying infection and prior radiation therapy; as well as deformities such as congenital pseudarthrosis of the tibia. Iatrogenic causes include compromised soft-tissue coverage at the osteotomy site, resulting from poor location selection; suboptimal osteotomy technique (e.g. thermal necrosis resulting from use of an oscillating saw); a persistent gap > 1 cm at the osteotomy site during the latency phase; application of a mechanically unstable frame configuration; a short latency phase (< 5 d); and rapid distraction (> 2 mm/d). A recent study of patients undergoing tibial lengthening noted the biologic superiority of Gigli saw osteotomy, especially in the presence of osteopenia[15,20].
There are different methods for improving the quality of regenerate during the consolidation phase; and removing the external fixators after the end of distraction phase during segment transfer.
Controlled, repetitive axial loading of healing fractures can stimulate callus formation and accelerate restoration of bone strength. Animal data exist to support the use of various means of modulating loading of the distraction regenerate (e.g. early weight bearing, temporary distraction, compression following osteotomy, dynamization following callus distraction). However, there are limited data regarding the effects of alteration of mechanical load on bone healing in humans[16,41]. Increasing the frequency of distraction while decreasing the amount of lengthening at each interval, whether manually[16], or with a motorized distractor, may improve bone formation and shorten the external fixation index. In a study of elderly patients with medial compartment osteoarthritis and hemicallotasis of the proximal tibia, Mizuta et al[17,20] reported increased bone mineral density and a shorter period of external fixation in patients who underwent distraction at a rate of 0.125 mm eight times per day (total, 1 mm/d), compared with those who underwent distraction at the standard frequency of 0.25 mm four times per day.
Technical variations on the conventional Ilizarov method have been developed with the goal of reducing the duration of external fixation or eliminating its use. New techniques include: segment transport over an intramedullary (IM) nail; segment transport followed by nailing; hemicorticotomy; tibiofibular synostosis; and fibular transport. These techniques are not without risk, and they may not be appropriate in patients with a history of local infection. Although these methods decrease the external fixation index, protect against refracture, and promote faster rehabilitation, they have not been shown to improve the bone healing index consistently. Other disadvantages include: the possibility of IM infection; the potential for customized instrumentation; increased surgical time, and excessive blood loss; additional surgery for removal of deep hardware; added cost; and a steep learning curve.
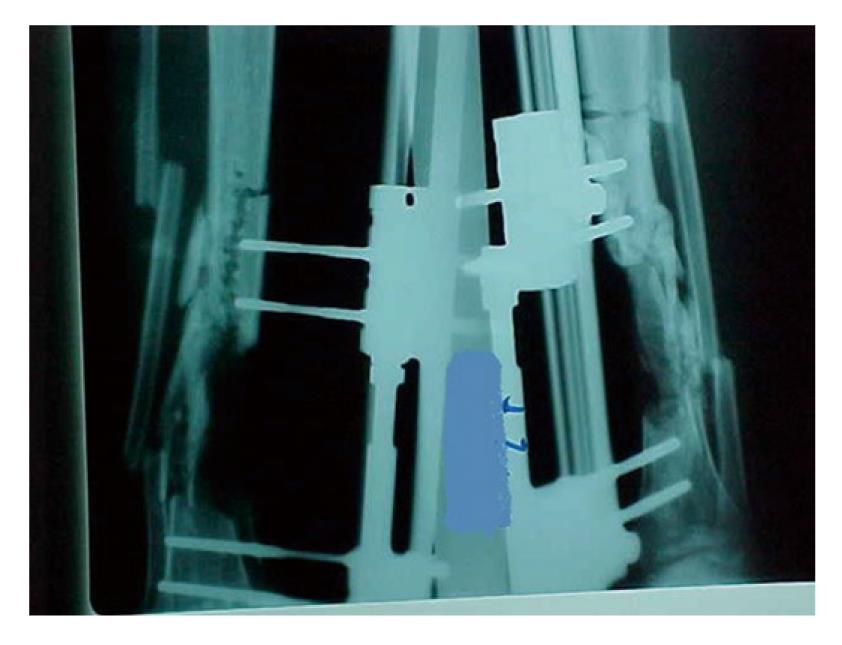
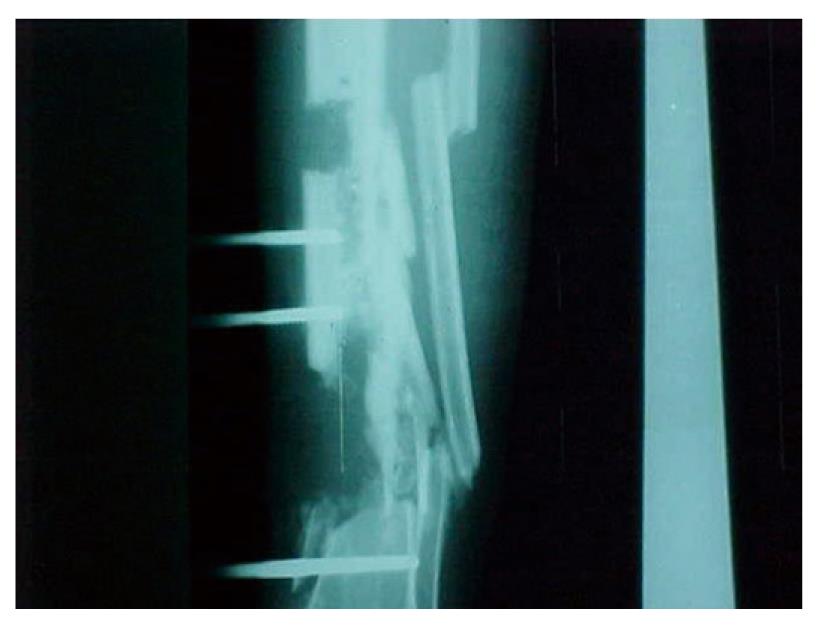
Hemicorticotomy[18]: After primary management which comprises debridement, excision of the sinus in the incision, and removal of the anterior half of the tibial cortex and any sequestrum, leaving the posterior cortex intact in its vascular muscle bed. Later, when the condition of the surgical wound allows, a partial corticotomy is performed of the anterior aspect of the tibia adjacent to the area from which bone has been removed. The hemi-corticotomy starts with a skin incision allowing a posteromedial approach to the tibia. The incision is deepened to expose the anteromedial surface of the tibia. The tibial periosteum is incised 1 cm anterior to the posterior tibial cortex, and periosteal stripping is avoided, multiple drill holes in an L-shaped configuration are then made, connected using an osteotome to separate the anterior half of the tibia from the posterior half (Figure 1), the hemicorticotomy is secured by using half pins, wires or both (Figure 2), and then gradual segment transfer is made to fill the defect[18].
Hemicorticotomy is indicated in chronic osteomyelitis involving the anterior tibial cortex with intact and healthy posterior cortex, and contraindicated in diffuse osteomyelitis involving both anterior and posterior tibial cortices.
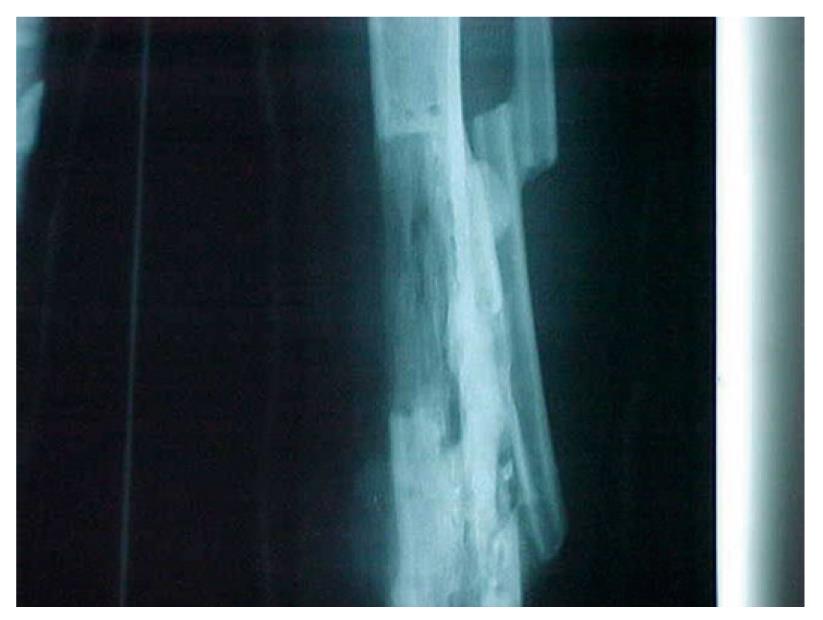
In our study of twenty patients with chronic osteomyelitis of the tibia hemicorticotomy and transfer by Ilizarov was performed. Of these patients 19 had complete healing of infection and the discharging sinus. Radiography confirmed consolidation of the new bone. The external fixator remained in place in 11 patients until full maturation of the new bone (average duration was 38 wk). Nine patients asked for removal of the frame immediately after segment transfer had been completed followed by a below knee cast for average period of 18 wk (Figure 3).
The technique did not work in one patient, whose sinus continued to discharge for 8 wk after surgery. As this was thought due to persistent infection related to insufficient removal of infected dead bone, segment transfer was discontinued and the transferred segment was returned to its original site. This was followed by another operation with complete excision of the segment with the suspected infection and classic segment transfer.
Ilizarov external fixation and then nailing: The plan consists of: segment transfer to reconstruct the defect resulting from the radical debridement; achievement of limb length equality, and deformity correction; use of Ilizarov external fixator frame; corticotomy of the tibia is performed in relatively normal bone; postoperative intravenous antibiotics, specific for culture and sensitivity, are given for 6 wk.
Removal of the external fixator and intramedullary fixation is done at 4th wk after finishing segment transfer. The 4 wk gap between finishing segment transfer and removal of the external fixator is to: (1) give a chance for the irritated skin at the pin site of segment transfer to heal; and (2) the docking site being compressed for some time to avoid gapping and decrease risk of regenerate spring-back after removal of the external fixator.
During nailing, the guide wire for the nail is passed gently through the proximal tibia, the soft distraction callus and the transferred bony segment to the distal tibial segment, followed by insertion of the nail. The statically locked nail fixes both the corticotomy distraction and the docking site while maintaining the length.
Ilizarov fixator followed by intramedullary nailing is indicated in infected nonunion of the tibial shaft, and contraindicated in prior intramedullary tibial nail insertion (to decrease risk of infection recurring after nail insertion, because any hidden infected focus along the medullary canal away from the fracture site could be missed in the debridement), as well as pin track infection during segment transfer[19].
In a study we had 33 patients[19] with infected nonunion of the tibia managed with segment transfer using an Ilizarov fixator; in 17 patients the fixator was replaced by nailing after segment transfer was completed.
The average duration of infected nonunion was 12.6 mo, in the patients who continued in the fixator where the average duration of the fixator was 8.5 mo; in the patients with nailing, the average duration of the fixator was 3.1 mo.
In the 17 patients with nailing, 15 had complete healing at the docking site and the corticotomy site with no residual infection. In one patient the nail was broken at the docking site level after seven weeks; in another there was recurrence of infection over the intramedullary nail.
Segment transfer over an intramedullary nail: The technique described involves radical debridement, application of a polymethylmethacrylate spacer with a monoplanar external fixator, followed by segment transfer using the Ilizarov technique over an intramedullary nail.
This method is indicated in Cierny-Mader Type IVA or IVB chronic osteomyelitis of the femoral and tibial metaphysis and diaphysis, and contraindicated in Cierny-Mader type IVC chronic osteomyelitis of the femoral and tibial metaphysis and diaphysis, Cierny-Mader Type I, II, or III chronic osteomyelitis of the femoral and tibial metaphysis and diaphysis[3].
In this method there are pitfalls such as: failure to achieve radical debridement of all dead tissue until the observation of the so-called paprika sign (live cortical bone); failure to determine debridement levels, decision-making which is assisted by intravenous contrast-enhanced magnetic resonance imaging of the whole long bone, which displays all necrotic tissues and skipped abscesses; failure to achieve good soft-tissue coverage of the debridement area which is managed by local or distant soft-tissue flaps; failure to include culture-specific, heat-stable antibiotics into the polymethylmethacrylate; failure to determine precisely the length and diameter of the intramedullary nail to be inserted and the level and number of custom locking holes which can be avoided by preoperative use of templates and standing orthoradiographs; failure to overream the medullary canal 1.5 mm larger than the diameter of the intramedullary nail to ensure easy gliding of bone segments over the nail; failure to ensure that the inserted Schanz screws or Kirschner wires are at least 1 mm away from the nail and failure to place the external fixator parallel to the intramedullary nail in both the frontal and sagittal planes.
In a study done by Eralp et al[21], this technique was used for 13 patients, the mean size of the defect was 7 cm, mean time of union at the docking site was 9 mo. Excellent results, in terms of both bone and functional assessment, were achieved for 11 patients, 2 patients had recurrence of infection which necessitated removal of the nail and revision with Ilizarov.
Fibular transport: The technique is a gradual ipsilateral fibular transport using a stable external fixator based on the Ilizarov principles. Proximal and distal fibular osteotomies are performed to allow medial translation of the central portion of the fibula. Olive wires with olives placed on the lateral aspect of the ipsilateral fibula are used to perform the medial fibular translation. This technique is indicated in patients with scarce bone regenerate obtained with the standard Ilizarov technique to replace the massive tibial loss as well as recurrence of osteomyelitis.
Catagni et al[22] used this technique in one patient with massive tibial bone loss and previous failed segment transfer. The Ilizarov ring fixation time to achieve fibular transport and bone union was 11 mo, the patient underwent additional multiple bone debridement with insertion of antibiotic beads. Using fibular transport, full replacement of tibial bone loss was obtained.
Two distinct forms of physical forces have been used to enhance DO: LIPU and PEMFs[23].
Low-intensity pulsed ultrasound: Low intensity pulsed ultrasound delivers high-frequency, low intensity pressure waves to the maturing distraction regenerate. An ultrasound transducer placed on the skin enhances endochondral ossification and callus formation, especially in acute fractures of the distal radius[24] and tibial shaft[25] managed with a cast. Most authors recommend Low intensity pulsed ultrasound over the healing fracture site for 20 min/d. The role of low intensity pulsed ultrasound in distraction osteogenesis is less well defined and is considered a physician-directed (off-label) application.
In a study of 20 young adults with segmental tibial defects who underwent distraction osteogenesis using external fixation, 10 underwent low intensity pulsed ultrasound applied to the distraction site during the consolidation phase[20,26]. The mean healing index was 30 d per centimeter in the ultrasound group compared with 48 d per centimeter in patients treated with rigid fixation (range, 27 to 36 d/cm and 42 to 75 d/cm, respectively).
Pulsed electromagnetic field: Electromagnetic stimulation is a noninvasive modality that has long been used to enhance fracture healing; however, a recent meta-analysis of randomized controlled trials revealed inconsistent outcomes[20,27].
A small double blind study of adolescent patients who underwent limb lengthening did not demonstrate a difference in the rate or amount of new bone formation in patients treated with an active pulsed electromagnetic field coil during distraction compared with control patients treated with an inactive coil[28]. However, when dual-energy X-ray absorptiometry was used to measure bone density, patients treated with pulsed electromagnetic field were found to have less bone loss adjacent to the distraction gap.
A variety of Bone morphogenic proteins (BMPs) play an active role in osteogenesis, including recruitment of stem cells, cellular proliferation and differentiation, and bone formation. Recombinant BMP-2 and BMP-7 have been used primarily in adults as an autograft-substitute for single-level spine fusions and certain open tibial shaft fractures and nonunions.
Currently, limited information exists on the clinical use of BMPs for distraction osteogenesis in humans.
In 2008, Burkhart et al[29] reported a case study of a 19-year-old woman with insufficient bone regeneration following tibial transport over an intramedullary nail. The patient was successfully treated with exchange reamed nailing and intramedullary application of recombinant BMP-7 mixed with reamed debris at the site of the atrophic regenerate. However, several simultaneous interventions were used in this case; thus, the role of BMPs in distraction osteogenesis remains unclear and needs further study.
Platelet-rich plasma contains a variety of osteoinductive growth factors that may play a role in the proliferation and differentiation of osteoprogenitor cells and thereby enhances new bone formation during distraction osteogenesis[30]. In a case series, three adolescent patients who underwent lower limb lengthening received an injection of culture-expanded bone marrow cells and platelet-rich plasma at the site of lengthening during the distraction and consolidation phases. The healing index was 23 days per centimeter, with no untoward effects. The same group of investigators applied a similar protocol in the treatment of a group of teenagers of short stature secondary to achondroplasia and hypochondroplasia who underwent lower limb lengthening[31]. Eleven patients (24 bones) were treated with transplantation of culture-expanded bone marrow cells and platelet-rich plasma during distraction osteogenesis.
These patients had a significantly lower average healing index compared with nine patients (32 bones) in the control group (27.1 ± 6.89 d/cm vs 36.2 ± 10.4 d/cm, respectively, P = 0.0005). The osteogenic effect was more pronounced with femoral lengthening than with tibial lengthening.
Although the initial results of injection of culture-expanded bone marrow cells and platelet-rich plasma (PRP) at the lengthening site seem encouraging, this treatment option requires additional visits to the operating room and special equipment, as well as additional training to harvest the stem cells. This technique must be studied in other patient populations, especially those with typically prolonged healing times, to evaluate whether these results can be duplicated[20].
Diphosphonates are a family of anticatabolic agents that prevent bone resorption. They may be administered orally or systemically. These agents have been used in a physician-directed (off-label) capacity in patients with osteopenia to reduce the rate of bone turnover, thereby improving bone mineralization and density. In 2007, Kiely et al[20,32] reported that six of seven children treated with parenteral diphosphonates for poor quality of regenerate following DO eventually healed without further intervention. No side effects related to diphosphonates therapy were reported. Other anticatabolic agents (e.g. calcitonin) may have a role to play in enhancement of bone formation during Distraction Osteogenesis.
Several other agents, such as parathyroid hormone[33], vitamin D analogs[34], and hyperbaric oxygen[35,36], have been studied in animal models of distraction osteogenesis with encouraging results. However, no human study of such therapies is available.
The docking site is prone to delayed healing in distraction osteogenesis following gradual transport of a bony fragment in patients with a skeletal defect. Satisfactory outcomes without the need for supplemental management of segmental defects of the tibia following sequestrectomy and bone transport have been reported in children with chronic hematogenous osteomyelitis. However, the potential exists for impaired bony union. Several options are available to minimize external fixation and enhance union at the docking site including: autologous bone graft; shingling or reshaping of the bony edges[37]; bifocal transport[38]; transport over an intramedullary nail; secondary intramedullary nailing[39]; and application of low intensity pulsed ultrasound to the docking site[40]. Acute shortening following segmental resection with lengthening using distraction osteogenesis at a distant site in the same bone is an effective alternative to the classic bone transport technique in some patients with moderate-sized skeletal defects[41].
Distraction osteogenesis is a clinically applicable method of new bone formation based on sound biologic and mechanical principles. Appropriate patient selection, surgical technique, and rate and frequency of distraction are important prerequisites for the timely formation of a robust distraction regenerate. Several methods can improve the rate and quality of bone formation while decreasing the external fixation index during distraction osteogenesis. These modalities include: mechanical stimulation; innovative surgical techniques that require a variety of intramedullary nails; exogenous application of low intensity pulsed ultrasonography or electromagnetic fields; and anticatabolic agents (e.g., diphosphonates, local injection of certain BMPs, pluripotent stem cells mixed with platelet-rich plasma). Few clinical studies exist supporting the use of such options.
Peer reviewers: Ashraf S Gorgey, Assistant Professor of Virginia Commonwealth University, Department of Physical Medicine and Rehabilitation, 1201 Broad Rock Blvd, Richmond, VA 23249, United States; Mohamed Kenawey, Dr., Sohag Faculty of Medicine, Orthopaedic Surgery, Sohag 82524, Egypt
S- Editor Yang XC L- Editor Hughes D E- Editor Zheng XM
| 1. | Cierny G. Infected tibial nonunions (1981-1995). The evolution of change. Clin Orthop Relat Res. 1999;97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Simpson AH, Deakin M, Latham JM. Chronic osteomyelitis. The effect of the extent of surgical resection on infection-free survival. J Bone Joint Surg Br. 2001;83:403-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Cierny G, Mader JT, Penninck JJ. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;7-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 464] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 4. | Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Relat Res. 1990;8-26. [PubMed] |
| 5. | Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res. 1990;81-104. [PubMed] |
| 6. | Fischgrund J, Paley D, Suter C. Variables affecting time to bone healing during limb lengthening. Clin Orthop Relat Res. 1994;31-37. [PubMed] |
| 7. | Aronson J. Limb-lengthening, skeletal reconstruction, and bone transport with the Ilizarov method. J Bone Joint Surg Am. 1997;79:1243-1258. [PubMed] |
| 8. | Hughes TH, Maffulli N, Green V, Fixsen JA. Imaging in bone lengthening. A review. Clin Orthop Relat Res. 1994;50-53. [PubMed] |
| 9. | Eski M, Ilgan S, Cil Y, Sengezer M, Ozcan A, Yapici K. Assessment of distraction regenerate using quantitative bone scintigraphy. Ann Plast Surg. 2007;58:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Catagni M. Imaging techniques: The radiographic classification of bone regenerate during distraction. Operative principles of ilizarov. London: Williams and Wilkins 1991; 53-57. |
| 11. | Forriol F, Iglesias A, Arias M, Aquerreta D, Cañadell J. Relationship between radiologic morphology of the bone lengthening formation and its complications. J Pediatr Orthop B. 1999;8:292-298. [PubMed] |
| 12. | Donnan LT, Saleh M, Rigby AS, McAndrew A. Radiographic assessment of bone formation in tibia during distraction osteogenesis. J Pediatr Orthop. 2002;22:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Simpson AH, Kenwright J. Fracture after distraction osteogenesis. J Bone Joint Surg Br. 2000;82:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | O’Carrigan T, Nocente C, Paley D, Herzenberg JE. Fractures complicating limb lengthening. J Bone Joint Surg Br. 2005;87-B:312. |
| 15. | Eralp L, Kocaoğlu M, Ozkan K, Türker M. A comparison of two osteotomy techniques for tibial lengthening. Arch Orthop Trauma Surg. 2004;124:298-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;263-285. [PubMed] |
| 17. | Mizuta H, Nakamura E, Kudo S, Maeda T, Takagi K. Greater frequency of distraction accelerates bone formation in open-wedge proximal tibial osteotomy with hemicallotasis. Acta Orthop Scand. 2004;75:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Emara KM. Hemi-corticotomy in the management of chronic osteomyelitis of the tibia. Int Orthop. 2002;26:310-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Emara KM, Allam MF. Ilizarov external fixation and then nailing in management of infected nonunions of the tibial shaft. J Trauma. 2008;65:685-691. [PubMed] |
| 20. | Sabharwal S. Enhancement of bone formation during distraction osteogenesis: pediatric applications. J Am Acad Orthop Surg. 2011;19:101-111. [PubMed] |
| 21. | Eralp L, Kocaoglu M, Rashid H. Reconstruction of segmental bone defects due to chronic osteomyelitis with use of an external fixator and an intramedullary nail. Surgical technique. J Bone Joint Surg Am. 2007;89 Suppl 2 Pt.2:183-195. [PubMed] |
| 22. | Catagni MA, Camagni M, Combi A, Ottaviani G. Medial fibula transport with the Ilizarov frame to treat massive tibial bone loss. Clin Orthop Relat Res. 2006;448:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Gebauer D, Correll J. Pulsed low-intensity ultrasound: a new salvage procedure for delayed unions and nonunions after leg lengthening in children. J Pediatr Orthop. 2005;25:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am. 1997;79:961-973. [PubMed] |
| 25. | Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am. 1994;76:26-34. [PubMed] |
| 26. | El-Mowafi H, Mohsen M. The effect of low-intensity pulsed ultrasound on callus maturation in tibial distraction osteogenesis. Int Orthop. 2005;29:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Mollon B, da Silva V, Busse JW, Einhorn TA, Bhandari M. Electrical stimulation for long-bone fracture-healing: a meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2008;90:2322-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Eyres KS, Saleh M, Kanis JA. Effect of pulsed electromagnetic fields on bone formation and bone loss during limb lengthening. Bone. 1996;18:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Burkhart KJ, Rommens PM. Intramedullary application of bone morphogenetic protein in the management of a major bone defect after an Ilizarov procedure. J Bone Joint Surg Br. 2008;90:806-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Kawasumi M, Kitoh H, Siwicka KA, Ishiguro N. The effect of the platelet concentration in platelet-rich plasma gel on the regeneration of bone. J Bone Joint Surg Br. 2008;90:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N. Distraction osteogenesis of the lower extremity in patients with achondroplasia/hypochondroplasia treated with transplantation of culture-expanded bone marrow cells and platelet-rich plasma. J Pediatr Orthop. 2007;27:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Kiely P, Ward K, Bellemore C M, Briody J, Cowell CT, Little DG. Bisphosphonate rescue in distraction osteogenesis: a case series. J Pediatr Orthop. 2007;27:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Kokoroghiannis C, Papaïoannou N, Lyritis G, Katsiri M, Kalogera P. Calcitonin administration in a rabbit distraction osteogenesis model. Clin Orthop Relat Res. 2003;286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Seebach C, Skripitz R, Andreassen TT, Aspenberg P. Intermittent parathyroid hormone (1-34) enhances mechanical strength and density of new bone after distraction osteogenesis in rats. J Orthop Res. 2004;22:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Yamane K, Okano T, Kishimoto H, Hagino H. Effect of ED-71 on modeling of bone in distraction osteogenesis. Bone. 1999;24:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Eralp L, Ozkan K, Kocaoglu M, Aktas S, Zihni M, Türker M, Ozkan FU. Effects of hyperbaric oxygen therapy on distraction osteogenesis. Adv Ther. 2007;24:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Dhar SA, Mir MR, Ahmed MS, Afzal S, Butt MF, Badoo AR, Dar IT, Hussain A. Acute peg in hole docking in the management of infected non-union of long bones. Int Orthop. 2008;32:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Griffith MH, Gardner MJ, Blyakher A, Widmann RF. Traumatic segmental bone loss in a pediatric patient treated with bifocal bone transport. J Orthop Trauma. 2007;21:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Oh CW, Song HR, Roh JY, Oh JK, Min WK, Kyung HS, Kim JW, Kim PT, Ihn JC. Bone transport over an intramedullary nail for reconstruction of long bone defects in tibia. Arch Orthop Trauma Surg. 2008;128:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Gold SM, Wasserman R. Preliminary results of tibial bone transports with pulsed low intensity ultrasound (Exogen). J Orthop Trauma. 2005;19:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |