Published online Feb 18, 2024. doi: 10.5312/wjo.v15.i2.170
Peer-review started: November 8, 2023
First decision: November 29, 2023
Revised: December 8, 2023
Accepted: January 5, 2024
Article in press: January 5, 2024
Published online: February 18, 2024
Processing time: 90 Days and 14.6 Hours
Prophylactic antibiotics have significantly led to a reduction in the risk of post-operative surgical site infections (SSI) in orthopaedic surgery. The aim of using antibiotics for this purpose is to achieve serum and tissue drug levels that exceed, for the duration of the operation, the minimum inhibitory concentration of the likely organisms that are encountered. Prophylactic antibiotics reduce the rate of SSIs in lower limb arthroplasty from between 4% and 8% to between 1% and 3%. Controversy, however, still surrounds the optimal frequency and dosing of anti
To evaluate the impact of introduction of a weight-adjusted antibiotic prophylaxis regime, combined with a reduction in the duration of administration of post-operative antibiotics on SSI incidence during the 2 years following primary elective total hip and knee arthroplasty
Following ethical approval, patients undergoing primary total hip arthroplasty (THA)/total knee arthroplasty (TKA) with the old regime (OR) of a preoperative dose [cefazolin 2 g intravenously (IV)], and two subsequent doses (2 h and 8 h), were compared to those after a change to a new regime (NR) of a weight-adjusted preoperative dose (cefazolin 2 g IV for patients < 120 kg; cefazolin 3g IV for patients > 120 kg) and a post-operative dose at 2 h. The primary outcome in both groups was SSI rates during the 2 years post-operatively.
A total of n = 1273 operations (THA n = 534, TKA n = 739) were performed in n = 1264 patients. There was no statistically significant difference in the rate of deep (OR 0.74% (5/675) vs NR 0.50% (3/598); fishers exact test P = 0.72), nor superficial SSIs (OR 2.07% (14/675) vs NR 1.50% (9/598); chi-squared test P = 0.44) at 2 years post-operatively. With propensity score weighting and an interrupted time series analysis, there was also no difference in SSI rates between both groups [RR 0.88 (95%CI 0.61 to 1.30) P = 0.46].
A weight-adjusted regime, with a reduction in number of post-operative doses had no adverse impact on SSI incidence in this population.
Core Tip: For patients in the study population undergoing primary lower limb arthroplasty, reducing the number of post-operative antibiotic doses had no adverse impact on surgical site infections incidence, at 2 years following surgery, if a weight-adjusted regime is used.
- Citation: Okoro T, Wan M, Mukabeta TD, Malev E, Gross M, Williams C, Manjra M, Kuiper JH, Murnaghan J. Assessment of the effectiveness of weight-adjusted antibiotic administration, for reduced duration, in surgical prophylaxis of primary hip and knee arthroplasty. World J Orthop 2024; 15(2): 170-179
- URL: https://www.wjgnet.com/2218-5836/full/v15/i2/170.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i2.170
Prophylactic antibiotics have significantly led to a reduction in the risk of post-operative surgical site infections (SSI) in orthopaedic surgery[1,2]. The aim of using antibiotics for this purpose is to achieve serum and tissue drug levels that exceed, for the duration of the operation, the minimum inhibitory concentration of the likely organisms that are encountered[3]. Prophylactic antibiotics reduce the rate of SSIs in lower limb arthroplasty from between 4% and 8% to between 1% and 3%[4]. Controversy, however, still surrounds the optimal frequency and dosing of antibiotic administration[5].
Periprosthetic joint infection (PJI) occurs following 1% to 2% of primary lower limb arthroplasties[6,7]. This complication is associated with significant morbidity for patients and the need for complex multidisciplinary treatment strategies[8]. In Canada, deep incisional and organ-space PJI rates are 0.96% for primary total hip arthroplasty (THA) and 0.71% for primary total knee arthroplasty (TKA)[9]. Ninety three percent of THA PJIs and 92% of TKA PJIs tend to be identified within 90 days following surgery, with an average diagnosis time of 21 d[9].
Various studies have analyzed the effect of antibiotic duration and infection, however, no benefit has been demonstrated beyond 24 h[10,11]. Prolonged postoperative prophylactic antibiotic administration should be discouraged due to the risk of additional toxicity, production of resistant organisms, and unnecessary expense incurred[12]. These findings are supported by guidance from the American Association (Academy) of Orthopedic Surgeons which recommend that the duration of prophylactic antibiotic administration should not exceed 24 h[13]. In the United Kingdom, the National Institute for Health and Care Excellence (NICE) recommends a single intravenous dose of antibiotic prophylaxis on induction of anesthesia, with a repeat dose if the surgical duration is longer than the half-life of the antibiotic, or if blood loss is significant[14]. The World Health Organization (WHO) also recommends against the prolongation of surgical antibiotic prophylaxis administration after completion of the operation for the purpose of preventing SSIs[15].
Obesity in patients represents a significant risk factor for SSI[16,17]. In 2016, a report from the WHO indicated that more than 39% of adults in world were considered overweight and of this group, around a third would be considered obese[18]. This report also highlighted that obese and overweight individuals represent a significant proportion of patients undergoing surgery worldwide[18]. However, dosing guidelines for antibiotic prophylaxis do not recommend adjustments based on weight[13-15]. The rationale for this is that the use of standardized doses is considered safe, effective, and convenient for the majority of the adult population[19]. Studies have suggested that doubling the dose of antibiotic prophylaxis for morbidly obese patients weighing at least 120 kg, or with a body mass index (BMI) of 40 kg/m2 or higher may reduce the risk of SSI[20,21]. According to the Centers for Disease Control and Prevention guidelines for SSI prevention, the issue of weight-adjusted antibiotic prophylaxis dosing is still considered unresolved[22].
At the time this study was conceived, the antibiotic prophylaxis regime at the study institution, a tertiary elective arthroplasty unit, comprised a single preoperative dose of cefazolin 2 g (irrespective of patient weight) followed by two postoperative doses within the first 24 h following surgery.
The aim of this study was to evaluate the impact of introduction of a weight-adjusted antibiotic prophylaxis regime, combined with a reduction in the duration of administration of post-operative antibiotics on SSI incidence during the 2 years following primary elective total hip and knee arthroplasty.
Following approval of the quality improvement project by the Sunnybrook Health Sciences Centre, Toronto, Research Ethics Board (September 2018), a prospective cohort study was performed. This study was granted an exemption from requiring informed consent by the Sunnybrook Health Sciences Centre Research Ethics Board.
A cohort of arthroplasty patients undergoing primary THA/TKA with a single pre-operative dose and two post-operative antibiotic doses (old regime, OR; September to December 2018), was compared to a group of patients undergoing primary THA/TKA after the regime had been changed to a weight-adjusted pre-operative dose and a single post-operative dose [new regime, (NR); January to April 2019].
In order to implement change to achieve the stated aims above, prescription order sets were developed with the NR of antibiotics, and this was performed with engagement of appropriate stakeholders to ensure buy-in. The involved stakeholders included Orthopedic Surgery, Pharmacy, Nursing, Anesthesia, Antimicrobial Stewardship and Infection Prevention and Control (IP&C). The majority of the discussions occurred via email. The previous antibiotic prophylaxis order-set had been in use since 2012. The group worked on developing a modified order set; with the main changes for the purpose of this QI project being the removal of the second post-operative dose of cefazolin, and the introduction of a weight-adjusted dosage regime for the pre-operative cefazolin that is administered. The modified order set was submitted for review and subsequently approved by the Forms Committee in November 2018. There was an active drive to notify all service areas, with communication sent to staff groups about the proposed change.
The old regime (OR) for antibiotic prophylaxis consisted of a single preoperative dose [cefazolin 2 g intravenously (IV)], and two subsequent antibiotic doses (cefazolin 2 g IV at 2 h and cefazolin 1g IV at 8 h). This was changed to the NR; which comprised a weight-adjusted preoperative dose (cefazolin 2 g IV for patients < 120 kg; cefazolin 3 g IV for patients > 120 kg) and a single subsequent dose (cefazolin 2 g IV at 2 h).
We included all adult patients undergoing primary hip or knee arthroplasty (THA/TKA) at our institution. Patients were also included if undergoing another primary THA/TKA within the study period. Patients undergoing revision arthroplasty surgery or return to theatre following primary procedures were not included in this cohort.
The primary outcome assessed in both groups was the incidence of SSI in the 2 years following the index operation. Surgical site infection for the purposes of this study was diagnosed as being superficial incisional, deep incisional, or organ-space in origin (Figure 1)[19,20].
Demographic information including age, sex, BMI, was also collected for every enrolled patient in each assessed group (OR and NR). Patient records were also interrogated for information on American Association of Anesthesiologists (ASA) grade, presence of diabetes mellitus and use of oral anticoagulants at the time of surgery.
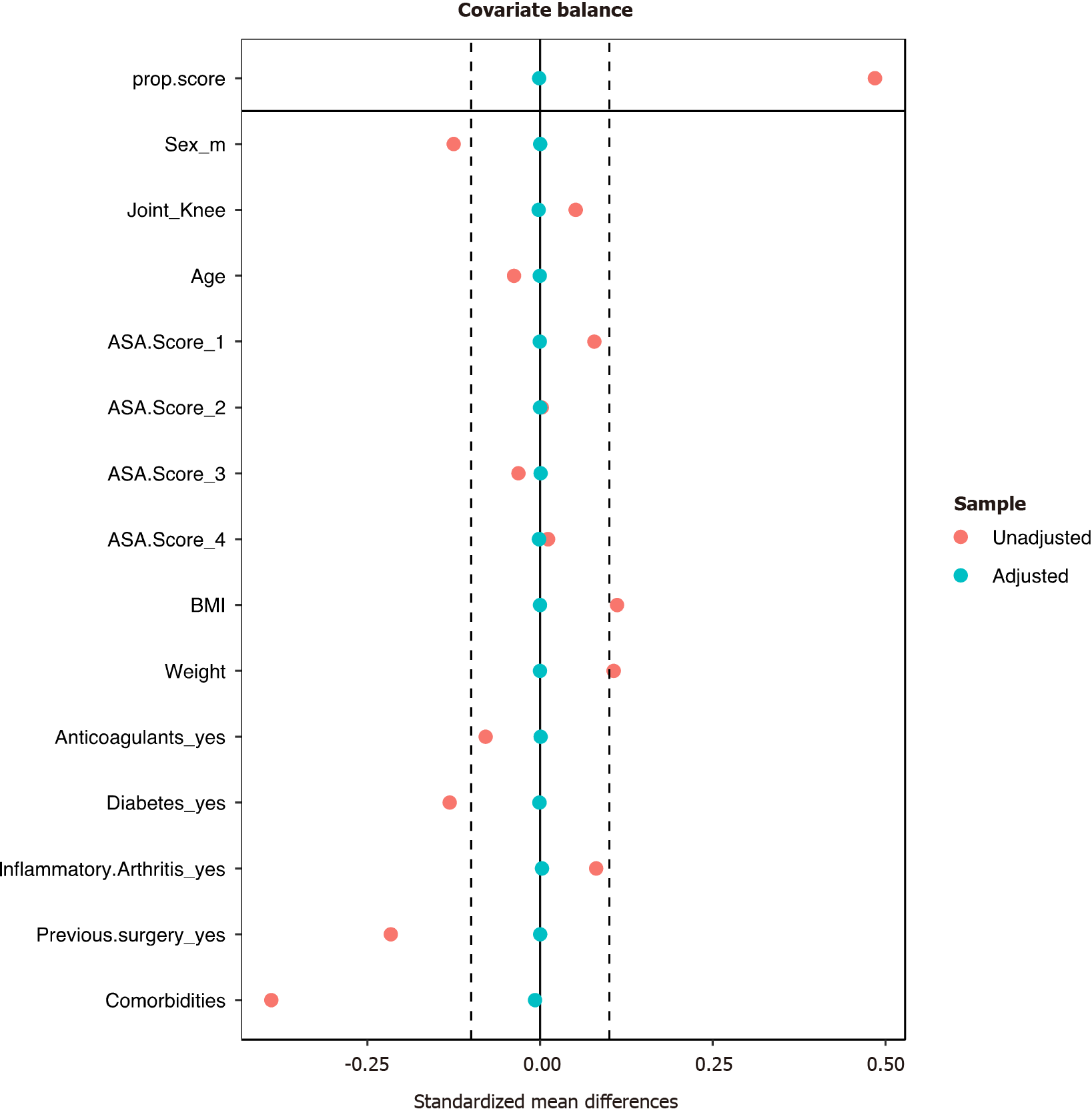
SSI rates were compared directly between the two regimes using Fisher’s exact test (where observed SSI infections < 5) or chi-squared tests (observed SSI infections > 5). We also used a covariate-balancing propensity score weighting method to reduce biases from baseline differences in our comparison between the two regimes[23]. In determining the propensity score, we used age, sex, joint (knee or hip), ASA grade, BMI, weight, anticoagulant use (yes or no), diabetes, inflammatory arthritis, previous surgery and number of comorbidities as covariates. Missing covariate values were imputed using a single imputation with added prediction error and parameter uncertainty (SI+PE+PU), a recommended method when comparing two treatments[24]. We used the standardized difference (SMD) to assess covariate balance and assumed that any imbalance above 10% would indicate a meaningful imbalance[25]. The average treatment effects were then estimated using a log-binomial model with a robust (sandwich) variance estimator. An inverse probability treatment weighting was used to implement the propensity weights[25]. The SSI rates under the old and NR were also analyzed as an interrupted time series, which was implemented as a segmented log-binomial regression with patient number ordered by operation time as independent variable[26]. Our hypothesis was that the introduction of a weight-adjusted regime of shorter duration in the NR would not lead to a change in the incidence of all SSI when compared to the old regime (OR). Statistical analysis was performed using ‘Jamovi’ (Version 1.6); retrieved from https://www.jamovi.org, and using R vs 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria) for the propensity score analysis (packages CBPS and mice) and the interrupted time series analysis. For all analyses, a P value below 0.05 was assumed to denote statistical significance.
A total of 1273 operations (THA n = 534, TKA n = 739) were performed over the study period in n = 1264 patients (males n = 493, females n = 771). Six hundred and sixty-nine (n = 669) patients had surgery under the old antibiotic prophylaxis regime (OR) whilst n = 595 had surgery under the NR. In the OR group the mean age was 69.3 years (SD ± 11.9), whilst for the NR cohort, the mean age was 68.8 years (SD ± 10.5). Table 1 illustrates the demographic characteristics of both groups in further detail. Complete data (demographic information, ASA grading, presence of diabetes mellitus, use of anticoagulation) was available for n = 310 patients in the OR group and n = 458 patients in the NR group (Table 2).
| Old regime | New regime | |
| n = 669 patients | n = 595 patients | |
| Age (mean ± SD) | 69.3 ± 11.9 yr | 68.8 ± 10.5 yr |
| Gender (male) | 279 (41.7) | 214 (40.0%) |
| BMI (mean ± SD) | 32.2 ± 11.0 | 31.8 ± 7.4 |
| Operation performed | ||
| THA | THA, n = 291 (43.1) | THA, n = 243 (40.6) |
| TKA | TKA, n = 384 (56.9) | TKA, n = 355 (59.4) |
| Old regime, n (%) | New regime, n (%) | |
| n = 310 | n = 458 | |
| Age (mean ± SD) | 67.5 ± 10.9 yr | 67.0 ± 10.5 yr |
| Gender (male) | 132 (42.6) | 165 (36.0) |
| Weight (kg; mean ± SD) | 84.82 ± 20.9 | 87.73 ± 22.3 |
| Weight > 120 kg | 19 (6.1) | 44 (9.6) |
| Operation performed | ||
| Total hip arthroplasty | 124 (40.0) | 198 (43.2) |
| Total knee arthroplasty | 186 (60.0) | 260 (56.8) |
| Comorbidities | ||
| ASA grade | ||
| 1 | 5 (1.6) | 12 (2.6) |
| 2 | 154 (49.7) | 212 (46.3) |
| 3 | 148 47.7) | 227 (50.0) |
| 4 | 3 (1.0) | 7 (1.5) |
| Diabetes mellitus | ||
| Yes | 49 (15.8) | 48 (10.5) |
| No | 261 (84.2) | 410 (89.5) |
| Anticoagulation on admission | ||
| Yes | 27 (8.7) | 29 (6.3) |
| No | 283 (91.3) | 429 (93.7) |
| Antibiotic prophylaxis | ||
| Cefazolin | 342 (50.7) | 449 (98.0) |
| Clindamycin | 11 (1.6) | 8 (1.7) |
| Vancomycin | 1 (0.1) | 1 (0.3) |
| Dose appropriate for weight? | ||
| Yes | 340 (50.3) | 458 (100) |
| No | 13 (2.0) | 0 (0.0) |
At final follow-up, there had been 5 episodes of deep incisional or organ space infection (5/675; 0.74%) in the OR cohort and 14 episodes of superficial incisional infection (14/675; 2.07%). For the NR group, at 2 years following surgery, there had been 3 episodes of deep incisional or organ space infection (3/598; 0.50%) and 9 episodes of superficial incisional infection (9/598; 1.67%). There was no statistically significant difference in the rate of deep incisional or organ space infection between the OR and NR groups (0.74% vs 0.50%; Fisher’s exact test P = 0.73), nor in the superficial incisional infection rate between the OR and NR groups (2.07% vs 1.67%; chi-squared test P = 0.44); Table 3. For the subgroup with complete demographic data, there was no significant difference in the rates of superficial (OR 2.6% vs NR 1.5%; chi-squared test P = 0.30) and deep infection (OR 0.3% vs NR 0.5%; Fisher’s exact test P = 1.0) between the OR and NR groups; Table 4. Supplementary Tables 1 and 2 provide further information on the patients in the whole cohort diagnosed with SSIs, whilst Supplementary Tables 3 and 4 provide the same for patients with SSIs from the subgroup with complete demographic information.
| Old regime, n = 669 patients | New regime, n = 595 patients | |
| (n = 675 THA/TKA) | (n = 598 THA/TKA) | |
| Superficial infection, n (%) | 14 (2.07) | 9 (1.50) |
| THA, n = 6 | THA, n = 2 | |
| TKA, n = 8 | TKA, n = 7 | |
| Deep/organ space infection, n (%) | 5 (0.74) | 3 (0.50) |
| THA, n = 1 | THA, n = 2 | |
| TKA, n = 4 | TKA, n = 1 |
| Old regime | New regime | |
| n = 310 joints | n = 458 joints | |
| Total number of infections, n (%) | 9 (2.9) | 9 (2.0) |
| Superficial infections, n (%) | 8 (2.6) | 7 (1.5) |
| THA, n = 2 | THA, n = 1 | |
| TKA, n = 6 | TKA, n = 6 | |
| Deep/organ space infections | 1 (0.3) | 2 (0.5) |
| THA, n = 1 | THA, n = 2 |
Before propensity score weighting, the dataset had meaningful imbalances (over 10% SMD) in the distribution of sex, BMI, weight, diabetes, previous surgery and the number of comorbidities (Figure 1). Propensity score weighting achieved a balanced dataset. The relative risks of overall or deep SSI were however comparable between the original and weighted dataset, with clearly overlapping 95% confidence intervals between the two types of analyses (Table 5).
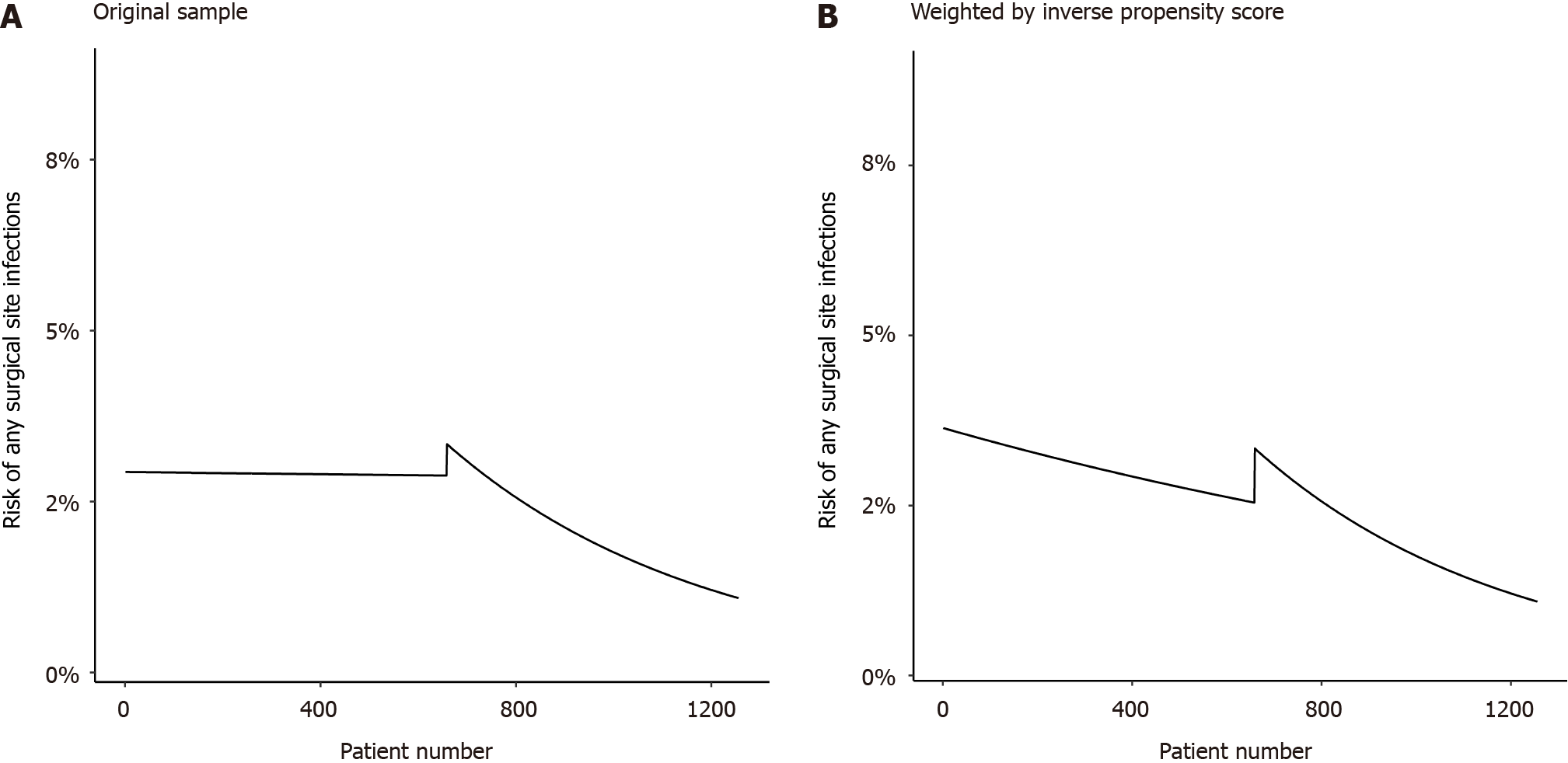
When analyzed as an interrupted time series, the overall infection rate in the original dataset seems constant under the old regime, whereas under the NR it seems to drop with patient number (Figure 2A). In the weighted dataset, the infection rate already seemed to drop under the old regime and after an initial rise continued to drop under the NR (Figure 2B). However, it is important to realize that none of the rate coefficients differed significantly from zero (Table 6), as can also be judged by the wide confidence intervals in the graphs.
| Parameter | Original dataset | Weighted dataset | ||
| RR (95%CI) | P value | RR (95%CI) | P value | |
| Slope of infection rate per 100 patients, old regime | 0.99 (0.79 to 1.3) | 0.98 | 0.95 (0.76 to 1.2) | 0.62 |
| Jump in infection rate, new vs old | 1.16 (0.30 to 4.4) | 0.82 | 1.3 (0.39 to 4.5) | 0.66 |
| Change in slope of infection rate per 100 patients, new vs old | 0.83 (0.55 to 1.2) | 0.37 | 0.88 (0.61 to 1.3) | 0.46 |
Weight-adjusted antibiotic prophylaxis dosing has not been evaluated in large patient cohorts and there is limited evidence for its use in SSI prophylaxis[13-15]. The findings from this study are in agreement with our null hypothesis that using a weight based antibiotic prophylaxis regime and shortening the duration of administered antibiotics, would not lead to a statistically significant increase in deep incisional/organ space SSI rates between the OR and NR groups. The use of a weight-adjusted regime led to a reduction in the rates of superficial SSIs [NR (1.67%) vs OR (2.07%)] but this was not found to be statistically significant. The incidence of superficial SSIs in TKAs were found to be greater than in THAs and this is likely to be due to the fact that there is less soft tissue overlying the operated joint in TKA as opposed to THA. Because of the oedema in the operated limb following surgery, TKA patients are perhaps more likely to be diagnosed and treated for a superficial SSI by their family care physician in the community.
No randomized trials exist in the literature comparing variable duration antibiotic prophylaxis in patients undergoing lower limb arthroplasty. In one study comparing a one-day regime of cefuroxime with a three-day regime in a pro
Evaluations of pre-and post-intervention periods have also been used to assess the impact of antibiotic duration on surgical prophylaxis. One such study showed that a change from one preoperative and two post-operative doses of intravenous cefuroxime every 8 h to a single preoperative dose of intravenous cefazolin for all clean orthopedic surgeries led to a deep wound infection rate of 1.1% for THA (95%CI 0%-3.3%), and 1.6% for TKA (95%CI 0%-3.8%) in the cefuroxime group, vs 1.1% for THA (95%CI 0%-2.2%) and 1.0% for TKA (95%CI 0.3%-1.7%) in the cefazolin group, with no statistically significant difference[27].
Obesity is associated with systemic low-grade inflammation, and this can be characterized by increased serum levels of pro-inflammatory cytokines, potentially resulting in an impaired immune response[28]. Conditions such as type 2 diabetes and dyslipidaemia, which are associated with obesity, may also increase the risk of postoperative infections[29]. Technical difficulties associated with surgery in obese patients may also result in prolonged operations, which are associated with higher SSI rates[30]. Standardized antibiotic prophylaxis doses do tend to provide lower antibiotic concentrations per kilogram in overweight and obese patients compared with patients of normal bodyweight. Various studies however, have suggested no difference in infection rates between standardized and weight-adjusted regimes[31,32]. We have used a weight-adjusted regime in this study and there appears to be a trend to reduction in the number of superficial SSIs, which is more likely to occur in TKA patients.
There were limitations to this study. Firstly, the data was derived from the observational analysis of a large cohort and, as such, the findings are subject to selection bias. However, the sample size was sufficient to yield meaningful analysis. Based on post priori-power calculation; the current power of the study is 11% vs 80% which would be the ideal scenario. Based on a difference between OR and NR regimes of 0.94%, for statistical power of 80%, to demonstrate a difference (or reduction in incidence of SSI) between the groups, n = 8862 patients would be required per group; a total of n = 17724. Such a number would be impractical to recruit to, and observational cohorts such as described in this study are the most pragmatic method to study SSI incidence because of the low rates typically observed. Another limitation is the missing demographic data (approximately 40%). However, the analysis of the subgroup data in terms of SSI incidence, yielded similar results in comparison to the whole cohort data.
In conclusion, reducing the number of post-operative antibiotic doses had no adverse impact on SSI incidence, at 2 years following surgery, in this patient population. A weight-adjusted regime appears to have a benefit (not statistically significant) in reducing the rate of superficial SSIs.
Antibiotic stewardship is important in everyday orthopaedic practice. Preventing surgical site infection (SSIs) with the use of prophylactic antibiotics has to take into account the impact of obesity. There is a growing consensus that a weight based regime may be efficacious in dealing with SSIs in everyday practice.
This study aimed to evaluate the impact of a weight based regime administered for a shorter duration, on the incidence of SSIs in a cohort of patients undergoing elective primary total hip and knee arthroplasty (THA/TKA).
The main objective of the study was to evaluate if there was no reduction in levels of prophylaxis for a weight based antibiotic regime, administered for a shorter duration, vs a standard regime in prevention of SSIs in a cohort of patients undergoing elective primary THA/TKA.
A cohort of arthroplasty patients undergoing primary THA/TKA with a single pre-operative dose and two post-operative antibiotic doses (old regime, OR; September to December 2018), was compared to a group of patients un
The findings from this study are in agreement with our null hypothesis that using a weight based antibiotic prophylaxis regime and shortening the duration of administered antibiotics, would not lead to a statistically significant increase in deep incisional/organ space SSI rates between the OR and NR groups. The use of a weight-adjusted regime led to a reduction in the rates of superficial SSIs [NR (1.67%) vs OR (2.07%)] but this was not found to be statistically significant.
It is important to consider use of a weight based regime for a shorter duration in patients undergoing elective primary THA/TKA as there is no increased SSI risk.
More studies on antibiotic prophylaxis stewardship for this group of patients is required.
Mr. Peter Craig and Mr. Caesar Wek for help in data collation and data analysis respectively. Research Staff at Sunnybrook Holland Orthopaedic and Arthritic Center, Toronto, for help in data collation.
CONSORT statement: The authors have read the CONSORT Statement—checklist of items, and the manuscript was prepared and revised according to the CONSORT Statement—checklist of items.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohapatra S, India S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY
| 1. | Lidwell OM. The cost implications of clean air systems and antibiotic prophylaxis in operations for total joint replacement. Infect Control. 1984;5:36-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Page CP, Bohnen JM, Fletcher JR, McManus AT, Solomkin JS, Wittmann DH. Antimicrobial prophylaxis for surgical wounds. Guidelines for clinical care. Arch Surg. 1993;128:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 301] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189:395-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 465] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 4. | Uçkay I, Hoffmeyer P, Lew D, Pittet D. Prevention of surgical site infections in orthopaedic surgery and bone trauma: state-of-the-art update. J Hosp Infect. 2013;84:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Bryson DJ, Morris DL, Shivji FS, Rollins KR, Snape S, Ollivere BJ. Antibiotic prophylaxis in orthopaedic surgery: difficult decisions in an era of evolving antibiotic resistance. Bone Joint J. 2016;98-B:1014-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Nehrer S, Thalhammer F, Schwameis E, Breyer S, Kotz R. Teicoplanin in the prevention of infection in total hip replacement. Arch Orthop Trauma Surg. 1998;118:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2193] [Cited by in RCA: 2171] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 8. | Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev. 2019;4:482-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 389] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 9. | Roth VR, Mitchell R, Vachon J, Alexandre S, Amaratunga K, Smith S, Vearncombe M, Davis I, Mertz D, Henderson E, John M, Johnston L, Lemieux C, Pelude L, Gravel D; Canadian Nosocomial Infection Surveillance Program. Periprosthetic Infection following Primary Hip and Knee Arthroplasty: The Impact of Limiting the Postoperative Surveillance Period. Infect Control Hosp Epidemiol. 2017;38:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Turano A. New clinical data on the prophylaxis of infections in abdominal, gynecologic, and urologic surgery. Multicenter Study Group. Am J Surg. 1992;164:16S-20S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Niederhäuser U, Vogt M, Vogt P, Genoni M, Künzli A, Turina MI. Cardiac surgery in a high-risk group of patients: is prolonged postoperative antibiotic prophylaxis effective? J Thorac Cardiovasc Surg. 1997;114:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Enzler MJ, Berbari E, Osmon DR. Antimicrobial prophylaxis in adults. Mayo Clin Proc. 2011;86:686-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | AAOS. Recommendations for the Use of Intravenous Antibiotic Prophylaxis in Primary Total Joint Arthroplasty. Am Acad Orthop Surg. 2014;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 14. | National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment NICE guideline (NG125). Published online 2019. Available from: https://www.nice.org.uk/guidance/ng125/resources/surgical-site-infections-prevention-and-treatment-pdf-66141660564421. |
| 15. | Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, de Jonge S, de Vries F, Gomes SM, Gans S, Wallert ED, Wu X, Abbas M, Boermeester MA, Dellinger EP, Egger M, Gastmeier P, Guirao X, Ren J, Pittet D, Solomkin JS; WHO Guidelines Development Group. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16:e288-e303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 538] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 16. | Thelwall S, Harrington P, Sheridan E, Lamagni T. Impact of obesity on the risk of wound infection following surgery: results from a nationwide prospective multicentre cohort study in England. Clin Microbiol Infect. 2015;21:1008.e1-1008.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 17. | Rondon AJ, Kheir MM, Tan TL, Shohat N, Greenky MR, Parvizi J. Cefazolin Prophylaxis for Total Joint Arthroplasty: Obese Patients Are Frequently Underdosed and at Increased Risk of Periprosthetic Joint Infection. J Arthroplasty. 2018;33:3551-3554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | WHO. World Health Organization (WHO): Obesity and overweight. World Health Organization 2021. Accessed 21st December 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. |
| 19. | Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA; American Society of Health-System Pharmacists; Infectious Disease Society of America; Surgical Infection Society; Society for Healthcare Epidemiology of America. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1364] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 20. | Decker BK, Nagrebetsky A, Lipsett PA, Wiener-Kronish JP, O'Grady NP. Controversies in Perioperative Antimicrobial Prophylaxis. Anesthesiology. 2020;132:586-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Edmiston CE, Krepel C, Kelly H, Larson J, Andris D, Hennen C, Nakeeb A, Wallace JR. Perioperative antibiotic prophylaxis in the gastric bypass patient: do we achieve therapeutic levels? Surgery. 2004;136:738-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Siddiqi A, Forte SA, Docter S, Bryant D, Sheth NP, Chen AF. Perioperative Antibiotic Prophylaxis in Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. J Bone Joint Surg Am. 2019;101:828-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Floor-Westerdijk M, Colier W, van der Burght R. Letter to the editor: re: "a new automated assessment method for contrast-detail images by applying support vector machine and its robustness to non-linear image processing" by Takei T, Ikeda M, Imai K, Yamauchi-Kawaura C, Kato K, Isoda H., APESM 36(3), pp 313-322, DOI 10.1007/s13246-013-0215-z. Australas Phys Eng Sci Med. 2014;37:471-472. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Coffman DL, Zhou J, Cai X. Comparison of methods for handling covariate missingness in propensity score estimation with a binary exposure. BMC Med Res Methodol. 2020;20:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661-3679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1759] [Cited by in RCA: 2848] [Article Influence: 284.8] [Reference Citation Analysis (0)] |
| 26. | Mascha EJ, Sessler DI. Segmented Regression and Difference-in-Difference Methods: Assessing the Impact of Systemic Changes in Health Care. Anesth Analg. 2019;129:618-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Tang WM, Chiu KY, Ng TP, Yau WP, Ching PT, Seto WH. Efficacy of a single dose of cefazolin as a prophylactic antibiotic in primary arthroplasty. J Arthroplasty. 2003;18:714-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Jin C, Henao-Mejia J, Flavell RA. Innate immune receptors: key regulators of metabolic disease progression. Cell Metab. 2013;17:873-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 29. | Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007;23:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 348] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 30. | Cheng H, Chen BP, Soleas IM, Ferko NC, Cameron CG, Hinoul P. Prolonged Operative Duration Increases Risk of Surgical Site Infections: A Systematic Review. Surg Infect (Larchmt). 2017;18:722-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 533] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 31. | Ho VP, Nicolau DP, Dakin GF, Pomp A, Rich BS, Towe CW, Barie PS. Cefazolin dosing for surgical prophylaxis in morbidly obese patients. Surg Infect (Larchmt). 2012;13:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Banoub M, Curless MS, Smith JM, Jarrell AS, Cosgrove SE, Rock C, Avdic E. Higher versus Lower Dose of Cefotetan or Cefoxitin for Surgical Prophylaxis in Patients Weighing One Hundred Twenty Kilograms or More. Surg Infect (Larchmt). 2018;19:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |