Published online Sep 18, 2024. doi: 10.5312/wjo.v15.i9.858
Revised: August 1, 2024
Accepted: August 28, 2024
Published online: September 18, 2024
Processing time: 178 Days and 0.8 Hours
Platelet-rich plasma (PRP) injection is used as an alternative non-operative mana
To assess and conclude the research-based study systematically to analyse the efficacy of PRP on DQT.
This systematic review used the Cochrane Handbook for Systematic Reviews and the guideline of preferred reporting items for systematic review and meta-analysis. A systematic literature search was applied to 11 databases. The authors assessed the study quality and risk of bias of each included study. Results of the meta-analysis were presented using mean difference (MD)/standardized mean difference (SMD) and 95% confidence interval (CI).
The authors evaluated 275 studies found in the literature search; 12 studies met the criteria for this review, and then the study quality and risk of bias were assessed. Pooled analysis of data from two studies involving 194 subjects with DQT showed that, compared with conservative treatment, PRP injection was associated with a greater reduction in visual analog scale pain in one month and six months after treatment (MD: -0.67, P value < 0.00001; MD: -1.16, P value < 0.00001) and the increase of Mayo’s wrist score in one month and six months after treatment (SMD: 3.72, P value < 0.00001; SMD: 4.44, P value < 0.00001).
PRP can be used as an alternative non-operative treatment for DQT due to the tissue regenerative effect of PRP.
Core Tip: De Quervain’s tenosynovitis (DQT) is a common tendon disorder characterized by wrist pain and tenderness at the first dorsal compartment of the wrist. Platelet-rich plasma (PRP) injection is currently used as an alternative non-operative management for DQT to regenerate tendon healing due to its tissue regenerative effect, which provides better pain mana
- Citation: Hidajat NN, Magetsari RMSN, Steven G, Budiman J, Prasetiyo GT. Platelet-rich plasma for de Quervain’s tenosynovitis: A systematic review and meta-analysis. World J Orthop 2024; 15(9): 858-869
- URL: https://www.wjgnet.com/2218-5836/full/v15/i9/858.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i9.858
Tendon disorders are frequent conditions that limit daily activities and influence the quality of life[1-4]. De Quervain’s tenosynovitis (DQT) is a common tendon disorder characterized by wrist pain and tenderness at the first dorsal compartment of the wrist; correlated with overworked, routine, and sustained activities that involve the tendons of abductor pollicis longus (APL) and extensor pollicis brevis (EPB)[5-14]. The incidence of DQT is 0.5% in males and 1.32% in females, and female between 30 to 50 years is the highest risk[6-9,11,12]. The diagnosis of DQT is confirmed with Finkelstein’s test or Eichhoff’s test and radiological examination if required[7,8,11].
Most patients respond well to non-surgical management, including activity modification, splinting, physical therapy, non-steroidal anti-inflammatory drugs (NSAIDs) medication, and corticosteroid injection[10-12]. In some cases where non-surgical management fails, primarily because of a false injection site and anatomical variation in the first dorsal compartment, a surgical release of the first dorsal compartment and decompression of the stenosed APL and EPB tendons is preferred[8,9,12,13,15]. Platelet-rich plasma (PRP) injection is currently used as an alternative non-operative mana
Many studies have explained PRP injection in DQT, but a systematic review focusing on PRP used for DQT is unavailable. This systematic review and meta-analysis aimed to systematically evaluate and conclude the research-based studies to report PRP’s efficacy for DQT.
This study’s protocol was registered on the International Prospective Register of Systematic Reviews with register number CRD42023466818. The Cochrane Handbook for systematic reviews and the preferred reporting items for systematic review and meta-analysis (PRISMA) were used as guidelines in this systematic review-meta-analysis[22-24].
Inclusion criteria: Publication type: Full-text manuscripts reported the efficacy of PRP for DQT primary research study; Articles published in English; Articles published in January 2000 - December 2023; The study used humans as the subject; The objective, methodology, and outcome of the study must discuss the efficacy of PRP for DQT.
Exclusion criteria: Review; Variables that were associated with the efficacy of PRP for DQT.
A systematic literature search was performed in these online scientific databases: ClinicalKey, Cochrane Library, EBSCOhost, Emerald Insight, Europe PMC, Google Scholar, JSTOR, ProQuest, PubMed, ScienceDirect, and Springer Link. The search used the following keywords for the title and abstract: (platelet-rich plasma OR PRP) AND (stenosing tenosynovitis OR de quervain tenosynovitis OR de quervain disease). The references of included studies were analysed to ensure that all published studies were included.
Articles were selected for evaluation after two authors (NNH and RSNM) had evaluated keywords from the online medical bibliographic databases. The results of the literature search were discussed with other authors (GS, JB and GTP), and any inconsistencies in results were deliberated. Three authors independently assessed selected full papers. Two authors independently evaluated selected studies for this systematic review to confirm the results (NNH and GS). The data from the included studies were presented in a summary table containing the key points of each study. The key points of each study were: First author, country, year, study design, sample, sample characteristic, outcome measure, and result.
The first author evaluated the study quality and risk of bias of each included article and deliberated them with other authors. Version 2 of the Cochrane risk-of-bias tool for randomized trials (ROB 2) was performed to evaluate randomized control trial studies in which the interpretation was high risk or some concerns or low risk[25]. Newcastle–Ottawa scale for the prospective study was applied to assess the quality and risk of bias of the prospective study; the explanation of the result was: ≥ 7 points were included in the good study, 5-6 points were included in the fair study, < 5 points were included in the poor study. The Joanna Briggs Institute (JBI) critical appraisal checklist was used to evaluate the descriptive study’s quality and risk of bias[25-27].
Review manager 5.4 was used to assess the overall effect size of PRP efficacy for DQT. A P value < 0.05 was considered statistically significant. Results were summarized using the mean difference (MD)/standardized mean difference (SMD) and 95% confidence interval (CI). Heterogeneity was evaluated using the I2 (inconsistency) value; < 25%, 26%-50%, and > 50% were considered low, moderate, and high heterogeneity. Funnel plots were performed to assess the risk of publication bias[24,28].
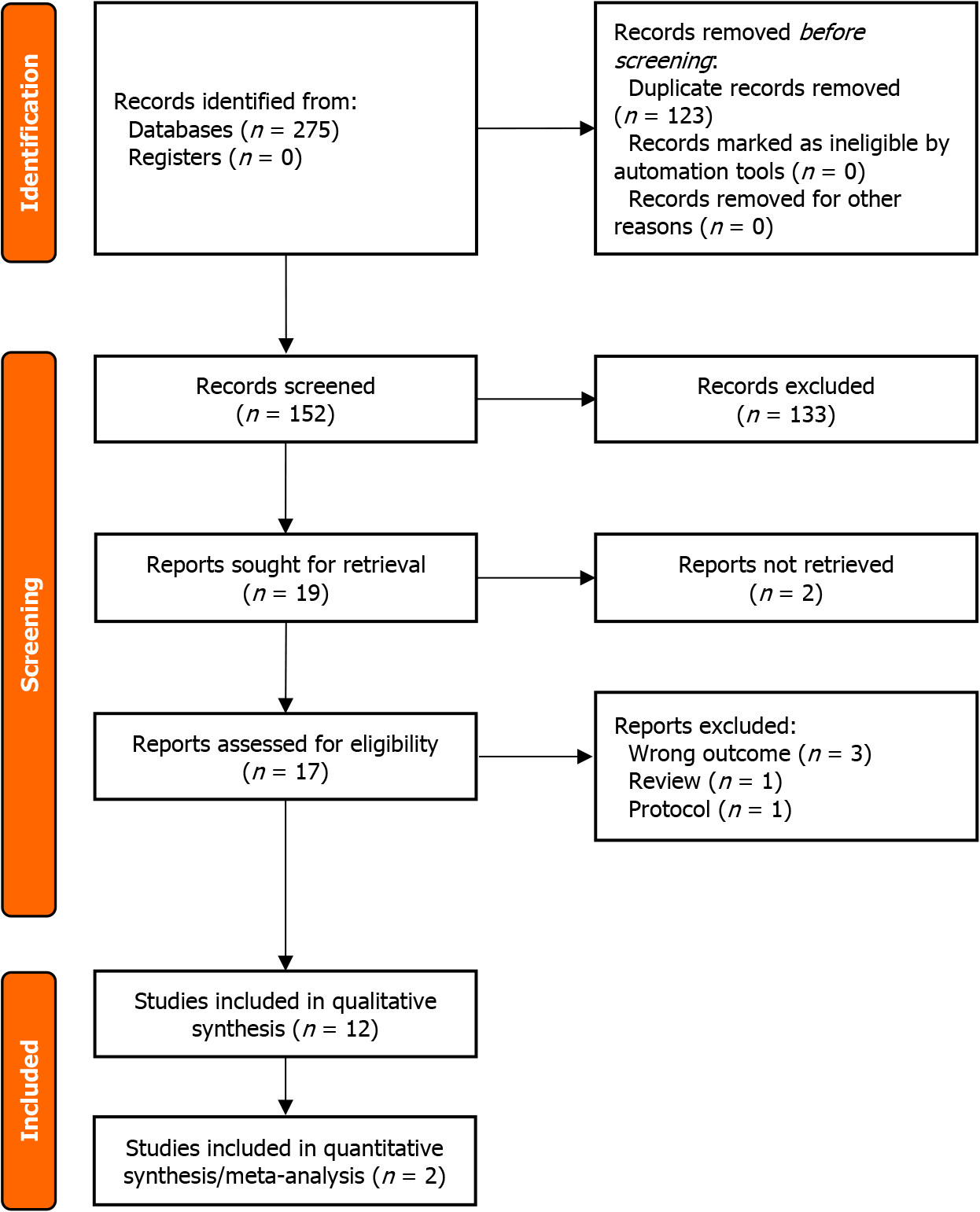
PRISMA flow diagram is presented in Figure 1. Initially, 275 peer-reviewed studies were identified from online medical research databases. After duplicates were removed, 152 studies were proceeded with the title and abstract screening. Seventeen articles were assessed for eligibility, of which 12 studies were included in the systematic review and two studies were included in the quantitative synthesis (meta-analysis).
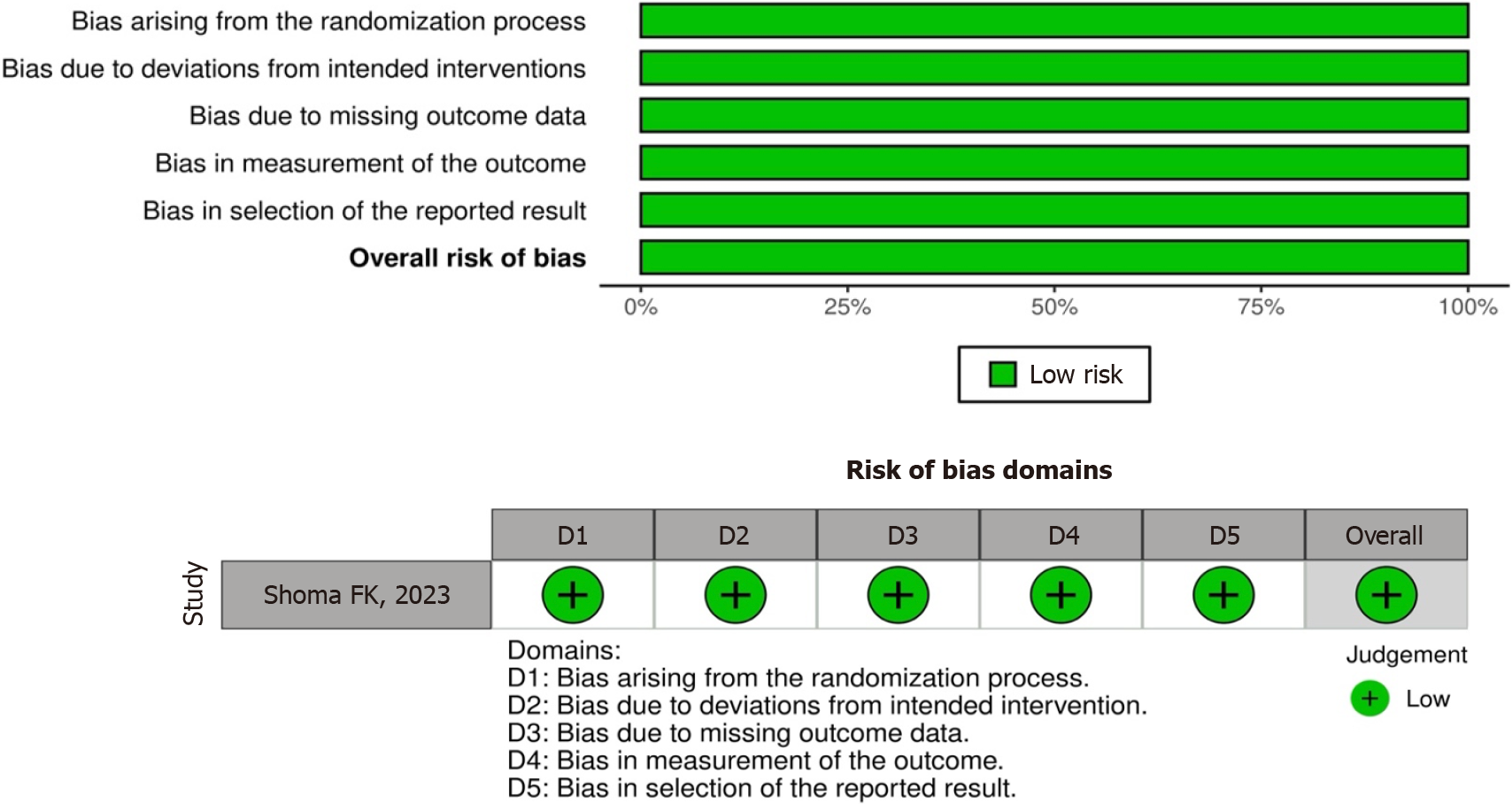
All included studies were associated with the efficacy of PRP for DQT. Figure 2 provides the quality scores for ran
| No. | Ref. | Selection | Comparability | Outcome | Total | |||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | ||||
| 1 | Asaad et al[9], 2023 | × | × | × | × | × | × | 6 | ||
| 2 | Deb et al[5], 2020 | × | × | × | × | × | × | × | × | 8 |
| 3 | Giroti et al[12], 2021 | × | × | × | × | ×× | × | × | × | 9 |
| 4 | Gulati and Ramesh[6], 2022 | × | × | × | × | ×× | × | × | × | 9 |
| 5 | Johurul et al[29], 2019 | × | × | × | × | ×× | × | × | × | 9 |
| 6 | Kumar et al[14], 2023 | × | × | × | × | ×× | × | × | × | 9 |
| 7 | Ramesh et al[30], 2018 | × | × | × | × | × | × | 6 | ||
| 8 | Sheikh et al[10], 2020 | × | × | × | × | ×× | × | × | × | 9 |
| No. | Major components | 1 | 2 |
| 1 | Were patient’s demographic characteristics clearly described? | Y | Y |
| 2 | Was the patient’s history clearly described and presented as a timeline? | Y | Y |
| 3 | Was the current clinical condition of the patient on presentation clearly described? | Y | Y |
| 4 | Were diagnostic tests or assessment methods and the results clearly described? | Y | Y |
| 5 | Was the intervention(s) or treatment procedure(s) clearly described? | Y | Y |
| 6 | Was the post-intervention clinical condition clearly described? | Y | Y |
| 7 | Were adverse events (harms) or unanticipated events identified and described? | Y | Y |
| 8 | Does the case report provide takeaway lessons? | Y | Y |
| Overall appraisal | I | I |
| No. | Major components | 1 |
| 1 | Were there clear criteria for inclusion in the case series? | Y |
| 2 | Was the condition measured in a standard, reliable way for all participants included in the case series? | Y |
| 3 | Were valid methods used for identification of the condition for all participants included in the case series? | Y |
| 4 | Did the case series have consecutive inclusion of participants? | Y |
| 5 | Did the case series have complete inclusion of participants? | Y |
| 6 | Was there clear reporting of the demographics of the participants in the study? | Y |
| 7 | Was there clear reporting of clinical information of the participants? | Y |
| 8 | Were the outcomes or follow-up results of cases clearly reported? | Y |
| 9 | Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | Y |
| 10 | Was statistical analysis appropriate? | NA |
| Overall appraisal | I |
Table 4 shows the study characteristics for the included studies. Most studies were prospective studies that discussed the efficacy of PRP in reducing pain in DQT subjects.
| No. | Ref. | Study design | Sample (n) | Sample characteristic: age (year), gender (male, female) | Outcome measure | Result |
| 1 | Mahdi Al-ardi[8], 2017, Iraq | Case series | PRP: 30 | Age: > 25 | Pain reduction (VAS) | PRP injection reduced pain in 1, 3, and 6 months after treatment (VAS in baseline: 5.92; 1 months: 2.11; 3 months: 2.01; 6 months: 2.01; P < 0.001) |
| 2 | Asaad et al[9], 2023, Iraq | Prospective study | PRP: 12 | Age: 43 (26-68); Gender: F | Pain reduction (VAS) and USG evaluation of tendon | PRP injection reduced pain significantly in 1 and 3 months after treatment (VAS in baseline: 8.66 ± 0.65; 1 months: 4.5 ± 1.97; 3 months: 1.91 ± 2.71; P < 0.001); decreased tendon sheath effusion significantly in 1 and 3 months after treatment (baseline: 2.07 ± 0.52; 1 months: 1.6 ± 0.75; 3 months: 0.73 ± 0.76; P < 0.001); decreased retinaculum thickness significantly in 1 and 3 months (baseline: 1.89 ± 0.5; 1 months: 1.3 ± 0.6; 3 months: 0.96 ± 0.56; P < 0.001); decreased peri-tendinous hyperemia significantly in 1 and 3 months (baseline: 58.3%; 1 months: 16.7%; 3 months: 0%; P < 0.001) |
| 3 | Chen et al[7], 2021, United States | Case report | PRP: 1 | Age: 38; Gender: F | Pain reduction | The PRP injection reduced pain after 2 weeks, completely resolved it after 4 weeks, and there were no recurrent pain or weakness symptoms after 6 months |
| 4 | Deb et al[5], 2020, India | Prospective study | PRP: 67; Conservative: 64; CS: 69 | PRP; Age: 53.96 ± 09.47; Gender: 38, 29; Conservative; Age: 51.85 ± 10.14; Gender: 43, 21; CS; Age: 57.49 ± 10.00; Gender: 33, 36 | Pain reduction (VAS) and functional outcome (Mayo’s wrist score) | PRP injection reduced pain significantly in 1, 6, 12 months after treatment compared to conservative and CS therapy (VAS in 1 months: 4.91 ± 1.01 vs 6.37 ± 2.45 vs 5.13 ± 2.07; 6 months: 3.96 ± 1.94 vs 5.01 ± 0.26 vs 6.09 ± 1.41; 12 months: 2.11 ± 0.28 vs 7.61 ± 0.72 vs 4.93 ± 1.95; P < 0.001) |
| PRP injection improved functional outcome significantly in 1, 6, 12 months after treatment compared to conservative and CS therapy (Mayo’s wrist score in 1 months: 73.61 ± 7.01 vs 39.71 ± 4.47 vs 63.45 ± 5.17, p: 0.045; 6 months: 83.47 ± 6.83 vs 51.43 ± 6.64 vs 70.94 ± 6.29, p: 0.003; 12 months: 87.24 ± 6.94 vs 64.78 ± 7.12 vs 72.01 ± 5.42, p: 0.001). | ||||||
| 5 | Giroti et al[12], 2021, India | Prospective study | PRP: 22 hand; CS: 28 hand | PRP; Age: 44.44 (27-60); Gender: 2, 20; CS; Age: 43.16 (31-59); Gender: 3, 24 | Pain reduction (VAS) and disability reduction (DASH score) | PRP injection reduced pain in 6 months after treatment compared to CS therapy (VAS: 1.4 vs 2.1) |
| PRP injection reduced disability compared to CS therapy (DASH score: 23.5 vs 39.7) | ||||||
| 6 | Gulati and Ramesh[6], 2022, India | Prospective study | PRP: 22; CS: 22 | PRP; Age: 46.3 ± 9.7; Gender: 6, 16; CS; Age: 42.3 ± 5.8; Gender: 7, 15 | Pain reduction (VAS) and disability reduction (DASH score) | PRP injection reduced pain significantly in 4, 12, and 24 weeks after treatment compared to CS therapy (VAS in 4 weeks: 5 vs 7; 12 weeks: 3.5 vs 5; 24 weeks: 1 vs 5; P < 0.001) |
| PRP injection reduced disability significantly in 4, 12, and 24 weeks compared to CS therapy (DASH score in 4 weeks: 61.3 vs 93.1; 12 weeks: 40.9 vs 87.2; 24 weeks: 13.6 vs 72.7; P < 0.001) | ||||||
| 7 | Johurul et al[29], 2019, Bangladesh | Prospective study | PRP: 25; CS: 35 | PRP; Age: 35 ± 2.1; Gender: 10, 15; CS; Age: 42 ± 7.3; Gender: 15, 20 | Pain reduction (wrist pain) | PRP injection reduced pain significantly in 60 days after treatment compared to CS therapy (wrist pain: 1.1 ± 1.0 vs 2.7 ± 1.0, p: 0.0001) |
| 8 | Kumar et al[14], 2023, India | Prospective study | PRP: 30; CS: 30 | PRP; Age: 35.83 ± 8.48; Gender: 8, 22; CS; Age: 37.80 ± 6.44; Gender: 10, 20 | Pain reduction (VAS), disability reduction (DASH score), and functional outcome (Mayo’s wrist score) | PRP injection reduced pain insignificantly in 3, 6, 12 months after treatment compared to CS therapy (VAS in 3 months: 1.87 ± 1.78 vs 2.30 ± 2.32, p: 0.420; 6 months: 0.83 ± 0.99 vs 1.23 ± 1.61, p: 0.251; 12 months: 0.40 ± 0.62 vs 0.47 ± 0.78; p: 0.715) |
| PRP injection reduced disability insignificantly in 3, 6, 12 months after treatment compared to CS therapy (DASH score in 3 months: 5.66 ± 6.56 vs 6.82 ± 8.70, p: 0.633; 6 months: 2.38 ± 3.87 vs 3.02 ± 5.13, p: 0.587; 12 months: 0.49 ± 0.85 vs 1.21 ± 2.83, p: 0.183) | ||||||
| PRP injection improved functional outcome insignificantly in 3, 6, 12 months after treatment compared to CS therapy (Mayo’s wrist score in 3 months: 82.83 ± 8.68 vs 82.00 ± 9.34, p: 0.722; 6 months: 88.83 ± 6.91 vs 86.83 ± 7.13, p: 0.274; 12 months: 92.50 ± 4.10 vs 90.83 ± 5.88, p: 0.208) | ||||||
| 9 | Peck and Ely[13], 2013, United States | Case report | PRP: 1 | Age: 74; Gender: F | Pain reduction (VAS) | PRP injection reduced pain in 3 and 6 months after treatment (VAS baseline: 38 of 100; 3 months: 10 of 100; 6 months: 14 of 100) |
| 10 | Ramesh et al[30], 2018, India | Prospective study | PRP: 141 | Age: 41.24 (21-59); Gender: 77 , 64 | Pain reduction (VAS) and functional outcome (Mayo’s wrist score) | PRP injection reduced pain significantly in 6 months after treatment (VAS: 9.42 vs 3.92, P < 0.001) |
| PRP injection improved functional outcome significantly in 6 months after treatment (Mayo’s wrist score: 22.71 vs 71.46, P < 0.001) | ||||||
| 11 | Sheikh et al[10], 2020, Egypt | Prospective study | PRP: 20 hand; CS: 20 hand | PRP; Age: 41.45 ± 11.54; Gender: 15, 2; CS; Age: 41.30 ± 8.06; Gender: 16, 2 | Pain reduction (VAS), disability reduction (qDASH score), functional outcome (JHFT), and USG evaluation of tendon | PRP injection reduced pain significantly in 6 months after treatment compared to CS therapy (VAS: 2.13 ± 2.75 vs 1.94 ± 3.04, p: 0.034) |
| PRP injection reduced disability significantly in 6 months after treatment compared to CS therapy (qDASH score: 10.90 ± 10.86 vs 9.38 ± 13.52, p: 0.729) | ||||||
| PRP injection improved functional outcome significantly in 6 months after treatment compared to CS therapy (JHFT: 49.56 ± 5.98 vs 50.39 ± 7.63, p: 0.735) | ||||||
| PRP injection decreased tendon thickness insignificantly in 6 months after treatment compared to CS therapy (LS: 2.35 ± 0.77 vs 1.99 ± 0.53, p: 0.133; TS: 2.54 ± 0.67 vs 2.42 ± 0.57, p: 0.571); decreased tendon and sheath thickness insignificantly in 6 months after treatment compared to CS therapy (LS: 3.23 ± 0.92 vs 2.91 ± 0.96, p: 0.335; TS: 3.53 ± 0.87 vs 3.18 ± 0.63, p: 0.183); decreased extensor retinaculum thickness significantly in 6 months after treatment compared to CS therapy (0.98 ± 0.35 vs 0.59 ± 0.25, P < 0.001) | ||||||
| 12 | Shoma et al[11], 2023, Bangladesh | RCT | PRP: 33; Conservative: 30; CS: 31 | PRP; Age: 45.6 ± 10.4; Gender: 12, 21; Conservative; Age: 42.4 ± 6.3; Gender: 7, 23; CS; Age: 46.9 ± 11.3; Gender: 9, 22 | Pain reduction (VAS) and functional outcome (Mayo’s wrist score) | PRP injection reduced pain significantly in 3 and 6 months after therapy compared to conservative and CS therapy (VAS: 3.5 ± 0.7 vs 4.4 ± 0.7 vs 3.9 ± 0.5, P < 0.001) |
| PRP injection improved functional outcome significantly in 3 and 6 months after therapy compared to conservative and CS therapy (Mayo’s wrist score: 87.9 ± 3.7 vs 65.2 ± 7.2 vs 73.7 ± 4.8, P < 0.001) |
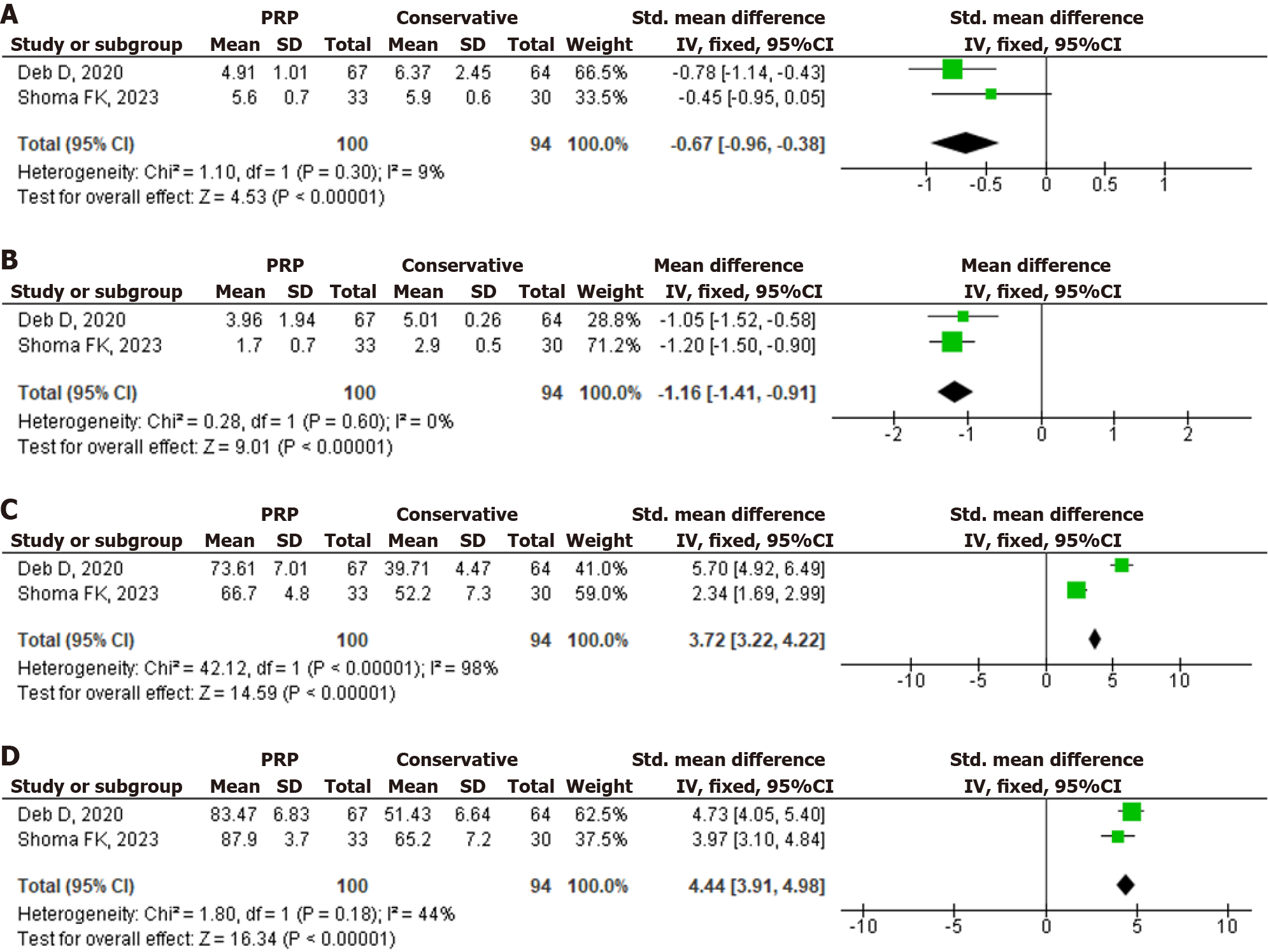
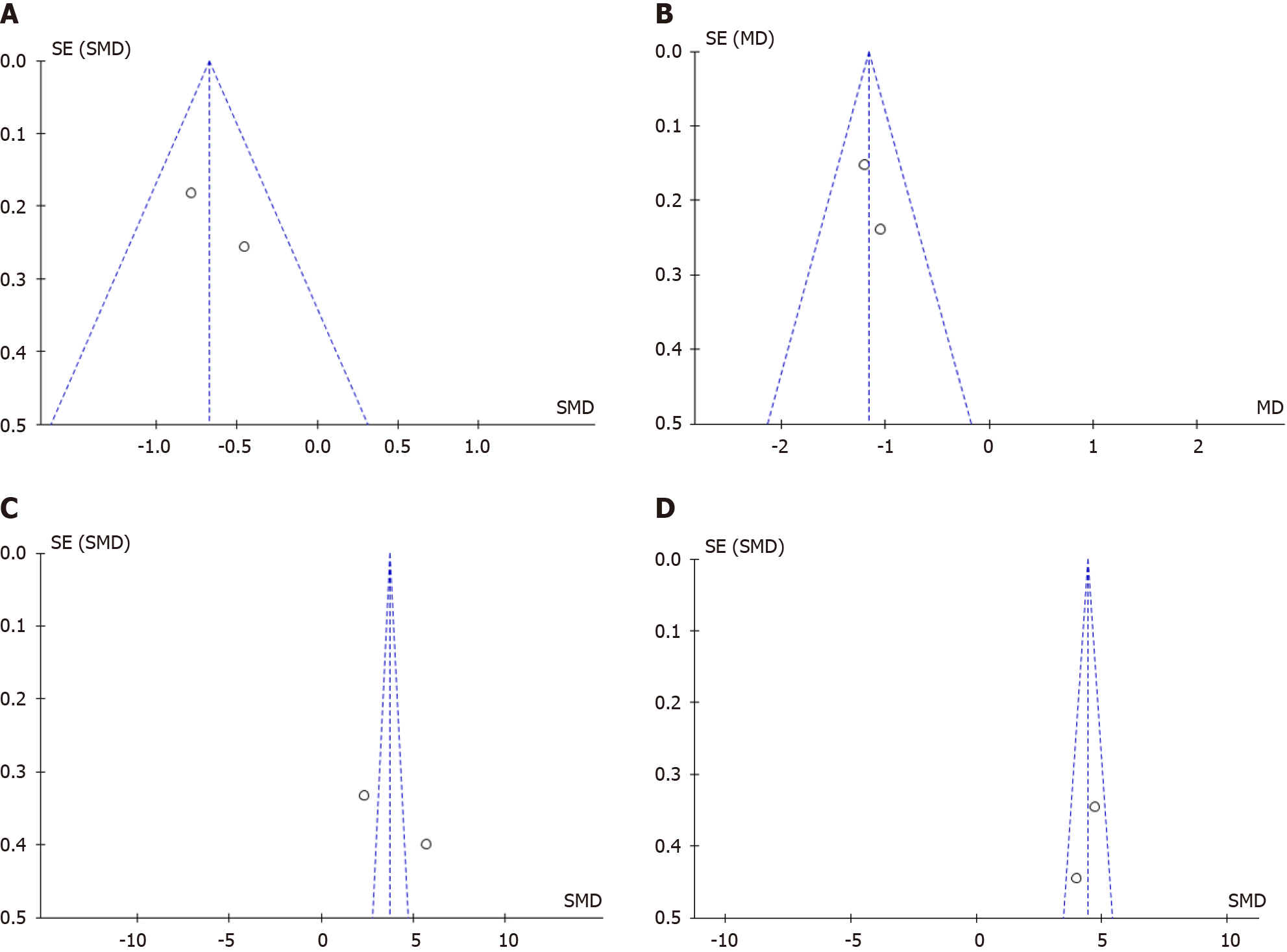
The forest plots of included studies for meta-analysis of the PRP efficacy for DQT are shown in Figure 3. Pooled analysis of data from two studies involving 194 subjects with DQT showed that, compared with conservative treatment, PRP injection was associated with a greater reduction in visual analogue scale (VAS) pain in one month and six months after treatment [MD: -0.67 (95%CI: -0.96 to -0.38), P value < 0.00001 and the heterogeneity (I2) was 9%; MD: -1.16 (95%CI: -1.41 to -0.91), P value < 0.00001 and the heterogeneity (I2) was 0%] and the increase of Mayo’s wrist score in one month and six months after treatment [SMD: 3.72 (95%CI: 3.22 to 4.22), P value < 0.00001 and the heterogeneity (I2) was 98% (high heterogeneity); SMD: 4.44 (95%CI: 3.91 to 4.98), P value < 0.00001 and the heterogeneity (I2) was 44% (moderate heterogeneity)]. The differences in demographic variance and occupational status influenced the heterogeneity of the included studies. Figure 4 shows the funnel plots, with the symmetry of the scatter plot in the triangle (low risk of bias).
Twelve studies had reported the efficacy of PRP as a treatment for DQT: Ten studies reported the reduction of VAS (scoring for assessing pain), four studies reported the improvement of Mayo’s wrist score (scoring for assessing wrist as functional outcome), four studies reported the reduction of disabilities of the arm, shoulder, and hand (DASH) score (scoring for assessing disability), one study reported the improvement of Jebsen-Taylor hand function test (scoring for assessing hand as functional outcome), and two studies reported the improvement of ultrasound findings (tendon thickness, combined tendon and sheath thickness, and extensor retinaculum thickness). Pooled analysis of included studies also supported these results, in which PRP was associated with a reduction in VAS and increasing Mayo’s wrist score (P < 0.00001). All these studies reported that PRP is an alternative treatment for DQT due to the initiation and production of growth factors like vascular endothelial growth factor, platelet-derived growth factor, transforming growth factor (TGF)-α, and TGF-β which supported the tissue regeneration through angiogenesis, chemotaxis, and cell proliferation that are activating intracellular signal transduction pathways[6-9,12,13,29-31].
Seven researches discussed about the comparison of PRP and corticosteroid (CS) as a treatment for DQT. All these studies concluded that PRP provided better outcomes than CS because PRP efficacy as an osteointegration and histopromotive agent had a long-term sustained effect with no adverse effect. In contrast, CS had a short-term effect[5,6,10-12,14,29]. According to Giroti et al[12], VAS reduction and DASH improvement were better after CS injection in comparison to PRP injection in the first month; however, after six months, PRP injection showed greater VAS reduction and DASH improvement in comparison to CS injection. PRP has four stages of treatment as a regenerative agent, making PRP has a late onset and long-term effect compared to CS injection. The first stage is an inflammatory stage, consisting of increased vascular permeability, initiation of angiogenesis, stimulation of tenocyte proliferation, and initiation of type III collagen synthesis in the injection site within two to three days. This inflammatory stage begins with a temporary improvement of the inflammatory response due to cyclooxygenase-2 induction, nuclear factor kappa B activation, and the secretion of pro-inflammatory cytokines. This step is continued by the production of an anti-inflammatory microenvironment through the secretion of prostaglandin E2. The second stage is the proliferative stage, where fibroblast proliferation and neo-angiogenesis accumulate. During this stage, water content and glycosaminoglycan levels are high, which lasts from one to six weeks. The remodelling stage is the third stage (six to ten weeks), with cellularity, collagen, and glycosaminoglycan production reduced, followed by the last stage, namely the maturation stage, where production of type I collagen is occurred which lasts from ten weeks to six months (in this stage, the metabolism of tenocyte remains high)[6,32-34]. According to this finding, PRP injection provides better pain management and stable improvements in functional results in the long term. In contrast, CS injection results in rapid recovery but temporary improvement (short-term effect)[29,33,35,36].
For PRP preparation, most studies used 10-30 cc of blood from the patient’s vein with an aseptic procedure and combined with an anticoagulant (citrate phosphate dextrose adenine-1). After that, the blood was placed into a PRP kit and centrifuge twice: The first time was four minutes at 3200 rpm, and the second time was three minutes at 3300 rpm to separate erythrocyte, platelet-poor plasma, and PRP. Then, 1.5-4 cc of PRP was collected. Prior to injection, 10% of calcium chloride solution was combined with autologous PRP in a ratio of 1: 10. First, the PRP injection was administered under local anaesthesia and injected along the inflamed tendon sheath of APL and EPB tendons; then, an antiseptic dressing was applied at the injection site after the procedure. The patient was observed for 10-15 minutes to monitor any adverse effects from the procedure and the patient was discharged with a recommendation to take a rest the next day, refrained from weight bearing and repetitive movements on the treated wrist for at least one week and the usage of wrist splint for 48-72 hours, the usage of cold compress or paracetamol for analgesia if necessary, and avoided the usage of NSAID[5-9,13,14,29,30].
Six studies discussed the usage of ultrasonography (USG) to guide the PRP injection to increase the accuracy of injection due to the anatomical variation of the first dorsal compartment of the wrist[5,7,9,10,13,30]. USG-guided PRP injection accurately directs the injection into the disorder area in cases of sub-compartmentalization, reduces the risk of subsequent tear, and prevents injection-associated complications, including superficial radial nerve injury[9,10]. Four studies used percutaneous needle tenotomy before the injection to create intra-tendinous micro tears and induce faster healing[9,37]. The fundamental theory of needle tenotomy is associated with the neovascularization and inflammatory response induced by perforation, which promotes tendon healing[38-41].
The dosage of PRP ranged from 1.5-4 cc from the included studies, with no differences in results in this dosage range[5-13,29,30]. However, with USG guided, the smaller volume of PRP injection (1.5 cc) can be effective in achieving good outcomes compared to PRP injection without USG guided due to the accuracy of injection location[8,29]. Most studies used single dose of PRP injection, but three studies used multiple doses of PRP injection, with the treatment interval being four weeks[5,6]. The interval between doses was required because of the initiation of fibroblast proliferation and neo-angiogenesis[30,42].
There was no complication associated with PRP injection in DQT treatment, however Asaad et al[9] reported two patients that experienced with mild vasovagal sign following the procedure due to the adverse event of lidocaine or pain at the time of procedure. According to Chen et al[7], the skin discoloration or atrophy caused by CS injection that has persisted for more than one year had utterly resolved after six weeks of PRP injection; this was probably due to the effect of PRP in tissue regeneration.
Despite PRP is a safe treatment with minimal side effects, some disadvantages and contraindications need to be considered. Adverse effects at the injection site (formation of scar tissue and calcification, infection, allergic reaction), no established and consistent method for preparing and administering PRP (ideal duration, method, frequency, volume and concentration of these components for maximizing beneficial effects are still unclear) are disadvantages for PRP treatment. While, critical thrombocytopenia, platelet dysfunction, hemodynamic instability, systemic infection, local inflammation at the injection site, and patients who are unwilling to accept risk are absolute contraindications for PRP treatment[43,44]. The relative contraindications of PRP treatments are the usage of NSAID within two days, glucocorticoid injection at the treatment site within a month, systemic glucocorticoid consumption within two weeks, recent fever or illness, malignancy especially bone or haematolymphoid malignancy, anaemia (haemoglobin less than 10 g/dL), thrombocytopenia (less than 150000 platelets per microliter), and smokers[45-47].
This systematic review consisted of 12 studies that discussed PPR efficacy for DQT. The majority of the studies were prospective studies that discussed the efficacy of PRP in reducing pain in DQT.
The limitations of this systematic review were that the majority of studies were observational studies, the baseline characteristics were varied, the demography variance and confounding variables in the human study were unknown, the sample size in some studies were small, the difference of PRP preparation and technique between included studies, there was limited follow-up time, and this systematic review only reviewed English-language articles.
The current systematic review can be a scientific publication for physicians, researchers, and all readers associated with the efficacy of PRP for DQT. Further research is needed with a larger sample size, diverse demographic variances, longer follow-up time, and standardization of PRP preparation and injection technique.
PRP can be used as an alternative non-operative treatment for DQT who did not respond to traditional therapy due to its tissue regenerative effect. USG-guided PRP with percutaneous needle tenotomy gives better outcome in DQT. Further research is needed related to standardization of PRP preparation and PRP injection technique.
| 1. | Gitto S, Draghi AG, Draghi F. Sonography of Non-neoplastic Disorders of the Hand and Wrist Tendons. J Ultrasound Med. 2018;37:51-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Miller LE, Parrish WR, Roides B, Bhattacharyya S. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Fitzpatrick J, Bulsara M, Zheng MH. The Effectiveness of Platelet-Rich Plasma in the Treatment of Tendinopathy: A Meta-analysis of Randomized Controlled Clinical Trials. Am J Sports Med. 2017;45:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 4. | Wesner M, Defreitas T, Bredy H, Pothier L, Qin Z, McKillop AB, Gross DP. A Pilot Study Evaluating the Effectiveness of Platelet-Rich Plasma Therapy for Treating Degenerative Tendinopathies: A Randomized Control Trial with Synchronous Observational Cohort. PLoS One. 2016;11:e0147842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Deb D, Singh YN, Singh NB, Das R. A study to compare the efficacy, feasibility and durability of conservative & physical therapy, corticosteroid therapy and platelet rich plasma therapy in patients suffering from de quervain’s tenosynovitis: A prospective cohort study. IJMSDR. 2020;4. [DOI] [Full Text] |
| 6. | Gulati S, Ramesh R. Comparative study between corticosteroids and platelet-rich plasma in the treatment of de Quervain’s tenosynovitis. Int J Orthop Sci. 2022;8:406-412. [DOI] [Full Text] |
| 7. | Chen S, Suarez N, Islam T, Trinley T, Urman I, Nampiaparampil DE. Using Platelet-Rich Plasma to Treat De Quervain’s Tenosynovitis and Cortisone-Induced Skin Discoloration and Atrophy: A Case Study. Pain Medicine Case Reports. 2021;5:127-131. |
| 8. | Mahdi Al-ardi I. Platelet-rich plasma as a treatment for DE Quervain's Disease. Qad Med J. 2018;13:197-201. [DOI] [Full Text] |
| 9. | Asaad SK, Mahmood KA, Arif SO, Abdalla BA, Salih AM, Kakamad FH, Mohammed SH, Salih RQ, Mohammed KK, Salih KM. Efficacy of ultrasoundguided platelet rich plasma injection for the management of de Quervain's tenosynovitis. Med Int (Lond). 2023;3:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Sheikh E, Mahmoud S, El-Rahim MIA. The Role of Platelet Rich Plasma in Comparison with Corticosteroids in the Treatment of De Quervain Tenosynovitis. Med J Cairo Univ. 2020;88:141-148. [DOI] [Full Text] |
| 11. | Shoma FK, Chowdhury ZR, Khan MM, Bhuiyan MK, Khandaker MN, Islam MT. Comparison of the Effect of Intralesional Injection of Corticosteroid and Platelet-rich Plasma in Patients with de Quervain’s Tenosynovitis. Bangladesh Med Res Counc Bull. 2023;49:32-38. [DOI] [Full Text] |
| 12. | Giroti C, Mittal A, Gotecha D. Comparison of steroid injection and platelet-rich plasma injection in the treatment of De-Quervain Tenosynovitis. Int J Orthop Sci. 2021;7:463-466. [DOI] [Full Text] |
| 13. | Peck E, Ely E. Successful treatment of de Quervain tenosynovitis with ultrasound-guided percutaneous needle tenotomy and platelet-rich plasma injection: a case presentation. PM R. 2013;5:438-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Kumar V, Talwar J, Rustagi A, Krishna LG, Sharma VK. Comparison of Clinical and Functional Outcomes after Platelet-Rich Plasma Injection and Corticosteroid Injection for the Treatment of de Quervain's Tenosynovitis. J Wrist Surg. 2023;12:135-142. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Jena D, Barman A, Sahoo J, Baral D. Platelet-rich Plasma in the Treatment of Recurrent Flexor Tenosynovitis of Wrist Complicated with Carpal Tunnel Syndrome in a Patient with Type 2 Diabetes Mellitus: A Case Report. J Orthop Case Rep. 2022;12:97-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Shala R. Platelet-rich plasma for tendinopathy and osteoarthritis: a narrative review. Bull Fac Phys Ther. 2021;26:10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Xue Y, Su X, Jiang M, Yu Z, Yang H, Qin L, Giannoudis PV, Guo JJ. Pure platelet-rich plasma facilitates the repair of damaged cartilage and synovium in a rabbit hemorrhagic arthritis knee model. Arthritis Res Ther. 2020;22:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Chen X, Jones IA, Park C, Vangsness CT Jr. The Efficacy of Platelet-Rich Plasma on Tendon and Ligament Healing: A Systematic Review and Meta-analysis With Bias Assessment. Am J Sports Med. 2018;46:2020-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 176] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 19. | Sharma R, Chaudhary NK, Karki M, Sunuwar DR, Singh DR, Pradhan PMS, Gyawali P, Duwal Shrestha SK, Bhandari KK. Effect of platelet-rich plasma versus steroid injection in plantar fasciitis: a randomized clinical trial. BMC Musculoskelet Disord. 2023;24:172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Thu AC. The use of platelet-rich plasma in management of musculoskeletal pain: a narrative review. J Yeungnam Med Sci. 2022;39:206-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Kalia RB, Singh V, Chowdhury N, Jain A, Singh SK, Das L. Role of Platelet Rich Plasma in Chronic Plantar Fasciitis: A Prospective Study. Indian J Orthop. 2021;55:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 39800] [Article Influence: 9950.0] [Reference Citation Analysis (2)] |
| 23. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4127] [Cited by in RCA: 4613] [Article Influence: 1153.3] [Reference Citation Analysis (0)] |
| 24. | Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V. Cochrane handbook for systematic reviews of intervention 6.4. United Kingdom: John Wiley and Sons. |
| 25. | Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 710] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 26. | JBI. Checklist for Case Reports. The Joanna Briggs Institute, 2020; 1-5. |
| 27. | JBI. Checklist for Case Series. The Joanna Briggs Institute, 2020; 1-7. |
| 28. | Ismiarto YD, Prasetiyo GT. Efficacy and Safety of Intra-Articular Botulinum Toxin A Injection for Knee Osteoarthritis: A Systematic Review, Meta-Analysis, and Meta-Regression of Clinical Trials. JB JS Open Access. 2023;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 29. | Johurul H, Kumar DR, Parvez AK, Emam ZM, Imtiaz F. Comparative study between Platelet -rich plasma and corticosteroid in De Quervain’s disease: A study in Zainul Haque Sikder Womens Medical College & Hospital, Dhaka, Bangladesh. Int J Med Health Res. 2019;5:10-14. |
| 30. | Ramesh R, Jeyaraman M, Prajwal G. The Prospective Study on Efficacy and Functional Outcome of Autologous Platelet Rich Plasma Injection in Musculoskeletal Disorders. EC Orthop. 2018;9:849-863. [DOI] [Full Text] |
| 31. | Yin J, Xu Z, Liu J. Alleviation of synovitis caused by joint instability with application of platelet-rich plasma. Thromb Res. 2020;186:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Hurley ET, Shimozono Y, Hannon CP, Smyth NA, Murawski CD, Kennedy JG. Platelet-Rich Plasma Versus Corticosteroids for Plantar Fasciitis: A Systematic Review of Randomized Controlled Trials. Orthop J Sports Med. 2020;8:2325967120915704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 439] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 34. | Xu P, Wu Y, Zhou L, Yang Z, Zhang X, Hu X, Yang J, Wang M, Wang B, Luo G, He W, Cheng B. Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization. Burns Trauma. 2020;8:tkaa028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 35. | Malahias MA, Chytas D, Mavrogenis AF, Nikolaou VS, Johnson EO, Babis GC. Platelet-rich plasma injections for carpal tunnel syndrome: a systematic and comprehensive review. Eur J Orthop Surg Traumatol. 2019;29:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Chong HH, Pradhan A, Dhingra M, Liong W, Hau MYT, Shah R. Advancements in de Quervain Tenosynovitis Management: A Comprehensive Network Meta-Analysis. J Hand Surg Am. 2024;49:557-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 37. | Hatamiya NS, Kobayashi Y, Gottschalk AW. Utility of Percutaneous Needle Tenotomy to Reduce Pain and Improve Function in Common Extensor Tendinosis of the Lateral Epicondyle. Ochsner J. 2021;21:326-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 38. | Koonen L, van Amerongen M, Smulders K, Mangesius S, Cerna G, Klauser A, Mur E, Obradov M. Added value of ultrasound-guided percutaneous needle tenotomy over hydrodissection and physiotherapy in chronic lateral elbow tendinopathy: a pilot randomized controlled trial. J Ultrason. 2023;23:e358-e364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 39. | Hung CY, Chang KV. Ultrasound-Guided Percutaneous Needle Tenotomy with Platelet-Rich Plasma Injection for an Uncommon Case of Proximal Gluteus Medius Tendinopathy. J Med Ultrasound. 2019;27:111-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Sconfienza LM, Albano D, Messina C, Gitto S, Guarrella V, Perfetti C, Taverna E, Arrigoni P, Randelli PS. Ultrasound-Guided Percutaneous Tenotomy of the Long Head of Biceps Tendon in Patients with Symptomatic Complete Rotator Cuff Tear: In Vivo Non-contRolled Prospective Study. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Finnoff JT, Fowler SP, Lai JK, Santrach PJ, Willis EA, Sayeed YA, Smith J. Treatment of chronic tendinopathy with ultrasound-guided needle tenotomy and platelet-rich plasma injection. PM R. 2011;3:900-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 42. | Jiang J, Xing F, Luo R, Liu M. Effectiveness of Platelet-Rich Plasma for Patients With Carpal Tunnel Syndrome: A Systematic Review and meta-Analysis of Current Evidence in Randomized Controlled Trials. Front Pharmacol. 2022;13:834213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Ramaswamy Reddy SH, Reddy R, Babu NC, Ashok GN. Stem-cell therapy and platelet-rich plasma in regenerative medicines: A review on pros and cons of the technologies. J Oral Maxillofac Pathol. 2018;22:367-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 44. | Szwedowski D, Szczepanek J, Paczesny Ł, Zabrzyński J, Gagat M, Mobasheri A, Jeka S. The Effect of Platelet-Rich Plasma on the Intra-Articular Microenvironment in Knee Osteoarthritis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 45. | Shahid M, Kundra R. Platelet-rich plasma (PRP) for knee disorders. EFORT Open Rev. 2017;2:28-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Hong P, Zheng Y, Rai S, Ding Y, Zhou Y, Liu R, Li J. Efficacy and safety of platelet-rich plasma in the treatment of carpal tunnel syndrome: A network meta-analysis of different injection treatments. Front Pharmacol. 2022;13:906075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Le ADK, Enweze L, DeBaun MR, Dragoo JL. Current Clinical Recommendations for Use of Platelet-Rich Plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 225] [Article Influence: 32.1] [Reference Citation Analysis (1)] |