Published online Aug 18, 2025. doi: 10.5312/wjo.v16.i8.108407
Revised: May 1, 2025
Accepted: July 3, 2025
Published online: August 18, 2025
Processing time: 117 Days and 6 Hours
Skeletal stem cells (SSCs) are tissue-specific stem cells characterized by their capacity for self-renewal and their position at the apex of the differentiation hierarchy. They can generate mature bone cell types essential for bone develop
Core Tip: Skeletal stem cells (SSCs) are tissue-specific stem cells characterized by their capacity for self-renewal and their position at the apex of the differentiation hierarchy. They can generate mature bone cell types essential for bone development, maintenance, and repair. Lineage tracing experiments have demonstrated that SSCs reside in the bone marrow, periosteum, and the resting zone of the growth plate. These findings not only enhance our understanding of bone growth and development mechanisms but also offer novel therapeutic strategies for conditions such as epiphyseal injuries, fractures, osteoarthritis (OA), and other orthopedic diseases. Recent advancements in biological scaffold technology, combined with 3D printing techniques, have facilitated bone tissue regeneration using bone stem cells. Additionally, bone stem cells have shown promise in cartilage regeneration therapy, particularly in treating degenerative diseases like OA and articular cartilage damage, thereby improving joint function. This review summarizes the latest research progress on the role of SSC in bone and joint injury regeneration and provides new insights into potential therapeutic approaches.
- Citation: Liu C, Jian J, Yi YF, Ding YT, Chen Y, Tang ZW, Wen J, Li YF. Skeletal stem cells, a new direction for the treatment of bone and joint diseases. World J Orthop 2025; 16(8): 108407
- URL: https://www.wjgnet.com/2218-5836/full/v16/i8/108407.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i8.108407
Bone and joint injuries are prevalent clinical conditions in orthopedics, with an increasing incidence observed in recent years[1-3]. Osteoarticular diseases encompass a range of conditions, including osteoarthritis (OA), rheumatoid arthritis, gouty arthritis, and others. The majority of these diseases are associated with varying degrees of cartilage damage. Epidemiological studies reveal distinct characteristics of cartilage injury, such as a significant gender disparity (higher incidence in females), a bimodal age distribution (predominantly affecting young and middle-aged individuals as well as the elderly), and anatomical specificity (primarily involving the knee and ankle joints). The rising incidence of cartilage injury is closely linked to population aging, obesity, and an increase in sports-related injuries[4-6]. Current treatment strategies for cartilage impairment can be categorized into nonoperative and surgical approaches[7-9]. For mild cartilage damage, such as superficial abrasions or wear, gradual repair and recovery can often be achieved through adequate rest, physical therapy (e.g., shortwave diathermy, electrical nerve stimulation), and pharmacological interventions (e.g., nonsteroidal anti-inflammatory drugs)[10]. These methods aim to alleviate inflammation, mitigate pain, and stimulate chondrocyte regeneration and repair[11]. However, complete recovery may prove challenging for more severe cartilage injuries, particularly those involving extensive cartilage defects or full-thickness cartilage lesions[12]. In such cases, surgical intervention becomes necessary, with options including cartilage repair surgery or cartilage transplantation. These procedures focus on restoring cartilage integrity and functionality, thereby reducing pain and enhancing joint performance[13,14]. Consequently, prompt treatment following articular cartilage injury is crucial for promoting effective cartilage repair and regeneration.
Stem cell therapy offers a novel therapeutic avenue for cartilage repair[15,16]. Stem cells, a unique population of undifferentiated cells characterized by their capacity for self-renewal, unlimited proliferation, and multidirectional differentiation, have been utilized in the treatment of various traumatic and degenerative bone and joint defects[17]. Skeletal stem cells (SSCs), also referred to as pre-chondrocytes, are adult stem cells located in the LaCroix ring of the metaphysis in the limbs of embryos or newborn animals. These cells exhibit the continuous proliferation and directed differentiation properties typical of stem cells[18]. In recent years, significant progress has been made in SSC research, particularly in the areas of bone regeneration, epiphyseal repair, and cartilage injury. In cartilage regeneration, SSCs secrete transforming growth factor-β1 to activate the downstream SMAD2/3 signaling pathway, thereby upregulating the expression of SOX9 and COL2A1, which in turn promotes chondrocyte proliferation and extracellular matrix synthesis[19,20]. Simultaneously, exosomes secreted by SSCs carrying miR-140-5p specifically target HDAC4 for inhibition, thereby enhancing chondrogenic differentiation capacity[21,22]. Moreover, SSCs inhibit the NF-κB pathway via interleukin (IL)-10 secretion, reducing the release of pro-inflammatory factors such as IL-1β and tumor necrosis factor-alpha (TNF-α), and thus alleviating the local inflammatory microenvironment[23,24]. In epiphyseal plate injury repair, SSCs activate Runx2 while inhibiting bone bridge formation through the BMP-2/Smad1/5 pathway. Additionally, they promote vascular endothelial cell migration and vascular remodeling via the VEGF-A/PI3K-Akt pathway[25-27]. Furthermore, their paracrine FGF2 regulates FGFR1 receptors, stimulating the orderly alignment of epiphyseal plate chondrocytes and preventing premature growth plate closure[28]. SSCs are tissue-specific stem cells that can self-renew and occupy the apex of their differentiation hierarchy, giving rise to mature bone cell types essential for bone growth, maintenance, and repair[29]. Research on SSCs not only enhances our understanding of bone growth and development mechanisms but also provides innovative therapeutic strategies for treating epiphyseal injuries[30]. This article reviews the application of SSCs in bone and joint injuries and lays the groundwork for their further clinical utilization.
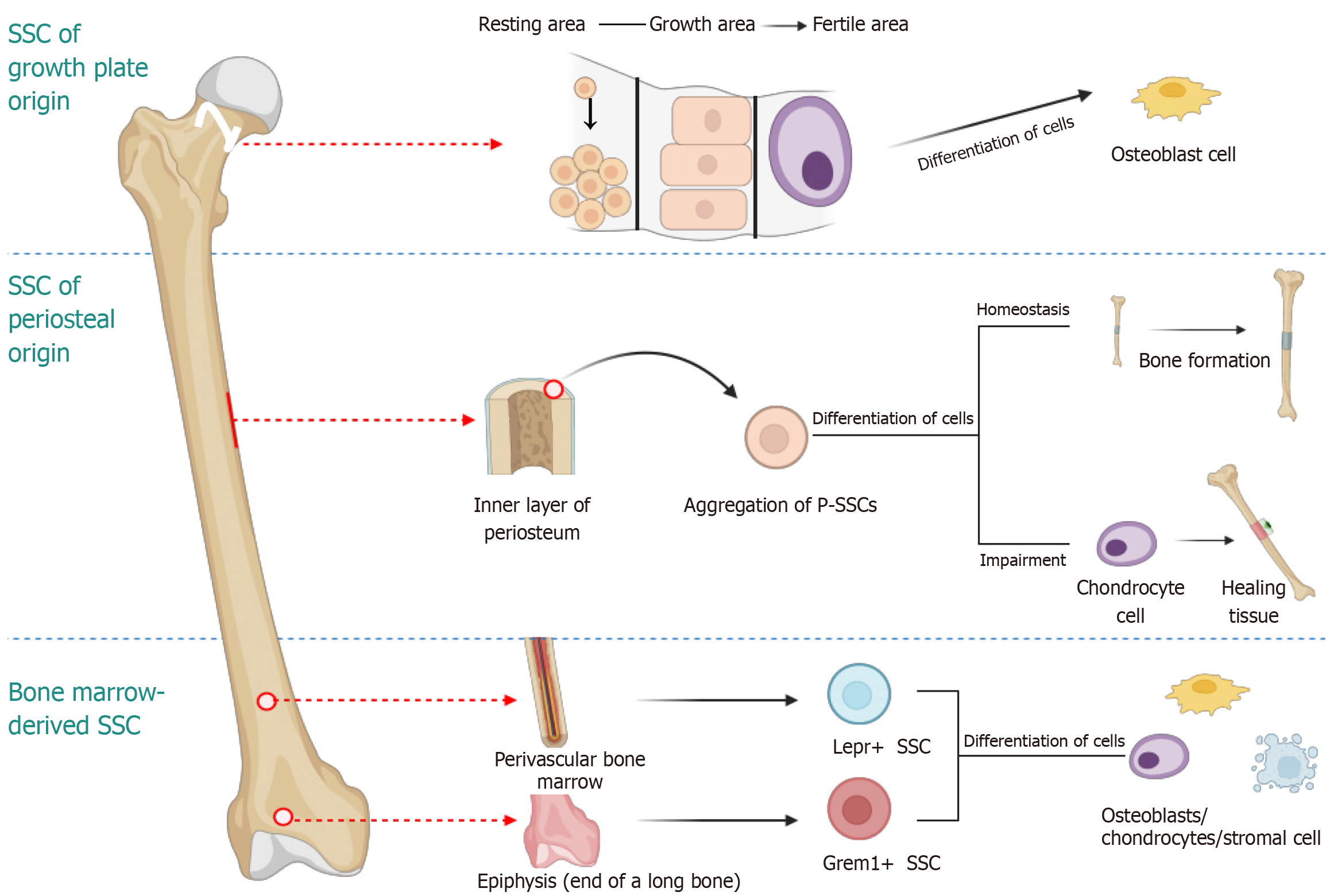
Bone development, maintenance, and repair rely on the self-renewal and differentiation of SSC[31]. The existence of colony-forming SSCs has been confirmed, and progenitor cells for bone, cartilage, and stroma have been isolated for rigorous functional characterization[32]. Researchers have proposed that SSCs reside in the pericardium surrounding the epiphysis and exhibit stem cell characteristics (Figure 1). Furthermore, they identified fibroblast growth factor receptor 3 as a marker antigen for SSCs, providing a theoretical foundation for their isolation, culture, and identification[33]. SSCs primarily originate from the epiphyseal plate region and include mesenchymal stem cells (MSCs) and chondroprogenitors[34]. Recent lineage tracing experiments have demonstrated that SSCs are present in the bone marrow, periosteum, and resting zone of the growth plate[35]. The isolation of SSCs is mainly achieved through specific cell surface markers (CD45-/TER119-/Tie2-/Thy1-/6C3-/CD105+/Alpha V+/CD200+)[36]. Some studies have further confirmed the presence of SSCs from the periosteum, growth plate, and bone marrow[37].
For the specific isolation of SSCS from broad mouse and human bone marrow stromal cell (BMSC) populations, cells were sorted based on the co-expression of the cell-surface markers CD146 (MCAM) and PDGFRα, while excluding cells positive for the hematopoietic lineage marker CD45 (PTPRC) and the endothelial lineage marker CD31 (PECAM1), bone marrow-derived SSC/stromal stem cells can be differentiated in vitro based on the positive cell surface markers CD146 and PDGFRα. Cells negative for the exclusion markers CD45 and CD31 were sorted out. In vivo, Cre-mediated DNA recombination techniques, such as gremlin 1 (Grem1)-CreER and Lepr-Cre transgenic mice, were utilized for identification[38]. Many markers associated with putative bone marrow SSCs, such as the BMP antagonist Grem1, have been identified in mice. Grem1+ cells are considered a type of SSC due to their multilineage differentiation capacity in vivo, their essential role in postnatal bone formation, and their long-term self-renewal ability. However, the intrinsic properties of these cells may be altered during isolation and in vitro culture, potentially due to sorting selection bias or non-physiological culture conditions, among them, Grem1+ cells are predominantly located in the metaphysis of long bones and differentiate into chondrocytes, osteoblasts, BMSC, and periosteal connective tissue cells shortly after birth. These cells play acritical role in bone formation and possess long-term self-renewal capacity. Lepr+ cells are primarily concentrated around blood vessels, generating bone marrow adipocytes in the early stages postnatally and gradually becoming the predominant source of osteoblasts with aging. A subset expressing Cxcl12 is also involved in bone injury repair, while certain Lepr+ BMSC subsets maintain an immature matrix phenotype to support hematopoiesis. The heterogeneity and functional diversity of these cells provide a crucial foundation for investigating bone development, repair, and associated diseases. However, it is important to consider the limitations of in vitro studies, as the characteristics of these cells may be altered under culture conditions[39].
The longitudinal growth mechanism of long bones primarily relies on the functional partitioning of the growth plate[40]. This dynamic process can be divided into several key stages: In the quiescent zone, slowly proliferating cells act as a reserve cell population that continuously generates chondroblasts; in the proliferative zone, these precursor cells form orderly columnar structures and rapidly divide to expand the growth plate; in the hypertrophic zone, mature chondrocytes undergo volume expansion and matrix remodeling before being eliminated by osteoclasts at the ossifying front, subsequently replaced by bone-marrow composite tissue[41,42]. Studies have shown that chondrocytes within the growth plate can transdifferentiate into osteoblasts[43]. Recent breakthroughs have been achieved regarding the spatiotemporal dynamics and functional heterogeneity of stem cells in the growth plate resting zone[44]. Lineage tracing using Col2a1-CreER and PTHrP-CreER revealed that chondrocytes undergo polyclonal-to-monoclonal evolution after birth, regulated by the MTORC1 signaling pathway[18]. PTHrP+ cells serve as the primary source for chondrogenesis and can differentiate into Col1a1+ osteoblasts and Cxcl12+stromal cells, supporting the hypothesis of trans differentiation through the hypertrophic cartilage stage.
However, "borderline chondrocytes" may transdifferentiate directly, indicating the potential existence of a bypass mechanism. Chan et al[37] isolated mouse and human growth plate cells capable of differentiating into multiple lineages using specific combinations of surface markers. Nevertheless, their functional homology to resting zone stem cells in vivo remains controversial and could be affected by culture-induced plasticity[37,45,46]. These findings offer a novel perspective for investigating bone development and regenerative medicine.
Recent studies have demonstrated the existence of a distinct population of stem cells, referred to as periosteal stem cells (P-SSCs), within the inner periosteum, which possess multi-directional differentiation potential[47]. When cathepsin K (Ctsk) was co-employed with surface markers (CD45-TER119-CD31-CD105-CD200+), P-SSCs mediating intramembranous ossification were successfully labeled[48]. Whereas other SSC utilize an endochondral pathway to form bone from an initial cartilage template, periosteal skeletal progenitor cells (PSCs) generate bone through a direct intramembranous pathway, thereby establishing a cellular basis for the divergence between intramembranous and endochondral developmental pathways. PSCs sustain cortical bone structure via intramembranous ossification. Specific knockout of Osx, a pivotal osteogenic transcription factor in PSCs, leads to severe defects in periosteal mineralization. Notably, PSCs display remarkable plasticity following fracture: They not only expand significantly in number but also switch from intramembranous to endochondral osteogenesis, contributing approximately 50% of callus chondrocytes. This unique adaptability renders them a central driver of fracture repair[48-50]. Ctsk, traditionally regarded as an osteoclast marker, has been identified as labeling P-SSCs involved in intramembranous ossification when combined with specific cell-surface markers[41]. Ctsk-Cre-labeled CD45-TER119-CD31-CD105-CD200+ cells isolated from the mouse femoral periosteum exhibit self-renewal properties and reside at the apex of the differentiation hierarchy, generating all Ctsk-Cre-labeled skeletal cell types. These P-SSCs also express markers previously identified by Chan et al[37], suggesting that this surface immunophenotype may be shared among various stem cell populations. The absence of Osx transcription factors in Ctsk-Cre-labeled cells results in impaired fracture repair and defective cortical bone formation. Human periosteum contains a population of immunophenotype conserved PSCs (Lin-CD90-CD200+CD105-) that exhibit multi-directional differentiation capacity and osteogenic potential in vivo. These findings not only elucidate the dual functions of PSCs in bone development and regeneration but also offer a theoretical foundation for the development of bone regeneration therapies focused on P-SSCs[51,52].
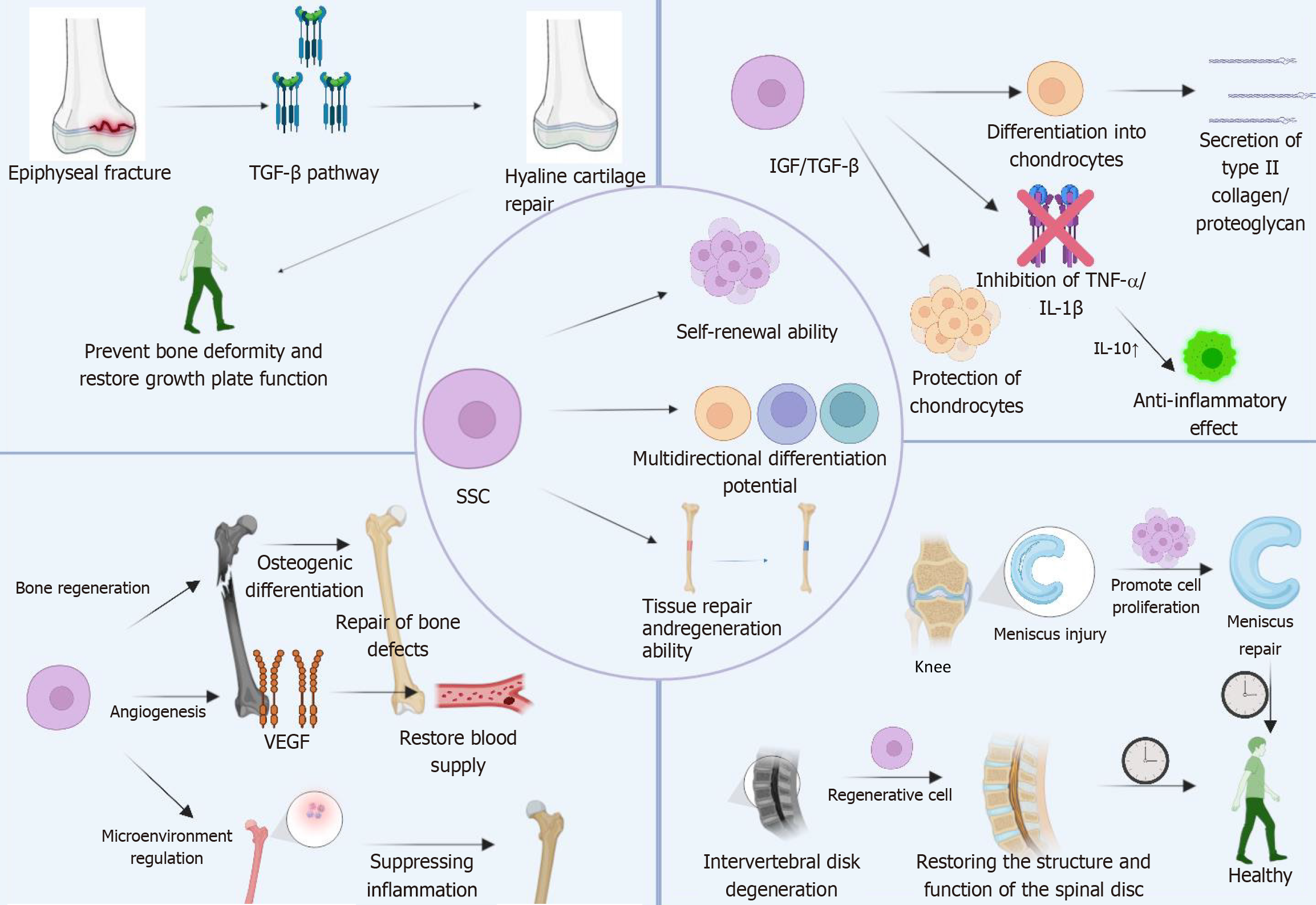
SSC have demonstrated significant therapeutic value in regenerative medicine over recent years, attributed to their distinctive self-renewal capacity and multi-lineage differentiation potential. Research has highlighted the broad application potential of SSCs in bone and cartilage tissue repair, disease modeling, and tissue engineering. SSCs exhibit notable advantages in treating orthopedic diseases by differentiating into osteoblasts and chondrocytes to promote bone and joint repair[53-61]. Furthermore, through precise regulation of their differentiation process, SSCs can generate functional chondrocytes, thereby enhancing joint function[56]. From a molecular perspective, the pluripotency of SSCs is rooted in their unique signaling pathway regulatory network. By modulating the inflammatory microenvironment, SSCs can exert therapeutic effects in inflammatory bone diseases[30,57]. Additionally, SSCs have been shown to promote angiogenesis[58] and, under specific conditions, differentiate into myocytes and neural cells[59], properties that position them as promising therapeutic candidates for various degenerative diseases (Figure 2).
Epiphyseal injury refers to damage at multiple sites affecting the longitudinal growth mechanism of bone, including critical regions such as the epiphyseal plate, Ranvier's groove, articular cartilage, and metaphyseal area[60,61]. As the most prevalent type of fracture in children[62], epidemiological studies indicate that it constitutes approximately 18% to 18.5% of all pediatric bone injuries[2,63]. Such injuries can result in structural disruption and vascular compromise of the growth plate, leading to heterotopic ossification of cartilage tissue and ultimately forming bone bridge, which impairs growth plate function[64,65]. Without timely intervention, severe developmental abnormalities may occur, resulting in irreversible consequences such as overgrowth, deformity, or growth arrest[66]. Since SSC are localized within the epiphyseal growth plate region and possess multipotent differentiation potential[67], they demonstrate significant therapeutic value in repairing epiphyseal injuries.
Through the BMP/Wnt signaling pathway, SSCs can differentiate into osteoblasts, thereby promoting bone matrix secretion and mineralization[68-71]. Simultaneously, via the TGF-β pathway, they differentiate into functional chondrocytes to reconstruct the joint surface[72]. Notably, SSCs can restore the normal structure and function of the growth plate by modulating the Ihh/PTHrP signaling pathway[73,74]. Proangiogenic factors such as VEGF secreted by SSCs significantly enhance blood supply to the injured area, providing critical nutritional support for tissue repair[75-78]. Furthermore, anti-inflammatory factors like IL-10secreted by SSCs effectively suppress the NF-κB signaling pathway, regulating local inflammatory responses and thus facilitating the repair process[79-82].
SSCs promote tissue repair after epiphyseal injury through multiple mechanisms, including osteogenesis, chondrogenesis, angiogenesis, and inflammation regulation. Future research will aim to further refine the application strategies of SSCs to enhance both the safety and efficacy of their clinical use.
OA is a degenerative joint disease characterized by progressive degradation of articular cartilage, subchondral bone sclerosis, and synovial inflammation[83-85]. Global epidemiological data indicate that OA affects approximately 528 million individuals worldwide, with its prevalence continuing to rise due to aging populations and increasing obesity rates[86-88]. The hallmark pathological features of OA include the gradual loss of articular cartilage, osteophyte formation, joint space narrowing, and chronic synovial inflammation[89-91]. Given the inherently limited regenerative capacity of articular cartilage[92],the treatment of OA remains clinically challenging[93]. SSC have recently been recognized as a promising cellular therapy for OA due to their exceptional potential for cartilage regeneration[94].
In OA, SSCs contribute to the treatment of OA primarily through their multifaceted mechanisms involving chondrogenesis, anti-inflammation, and tissue repair[95]. SSCs differentiate into functional chondrocytes via the Wnt/TGF-β/BMP signaling pathway[72,96] and synthesize critical matrix components, such as collagen type II and proteoglycan, thereby effectively reconstructing the articular cartilage structure[3,97]. This process significantly enhances joint function and slows disease progression. By secreting paracrine growth factors like IGF and TGF-β[98-100], SSCs counteract chondrocyte apoptosis induced by pro-inflammatory factors such as TNF-α and IL-1β[101,102]. Additionally, the secretion of matrix metalloproteinase inhibitors helps maintain cartilage matrix homeostasis[103,104]. Moreover, SSCs promote angiogenesis and repair, improve the function of the joint synovial membrane, reduce synovial inflammation, regulate the microenvironment within the joint cavity, and enhance the production of lubricating fluid in the joint cavity, thereby alleviating joint friction and relieving pain[105].
The application of SSCs in OA demonstrates a promising and extensive potential. SSCs facilitate cartilage repair, suppress inflammatory responses, and enhance the joint microenvironment via the synergistic effects of multiple mechanisms (such as differentiation, paracrine signaling, anti-inflammatory actions, and metabolic regulation). These actions not only alleviate the symptoms of OA but also offer a potential novel strategy for its treatment.
The application of SSCs in avascular necrosis of the femoral head primarily involves promoting bone tissue regeneration, enhancing blood supply, and modulating the micro-environment[106]. SSCs possess multilineage differentiation potential and can differentiate into osteoblasts to facilitate bone matrix deposition and mineralization, thereby repairing bone defects caused by ischemic necrosis[107]. Moreover, SSCs secrete a range of growth factors that stimulate angiogenesis, improve blood circulation, restore local oxygen and nutrient supply, and expedite bone healing[103].
SSCs also possess immunomodulatory functions, creating favorable conditions for bone repair by suppressing inflammatory responses and optimizing the immune microenvironment, thereby reducing local inflammation levels[108]. Simultaneously, SSCs can regulate the synthesis and remodeling of the extracellular matrix, promoting cell migration and proliferation while providing scaffolds for bone tissue regeneration[109]. Through the secretion of cytokines and chemokines, SSCs interact with surrounding cells, such as bone marrow MSC and osteoblasts, to modulate bone tissue growth and repair[76]. Moreover, SSCs can activate the body's self-healing mechanisms, enhancing endogenous stem cell activity and accelerating bone defect repair[110]. Studies have demonstrated that SSCs can form regenerative tissue resembling normal trabecular bone in the treatment of early focal avascular necrosis of the femoral head, further supporting their potential application in tissue-engineered bone[111]. Additionally, the combination of SSCs with impacted bone graft has been proven to be an effective therapeutic strategy. Analysis of recovered femoral head samples indicates that this tissue engineering approach can regenerate tissue with structure and function closely approximating normal trabecular bone, offering significant value for repairing a broader spectrum of bone defects[112].
The application of SSCs in the treatment of avascular necrosis of the femoral head can not only promote bone regeneration and repair, but also improve the therapeutic effect through multiple mechanisms such as improving the microenvironment and immune regulation. With further research, a better understanding of the mechanisms of stem cell therapy may drive its breadth and effectiveness in clinical applications.
Cartilage damage encompasses any structural impairment to cartilage tissue, including surface fissures, fibrosis, or localized shedding. Articular cartilage lacks intrinsic regenerative capacity and is typically replaced by mechanically inferior fibrocartilage following injury. Current interventions, such as subchondral bone drilling and osteochondral transplantation, offer only temporary relief[113]. Effective cartilage repair necessitates the coordinated action of cells, extracellular matrix scaffolds, and bioactive factors. While MSC possess the ability to differentiate into chondrocytes, their chondrogenic differentiation potential is constrained by variability in cell sources, passage-related attenuation, and imprecise regulation of growth factors[114-116]. In contrast, SSC exhibit distinct advantages in cartilage repair due to their robust self-renewal capacity and directed differentiation potential toward chondrogenesis. Cartilage injuries, encompassing conditions such as degenerative joint disease, traumatic cartilage damage, meniscus avascular zone repair, chondromalacia patellae, and intervertebral disc degeneration, involve the destruction of cartilage or fibrocartilage structures[117-119]. SSCs not only facilitate cartilage regeneration and minimize fibrous scar formation but also restore tissue architecture and function by enhancing extracellular matrix metabolism, inhibiting apoptosis, and delaying calcification[120-122]. Consequently, SSC-based therapies hold promise for restoring joint function in patients with cartilage-related disorders.
In the field of cartilage defects, partial or full-thickness defects are frequently associated with exposure of subchondral bone. Defects exceeding a critical size necessitate surgical intervention due to the loss of self-repair capability[123-125]. Traditional bone grafting methods exhibit limited efficacy in repairing such defects, and some studies have demonstrated that bone tissue engineering holds promise as an alternative approach for treating bone defects[126]. Researchers have enhanced the directed regeneration of MSC by encapsulating them within a 3D bio scaffold composed of alginate gel[127,128]. Furthermore, studies indicate that SSCs, owing to their robust chondrogenic differentiation potential and minimal immunogenicity[37], may address the limitations of MSCs when integrated with biological scaffolds, thereby offering a superior solution for cartilage defect repair.
SSC exhibit promising application value in the treatment of various diseases due to their multi-directional differentiation potential, self-renewal capacity, and immunomodulatory properties. In osteoporosis therapy, SSCs can differentiate into osteoblasts and suppress osteoclast activity, thereby restoring bone metabolic balance. Studies using animal models have demonstrated that SSC transplantation increases bone mineral density and mitigates trabecular microstructure damage. Furthermore, when combined with β-tricalcium phosphate scaffolds, the efficiency of osteogenic differentiation is significantly enhanced[129]. For intervertebral disc degeneration in spinal degenerative diseases, SSCs can differentiate into nucleus pulposus-like cells, promoting the synthesis of collagen type II and proteoglycan. Animal experiments indicate that SSC transplantation delays intervertebral disc degeneration, while its integration with a hydrogel delivery system improves cell survival within the disc environment[130]. In response to muscle atrophy or injury, SSCs stimulate muscle satellite cell activation and muscle fiber regeneration through paracrine signaling of IGF-1, HGF, and other factors. In muscular dystrophy models, SSC transplantation partially restores muscle function, and co-culture with myogenic precursor cells enhances myogenic differentiation[131,132]. In skin trauma and chronic ulcer treatment, SSCs secrete EGF, FGF, and other growth factors to promote keratinocyte migration and angiogenesis[133]. In diabetic foot ulcer models, local injection reduces inflammation and promotes granulation tissue formation. When combined with collagen scaffolds, SSCs improve retention rates and enhance repair outcomes[134,135]. In immune-related diseases, such as rheumatoid arthritis, SSCs regulate the Treg/Th17 balance, suppress proinflammatory cytokines, alleviate synovial hyperplasia and bone erosion, and exhibit superior cartilage protection compared to MSC[136,137]. In tumor-related bone diseases ,such as multiple myeloma or bone metastases, SSCs inhibit tumor cell proliferation and promote normal bone remodeling by competitively occupying the bone marrow microenvironment. Additionally, they may secrete DKK-1 antagonists to reverse osteolytic lesions. Genetically engineered SSCs can further enhance the anti-tumor bone destruction effect[138,139].
Stem cell technology has achieved remarkable advancements in the field of bone and joint injury repair, with a variety of emerging technologies offering novel directions for precision treatment. Gene editing technologies, such as CRISPR, can enhance the differentiation potential of stem cells into osteoblasts or chondrocytes by either knocking out or overexpressing specific genes, while simultaneously inhibiting inflammatory or fibrosis-related pathways. Epigenetic regulation technologies employ small molecule compounds or miRNAs to modulate chromatin accessibility in stem cells, thereby promoting osteogenic differentiation and suppressing adipogenic differentiation[140,141]. The HA/PLA composite scaffold fabricated via 3D bioprinting mimics the natural bone-cartilage interface through its gradient pore structure. When loaded with BMSCs, it induces directional osteogenesis via the integrin β1-FAK signaling pathway under dynamic compressive stress conditions in a bioreactor[142]. Exosome therapy functions by delivering functional molecules, such as synovial MSC-derived exosomes carrying miR-140-5p to inhibit MMP-13 expression. Engineered exosomes, modified with CD44 targeting molecules, are enriched on injured cartilage surfaces, enabling cell-free inflammation modulation and matrix repair[6,143]. Biomechanical regulation technology stimulates stem cell polarization toward the osteogenic lineage by upregulating mechanosensitive factors such as YAP/TAZ through in vitro fluid shear stress stimulation. This process is closely associated with chromatin remodeling, including H3K27acmodifications that enhance the activity of osteogenic gene promoters[144]. Collectively, these innovative technologies provide new opportunities for bone and joint injury repair by improving the efficiency of directed stem cell differentiation, enhancing local microenvironment regulation capabilities, and optimizing delivery strategies.
Bone and joint injuries are prevalent in orthopedics, and the treatment of cartilage injuries remains a significant challenge. While traditional non-surgical and surgical treatments have demonstrated some efficacy for mild injuries, their therapeutic effects are still limited for large cartilage defects or full-thickness injuries. In recent years, stem cell therapy, particularly the application of SSC, has emerged as a promising strategy for repairing bone and joint injuries. SSCs possess the capacity for self-renewal and multi-lineage differentiation, enabling them to differentiate into osteoblasts and chondrocytes, thereby promoting bone and cartilage regeneration. In conditions such as epiphyseal injury, OA, avascular necrosis of the femoral head, and cartilage damage, SSCs exert their effects through multiple mechanisms, including enhancing cartilage and bone tissue regeneration, modulating inflammatory responses, improving local blood supply, and inhibiting cell apoptosis. Notably, in the treatment of OA, SSCs not only promote cartilage regeneration but also exhibit anti-inflammatory and tissue repair properties, which improve joint function and slow disease progression. Furthermore, SSCs have shown superior bone regeneration capabilities in treating avascular necrosis of the femoral head, offering a novel therapeutic option for this condition.
Although SSCs have demonstrated significant potential in treating bone and joint injuries, their clinical application still encounters numerous challenges. First, achieving large-scale acquisition of SSCs while preserving their stemness remains an urgent issue to address, as this may compromise their biological functions during in vitro expansion. Second, the survival rate, differentiation efficiency, and integration of SSCs into host tissues in vivo require further optimization. Moreover, long-term safety concerns, such as the risks of heterotopic ossification or tumorigenesis, necessitate prolonged monitoring to ensure the feasibility and safety of clinical translation.
In the future, three key strategies should be implemented to optimize the clinical application of SSCs: (1) Utilizing biomimetic 3D systems and hypoxic conditions during culture, sequentially adding FGF-2/BMP-4 for expansion and Wnt3a/BMP-2 for differentiation, and employing small molecule regulation; (2) Establish a comprehensive system including genome monitoring, telomere detection, and cell purification in terms of safety, introducing suicide genes and prioritize autologous transplantation; and (3) Complete animal experiments prior to clinical translation, and establish a GMP-standard serum-free culture process to ensure consistent and controllable quality. Through these strategies, SSCs are expected to become a critical approach for treating bone and joint injuries. Moreover, an in-depth exploration of the molecular mechanisms and signaling pathways underlying SSCs will facilitate the development of more efficient stem cell therapies, thereby accelerating the clinical translation of regenerative medicine in orthopedics.
In summary, SSCs hold significant clinical potential for addressing bone and joint injuries. As technological advancements continue and research deepens, their application prospects will expand, offering patients more effective treatment options and ultimately enhancing their quality of life.
| 1. | Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 451] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | Mizuta T, Benson WM, Foster BK, Paterson DC, Morris LL. Statistical analysis of the incidence of physeal injuries. J Pediatr Orthop. 1987;7:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 183] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Massengale M, Massengale JL, Benson CR, Baryawno N, Oki T, Steinhauser ML, Wang A, Balani D, Oh LS, Randolph MA, Gill TJ 3rd, Kronenberg HM, Scadden DT. Adult Prg4+ progenitors repair long-term articular cartilage wounds in vivo. JCI Insight. 2023;8:e167858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Wang W, Rigueur D, Lyons KM. TGFβ signaling in cartilage development and maintenance. Birth Defects Res C Embryo Today. 2014;102:37-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 5. | Haseeb A, Kc R, Angelozzi M, de Charleroy C, Rux D, Tower RJ, Yao L, Pellegrino da Silva R, Pacifici M, Qin L, Lefebvre V. SOX9 keeps growth plates and articular cartilage healthy by inhibiting chondrocyte dedifferentiation/osteoblastic redifferentiation. Proc Natl Acad Sci U S A. 2021;118:e2019152118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 6. | Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 550] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 7. | Roelofs AJ, De Bari C. Osteoarthritis year in review 2023: Biology. Osteoarthritis Cartilage. 2024;32:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 8. | Caine D, Patel V, Nguyen JC. Overuse Injury of the Epiphyseal Primary Physis. Semin Musculoskelet Radiol. 2024;28:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Olson SA, Horne P, Furman B, Huebner J, Al-Rashid M, Kraus VB, Guilak F. The role of cytokines in posttraumatic arthritis. J Am Acad Orthop Surg. 2014;22:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Tu YK, On Tong G, Wu CH, Sananpanich K, Kakinoki R. Soft-tissue injury in orthopaedic trauma. Injury. 2008;39 Suppl 4:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 1014] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 12. | Watson TS, Shurnas PS, Denker J. Treatment of Lisfranc joint injury: current concepts. J Am Acad Orthop Surg. 2010;18:718-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage. 2013;21:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 442] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 14. | Riordan EA, Little C, Hunter D. Pathogenesis of post-traumatic OA with a view to intervention. Best Pract Res Clin Rheumatol. 2014;28:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Madry H, Grün UW, Knutsen G. Cartilage repair and joint preservation: medical and surgical treatment options. Dtsch Arztebl Int. 2011;108:669-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Zhang W, Ouyang H, Dass CR, Xu J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 336] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 17. | Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Tian Z, Yu T, Liu J, Wang T, Higuchi A. Introduction to stem cells. Prog Mol Biol Transl Sci. 2023;199:3-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 19. | Adkar SS, Wu CL, Willard VP, Dicks A, Ettyreddy A, Steward N, Bhutani N, Gersbach CA, Guilak F. Step-Wise Chondrogenesis of Human Induced Pluripotent Stem Cells and Purification Via a Reporter Allele Generated by CRISPR-Cas9 Genome Editing. Stem Cells. 2019;37:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Gopalarethinam J, Nair AP, Iyer M, Vellingiri B, Subramaniam MD. Advantages of mesenchymal stem cell over the other stem cells. Acta Histochem. 2023;125:152041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 21. | Newton PT, Li L, Zhou B, Schweingruber C, Hovorakova M, Xie M, Sun X, Sandhow L, Artemov AV, Ivashkin E, Suter S, Dyachuk V, El Shahawy M, Gritli-Linde A, Bouderlique T, Petersen J, Mollbrink A, Lundeberg J, Enikolopov G, Qian H, Fried K, Kasper M, Hedlund E, Adameyko I, Sävendahl L, Chagin AS. A radical switch in clonality reveals a stem cell niche in the epiphyseal growth plate. Nature. 2019;567:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 22. | Gu X, Li F, Gao Y, Che X, Li P. HDAC4 mutant represses chondrocyte hypertrophy by locating in the nucleus and attenuates disease progression of posttraumatic osteoarthritis. BMC Musculoskelet Disord. 2022;23:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Müller L, Tunger A, Wobus M, von Bonin M, Towers R, Bornhäuser M, Dazzi F, Wehner R, Schmitz M. Immunomodulatory Properties of Mesenchymal Stromal Cells: An Update. Front Cell Dev Biol. 2021;9:637725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 24. | Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023-17023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2830] [Cited by in RCA: 5571] [Article Influence: 696.4] [Reference Citation Analysis (0)] |
| 25. | Huang RL, Yuan Y, Tu J, Zou GM, Li Q. Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis. 2014;5:e1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 26. | Vimalraj S, Arumugam B, Miranda PJ, Selvamurugan N. Runx2: Structure, function, and phosphorylation in osteoblast differentiation. Int J Biol Macromol. 2015;78:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 278] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 27. | Burger MG, Grosso A, Briquez PS, Born GME, Lunger A, Schrenk F, Todorov A, Sacchi V, Hubbell JA, Schaefer DJ, Banfi A, Di Maggio N. Robust coupling of angiogenesis and osteogenesis by VEGF-decorated matrices for bone regeneration. Acta Biomater. 2022;149:111-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 28. | Javidi-Sharifi N, Martinez J, English I, Joshi SK, Scopim-Ribeiro R, Viola SK, Edwards DK 5th, Agarwal A, Lopez C, Jorgens D, Tyner JW, Druker BJ, Traer E. Correction: FGF2-FGFR1 signaling regulates release of leukemia-protective exosomes from bone marrow stromal cells. Elife. 2019;8:e47174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Ono N, Balani DH, Kronenberg HM. Stem and progenitor cells in skeletal development. Curr Top Dev Biol. 2019;133:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Mizuhashi K, Ono W, Matsushita Y, Sakagami N, Takahashi A, Saunders TL, Nagasawa T, Kronenberg HM, Ono N. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature. 2018;563:254-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 286] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 31. | Tsukasaki M, Komatsu N, Negishi-Koga T, Huynh NC, Muro R, Ando Y, Seki Y, Terashima A, Pluemsakunthai W, Nitta T, Nakamura T, Nakashima T, Ohba S, Akiyama H, Okamoto K, Baron R, Takayanagi H. Periosteal stem cells control growth plate stem cells during postnatal skeletal growth. Nat Commun. 2022;13:4166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Serowoky MA, Arata CE, Crump JG, Mariani FV. Skeletal stem cells: insights into maintaining and regenerating the skeleton. Development. 2020;147:dev179325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 33. | Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117:5281-5288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 34. | Chang YP, Hong HP, Lee YH, Liu IH. The canine epiphyseal-derived mesenchymal stem cells are comparable to bone marrow derived-mesenchymal stem cells. J Vet Med Sci. 2015;77:273-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Sanghani A, Chimutengwende-Gordon M, Adesida A, Khan W. Applications of stem cell therapy for physeal injuries. Curr Stem Cell Res Ther. 2013;8:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Zhu Q, Ding L, Yue R. Skeletal stem cells: a game changer of skeletal biology and regenerative medicine? Life Med. 2022;1:294-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T, Lu WJ, Senarath-Yapa K, Chung MT, Marecic O, Tran M, Yan KS, Upton R, Walmsley GG, Lee AS, Sahoo D, Kuo CJ, Weissman IL, Longaker MT. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160:285-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 575] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 38. | Matsushita Y, Ono W, Ono N. Skeletal Stem Cells for Bone Development and Repair: Diversity Matters. Curr Osteoporos Rep. 2020;18:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Ono N, Ono W, Mizoguchi T, Nagasawa T, Frenette PS, Kronenberg HM. Vasculature-associated cells expressing nestin in developing bones encompass early cells in the osteoblast and endothelial lineage. Dev Cell. 2014;29:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 40. | Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, Levin D, Schwartz MG, Uygur A, Hayakawa Y, Gross S, Renz BW, Setlik W, Martinez AN, Chen X, Nizami S, Lee HG, Kang HP, Caldwell JM, Asfaha S, Westphalen CB, Graham T, Jin G, Nagar K, Wang H, Kheirbek MA, Kolhe A, Carpenter J, Glaire M, Nair A, Renders S, Manieri N, Muthupalani S, Fox JG, Reichert M, Giraud AS, Schwabe RF, Pradere JP, Walton K, Prakash A, Gumucio D, Rustgi AK, Stappenbeck TS, Friedman RA, Gershon MD, Sims P, Grikscheit T, Lee FY, Karsenty G, Mukherjee S, Wang TC. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 522] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 41. | Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2063] [Cited by in RCA: 2077] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 42. | Salhotra A, Shah HN, Levi B, Longaker MT. Mechanisms of bone development and repair. Nat Rev Mol Cell Biol. 2020;21:696-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 663] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 43. | Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A. 2014;111:12097-12102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 547] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 44. | Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 703] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 45. | Bianco P, Cancedda FD, Riminucci M, Cancedda R. Bone formation via cartilage models: the "borderline" chondrocyte. Matrix Biol. 1998;17:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 129] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Böhm AM, Dirckx N, Tower RJ, Peredo N, Vanuytven S, Theunis K, Nefyodova E, Cardoen R, Lindner V, Voet T, Van Hul M, Maes C. Activation of Skeletal Stem and Progenitor Cells for Bone Regeneration Is Driven by PDGFRβ Signaling. Dev Cell. 2019;51:236-254.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 47. | Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1158] [Article Influence: 115.8] [Reference Citation Analysis (0)] |
| 48. | Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24:274-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 413] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 49. | Colnot C, Zhang X, Knothe Tate ML. Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches. J Orthop Res. 2012;30:1869-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 50. | Duchamp de Lageneste O, Julien A, Abou-Khalil R, Frangi G, Carvalho C, Cagnard N, Cordier C, Conway SJ, Colnot C. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun. 2018;9:773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 378] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 51. | Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 754] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 52. | Debnath S, Yallowitz AR, McCormick J, Lalani S, Zhang T, Xu R, Li N, Liu Y, Yang YS, Eiseman M, Shim JH, Hameed M, Healey JH, Bostrom MP, Landau DA, Greenblatt MB. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562:133-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 452] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 53. | Tack P, Victor J, Gemmel P, Annemans L. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online. 2016;15:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 811] [Cited by in RCA: 602] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 54. | Dai H, Zhang H, Qiu Z, Shi Q. Periosteum-derived skeletal stem cells encapsulated in platelet-rich plasma enhance the repair of bone defect. Tissue Cell. 2023;83:102144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Ishihara A, Ohmine K, Weisbrode SE, Bertone AL. Effect of Intra-Medullar and Intra-Venous Infusions of Mesenchymal Stem Cells on Cell Engraftment by In-Vivo Cell Tracking and Osteoinductivity in Rabbit Long Bones: A Pilot Study. Orthop Muscular Syst. 2014;3:1000172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Tuan RS, Chen AF, Klatt BA. Cartilage regeneration. J Am Acad Orthop Surg. 2013;21:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 57. | Berthelot JM, Sibilia J. Rampant infections of bone marrow stem cell niches as triggers for spondyloarthropathies and rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:329-336. [PubMed] |
| 58. | Mo C, Guo J, Qin J, Zhang X, Sun Y, Wei H, Cao D, Zhang Y, Zhao C, Xiong Y, Zhang Y, Sun Y, Shen L, Yue R. Single-cell transcriptomics of LepR-positive skeletal cells reveals heterogeneous stress-dependent stem and progenitor pools. EMBO J. 2022;41:e108415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 59. | Yamakawa H, Kusumoto D, Hashimoto H, Yuasa S. Stem Cell Aging in Skeletal Muscle Regeneration and Disease. Int J Mol Sci. 2020;21:1830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 60. | Iannotti JP. Growth Plate Physiology and Pathology. Orthop Clin North Am. 1990;21:1-17. [DOI] [Full Text] |
| 61. | Shaw N, Erickson C, Bryant SJ, Ferguson VL, Krebs MD, Hadley-Miller N, Payne KA. Regenerative Medicine Approaches for the Treatment of Pediatric Physeal Injuries. Tissue Eng Part B Rev. 2018;24:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 62. | Mann DC, Rajmaira S. Distribution of physeal and nonphyseal fractures in 2,650 long-bone fractures in children aged 0-16 years. J Pediatr Orthop. 1990;10:713-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 182] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Worlock P, Stower M. Fracture patterns in Nottingham children. J Pediatr Orthop. 1986;6:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 128] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Macsai CE, Hopwood B, Chung R, Foster BK, Xian CJ. Structural and molecular analyses of bone bridge formation within the growth plate injury site and cartilage degeneration at the adjacent uninjured area. Bone. 2011;49:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Blondin E, Stourbe O, Plancq MC, Deroussen F, Gouron R, Klein C. Outcomes of pediatric distal tibial physeal fractures. Orthop Traumatol Surg Res. 2022;108:103199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 66. | Meyers AB. Physeal bridges: causes, diagnosis, characterization and post-treatment imaging. Pediatr Radiol. 2019;49:1595-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Solidum JGN, Jeong Y, Heralde F 3rd, Park D. Differential regulation of skeletal stem/progenitor cells in distinct skeletal compartments. Front Physiol. 2023;14:1137063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Lerbs T, Cui L, Muscat C, Saleem A, van Neste C, Domizi P, Chan C, Wernig G. Expansion of Bone Precursors through Jun as a Novel Treatment for Osteoporosis-Associated Fractures. Stem Cell Reports. 2020;14:603-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Muruganandan S, Pierce R, Teguh DA, Perez RF, Bell N, Nguyen B, Hohl K, Snyder BD, Grinstaff MW, Alberico H, Woods D, Kong Y, Sima C, Bhagat S, Ho K, Rosen V, Gamer L, Ionescu AM. A FoxA2+ long-term stem cell population is necessary for growth plate cartilage regeneration after injury. Nat Commun. 2022;13:2515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 70. | Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1267] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 71. | Ghodadra N, Singh K. Recombinant human bone morphogenetic protein-2 in the treatment of bone fractures. Biologics. 2008;2:345-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 72. | Wu M, Chen G, Li YP. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 734] [Cited by in RCA: 1173] [Article Influence: 130.3] [Reference Citation Analysis (0)] |
| 73. | Mak KK, Kronenberg HM, Chuang PT, Mackem S, Yang Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development. 2008;135:1947-1956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 74. | Abad V, Meyers JL, Weise M, Gafni RI, Barnes KM, Nilsson O, Bacher JD, Baron J. The role of the resting zone in growth plate chondrogenesis. Endocrinology. 2002;143:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 75. | Liu W, Zou M, Chen M, Zhang Z, Mao Y, Yang Y, Liu Y, Shi Q, Wang X, Zhang F. Hypoxic environment promotes angiogenesis and bone bridge formation by activating Notch/RBPJ signaling pathway in HUVECs. Genomics. 2024;116:110838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 76. | Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1501] [Cited by in RCA: 1469] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 77. | Fischerauer E, Heidari N, Neumayer B, Deutsch A, Weinberg AM. The spatial and temporal expression of VEGF and its receptors 1 and 2 in post-traumatic bone bridge formation of the growth plate. J Mol Histol. 2011;42:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Su YW, Chung R, Ruan CS, Chim SM, Kuek V, Dwivedi PP, Hassanshahi M, Chen KM, Xie Y, Chen L, Foster BK, Rosen V, Zhou XF, Xu J, Xian CJ. Neurotrophin-3 Induces BMP-2 and VEGF Activities and Promotes the Bony Repair of Injured Growth Plate Cartilage and Bone in Rats. J Bone Miner Res. 2016;31:1258-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 79. | Shkhyan R, Flynn C, Lamoure E, Sarkar A, Van Handel B, Li J, York J, Banks N, Van der Horst R, Liu NQ, Lee S, Bajaj P, Vadivel K, Harn HI, Tassey J, Lozito T, Lieberman JR, Chuong CM, Hurtig MS, Evseenko D. Inhibition of a signaling modality within the gp130 receptor enhances tissue regeneration and mitigates osteoarthritis. Sci Transl Med. 2023;15:eabq2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 80. | Li PL, Chen DF, Li XT, Hao RC, Zhao ZD, Li ZL, Yin BF, Tang J, Luo YW, Wu CT, Nie JJ, Zhu H. Microgel-based carriers enhance skeletal stem cell reprogramming towards immunomodulatory phenotype in osteoarthritic therapy. Bioact Mater. 2024;34:204-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 81. | Chen X, Ji Y, Liu R, Zhu X, Wang K, Yang X, Liu B, Gao Z, Huang Y, Shen Y, Liu H, Sun H. Mitochondrial dysfunction: roles in skeletal muscle atrophy. J Transl Med. 2023;21:503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 116] [Reference Citation Analysis (0)] |
| 82. | Benjamin DI, Brett JO, Both P, Benjamin JS, Ishak HL, Kang J, Kim S, Chung M, Arjona M, Nutter CW, Tan JH, Krishnan AK, Dulay H, Louie SM, de Morree A, Nomura DK, Rando TA. Multiomics reveals glutathione metabolism as a driver of bimodality during stem cell aging. Cell Metab. 2023;35:472-486.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 83. | Buckwalter JA, Brown TD. Joint Injury, Repair, and Remodeling. Clinical Orthop Relat Res. 2004;423:7-16. [DOI] [Full Text] |
| 84. | Abramoff B, Caldera FE. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med Clin North Am. 2020;104:293-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 675] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 85. | Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, Goldring SR, Jones G, Teichtahl AJ, Pelletier JP. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 1214] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 86. | Peat G, Thomas MJ. Osteoarthritis year in review 2020: epidemiology & therapy. Osteoarthritis Cartilage. 2021;29:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 87. | Pereira D, Ramos E, Branco J. Osteoarthritis. Acta Med Port. 2015;28:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 88. | Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33:2271-2279. [PubMed] |
| 89. | Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 940] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 90. | Brandt KD, Radin EL, Dieppe PA, van de Putte L. Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis. 2006;65:1261-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 314] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 91. | Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 531] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 92. | Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 879] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 93. | Cho Y, Jeong S, Kim H, Kang D, Lee J, Kang SB, Kim JH. Disease-modifying therapeutic strategies in osteoarthritis: current status and future directions. Exp Mol Med. 2021;53:1689-1696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 162] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 94. | Leijten JC, Emons J, Sticht C, van Gool S, Decker E, Uitterlinden A, Rappold G, Hofman A, Rivadeneira F, Scherjon S, Wit JM, van Meurs J, van Blitterswijk CA, Karperien M. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 2012;64:3302-3312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 95. | Zhang FX, Dou Y, Zhang B, Zhang Z, Du MZ, Chien MH, Du JK, Ai LY, Chen R, Jiang D. Skeletal Stem Cell-Derived Exosomes Promote Meniscal Tear Healing and Ameliorate Secondary Osteoarthritis. Am J Sports Med. 2024;52:2512-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 96. | Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1162] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 97. | Blair HC, Zaidi M, Schlesinger PH. Mechanisms balancing skeletal matrix synthesis and degradation. Biochem J. 2002;364:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 98. | Zhu J, Yang S, Qi Y, Gong Z, Zhang H, Liang K, Shen P, Huang YY, Zhang Z, Ye W, Yue L, Fan S, Shen S, Mikos AG, Wang X, Fang X. Stem cell-homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model. Sci Adv. 2022;8:eabk0011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 108] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 99. | Tan SSH, Tjio CKE, Wong JRY, Wong KL, Chew JRJ, Hui JHP, Toh WS. Mesenchymal Stem Cell Exosomes for Cartilage Regeneration: A Systematic Review of Preclinical In Vivo Studies. Tissue Eng Part B Rev. 2021;27:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 100. | Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol Biol. 2016;1416:123-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 304] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 101. | Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 798] [Cited by in RCA: 1135] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 102. | Vincent TL, Alliston T, Kapoor M, Loeser RF, Troeberg L, Little CB. Osteoarthritis Pathophysiology: Therapeutic Target Discovery may Require a Multifaceted Approach. Clin Geriatr Med. 2022;38:193-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 103. | Chan CKF, Gulati GS, Sinha R, Tompkins JV, Lopez M, Carter AC, Ransom RC, Reinisch A, Wearda T, Murphy M, Brewer RE, Koepke LS, Marecic O, Manjunath A, Seo EY, Leavitt T, Lu WJ, Nguyen A, Conley SD, Salhotra A, Ambrosi TH, Borrelli MR, Siebel T, Chan K, Schallmoser K, Seita J, Sahoo D, Goodnough H, Bishop J, Gardner M, Majeti R, Wan DC, Goodman S, Weissman IL, Chang HY, Longaker MT. Identification of the Human Skeletal Stem Cell. Cell. 2018;175:43-56.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 425] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 104. | Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18:258-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 503] [Article Influence: 167.7] [Reference Citation Analysis (0)] |
| 105. | Zhang F, Wang L, Li Y, Liu W, Duan F, Huang R, Chen X, Chang SC, Du Y, Na J. Optimizing mesoderm progenitor selection and three-dimensional microniche culture allows highly efficient endothelial differentiation and ischemic tissue repair from human pluripotent stem cells. Stem Cell Res Ther. 2017;8:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 106. | Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3539] [Cited by in RCA: 3300] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 107. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15203] [Article Influence: 584.7] [Reference Citation Analysis (0)] |
| 108. | Rong L, Zhang L, Yang Z, Xu L. New insights into the properties, functions, and aging of skeletal stem cells. Osteoporos Int. 2023;34:1311-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 109. | Tare RS, Babister JC, Kanczler J, Oreffo RO. Skeletal stem cells: phenotype, biology and environmental niches informing tissue regeneration. Mol Cell Endocrinol. 2008;288:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Gulati GS, Murphy MP, Marecic O, Lopez M, Brewer RE, Koepke LS, Manjunath A, Ransom RC, Salhotra A, Weissman IL, Longaker MT, Chan CKF. Isolation and functional assessment of mouse skeletal stem cell lineage. Nat Protoc. 2018;13:1294-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 111. | Aarvold A, Smith JO, Tayton ER, Jones AM, Dawson JI, Lanham S, Briscoe A, Dunlop DG, Oreffo RO. A tissue engineering strategy for the treatment of avascular necrosis of the femoral head. Surgeon. 2013;11:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 112. | Aarvold A, Smith JO, Tayton ER, Jones AM, Dawson JI, Lanham S, Briscoe A, Dunlop DG, Oreffo RO. From bench to clinic and back: skeletal stem cells and impaction bone grafting for regeneration of bone defects. J Tissue Eng Regen Med. 2014;8:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 113. | Ghivizzani SC, Oligino TJ, Robbins PD, Evans CH. Cartilage Injury and Repair. Phys Med Rehabil Clin North Am. 2000;11:289-307. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 114. | Toosi S, Behravan N, Behravan J. Nonunion fractures, mesenchymal stem cells and bone tissue engineering. J Biomed Mater Res A. 2018;106:2552-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 115. | Kim YG, Choi J, Kim K. Mesenchymal Stem Cell-Derived Exosomes for Effective Cartilage Tissue Repair and Treatment of Osteoarthritis. Biotechnol J. 2020;15:e2000082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 116. | Ciuffreda MC, Malpasso G, Musarò P, Turco V, Gnecchi M. Protocols for in vitro Differentiation of Human Mesenchymal Stem Cells into Osteogenic, Chondrogenic and Adipogenic Lineages. Methods Mol Biol. 2016;1416:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 117. | Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1459] [Cited by in RCA: 1381] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 118. | Gee SM, Posner M. Meniscus Anatomy and Basic Science. Sports Med Arthrosc Rev. 2021;29:e18-e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 119. | Zhang H, Kong XQ, Cheng C, Liang MH. A correlative study between prevalence of chondromalacia patellae and sports injury in 4068 students. Chin J Traumatol. 2003;6:370-374. [PubMed] |
| 120. | Feehan J, Macfarlane C, Vaughan B. Conservative management of a traumatic meniscal injury utilising osteopathy and exercise rehabilitation: A case report. Complement Ther Med. 2017;33:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 121. | Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 1157] [Article Influence: 144.6] [Reference Citation Analysis (0)] |
| 122. | Tian Y, Yuan W, Fujita N, Wang J, Wang H, Shapiro IM, Risbud MV. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-κB. Am J Pathol. 2013;182:2310-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 123. | Garimella A, Ghosh SB, Bandyopadhyay-Ghosh S. Biomaterials for bone tissue engineering: achievements to date and future directions. Biomed Mater. 2024;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 124. | Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1444] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 125. | Schmitz JP, Hollinger JO. The Critical Size Defect as an Experimental Model for Craniomandibulofacial Nonunions. Clin Orthop Relat Res. 1986;205:299-308. [RCA] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 449] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 126. | El-Rashidy AA, Roether JA, Harhaus L, Kneser U, Boccaccini AR. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017;62:1-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 385] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 127. | Rastogi P, Kandasubramanian B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019;11:042001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 326] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 128. | de Souza JB, Rosa GDS, Rossi MC, Stievani FC, Pfeifer JPH, Krieck AMT, Bovolato ALC, Fonseca-Alves CE, Borrás VA, Alves ALG. In Vitro Biological Performance of Alginate Hydrogel Capsules for Stem Cell Delivery. Front Bioeng Biotechnol. 2021;9:674581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 129. | Takegahara N, Kim H, Choi Y. Unraveling the intricacies of osteoclast differentiation and maturation: insight into novel therapeutic strategies for bone-destructive diseases. Exp Mol Med. 2024;56:264-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 130. | Carballo-Pedrares N, Sanjurjo-Rodriguez C, Señarís J, Díaz-Prado S, Rey-Rico A. Chondrogenic Differentiation of Human Mesenchymal Stem Cells via SOX9 Delivery in Cationic Niosomes. Pharmaceutics. 2022;14:2327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 131. | Ribeiro AF Jr, Souza LS, Almeida CF, Ishiba R, Fernandes SA, Guerrieri DA, Santos ALF, Onofre-Oliveira PCG, Vainzof M. Muscle satellite cells and impaired late stage regeneration in different murine models for muscular dystrophies. Sci Rep. 2019;9:11842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 132. | Ren S, Fu X, Guo W, Bai R, Li S, Zhang T, Liu J, Wang Z, Zhao H, Suo S, Zhang W, Jia M, Ji W, Hu P, Chen Y. Profound cellular defects attribute to muscular pathogenesis in the rhesus monkey model of Duchenne muscular dystrophy. Cell. 2024;187:6669-6686.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 133. | Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol. 2007;25:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 134. | Cao Y, Gang X, Sun C, Wang G. Mesenchymal Stem Cells Improve Healing of Diabetic Foot Ulcer. J Diabetes Res. 2017;2017:9328347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 135. | Abolgheit S, Abdelkader S, Aboushelib M, Omar E, Mehanna R. Bone marrow-derived mesenchymal stem cells and extracellular vesicles enriched collagen chitosan scaffold in skin wound healing (a rat model). J Biomater Appl. 2021;36:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 136. | Feng Z, Zhai Y, Zheng Z, Yang L, Luo X, Dong X, Han Q, Jin J, Chen ZN, Zhu P. Loss of A20 in BM-MSCs regulates the Th17/Treg balance in Rheumatoid Arthritis. Sci Rep. 2018;8:427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 137. | Su R, Li B, Wu R, Xie Y, Gao A, Gao C, Li X, Wang C. Stratified distribution of Th17 and Treg cells in patients with multi-stage rheumatoid arthritis. Arthritis Res Ther. 2023;25:55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 138. | Buenrostro D, Park SI, Sterling JA. Dissecting the role of bone marrow stromal cells on bone metastases. Biomed Res Int. 2014;2014:875305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 139. | Shu B, Zhao Y, Zhao S, Pan H, Xie R, Yi D, Lu K, Yang J, Xue C, Huang J, Wang J, Zhao D, Xiao G, Wang Y, Chen D. Inhibition of Axin1 in osteoblast precursor cells leads to defects in postnatal bone growth through suppressing osteoclast formation. Bone Res. 2020;8:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 140. | Yang S, Guo S, Tong S, Sun X. Promoting Osteogenic Differentiation of Human Adipose-Derived Stem Cells by Altering the Expression of Exosomal miRNA. Stem Cells Int. 2019;2019:1351860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 141. | Taherian M, Bayati P, Mojtabavi N. Stem cell-based therapy for fibrotic diseases: mechanisms and pathways. Stem Cell Res Ther. 2024;15:170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 142. | Wang W, Zhang B, Li M, Li J, Zhang C, Han Y, Wang L, Wang K, Zhou C, Liu L, Fan Y, Zhang X. 3D printing of PLA/n-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering. Compos Part B: Eng. 2021;224:109192. [DOI] [Full Text] |
| 143. | Lim GT, You DG, Han HS, Lee H, Shin S, Oh BH, Kumar EKP, Um W, Kim CH, Han S, Lee S, Lim S, Yoon HY, Kim K, Kwon IC, Jo DG, Cho YW, Park JH. Bioorthogonally surface-edited extracellular vesicles based on metabolic glycoengineering for CD44-mediated targeting of inflammatory diseases. J Extracell Vesicles. 2021;10:e12077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 144. | Chen X, Ji X, Lao Z, Pan B, Qian Y, Yang W. Role of YAP/TAZ in bone diseases: A transductor from mechanics to biology. J Orthop Translat. 2025;51:13-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |