Published online May 18, 2023. doi: 10.5312/wjo.v14.i5.362
Peer-review started: January 16, 2023
First decision: January 31, 2023
Revised: February 25, 2023
Accepted: March 29, 2023
Article in press: March 29, 2023
Published online: May 18, 2023
Processing time: 122 Days and 2 Hours
Myositis ossificans (MO) is an uncommon disorder characterized by heterotopic ossification within soft tissues. Only a few cases of intra-abdominal MO (IMO) have been described in the literature. Histology could be difficult to understand and a wrong diagnosis could lead to an improper cure.
We herein report the case of IMO in a healthy 69-year-old man. The patient presented with an abdominal mass in the left lower quadrant. A computed tomography scan showed an inhomogeneous mass with multiple calcifications. The patient underwent radical excision of the mass. Histopathological findings were compatible with MO. Five months later the patient showed a recurrence causing hemorrhagic shock due to intractable intralesional bleeding. The patients eventually died within three months since recurrence.
The case described could be classified as post-traumatic MO that developed close to the previously fractured iliac bone. The subsequent surgical procedure was ineffective and the disease rapidly recurred. The misleading intraoperative diagnosis led to improper surgical treatment with a dramatic evolution.
Core Tip: Myositis ossificans (MO) is a rare condition of heterotopic bone formation within muscle or soft tissues. Intra-abdominal MO is even rarer usually arousing following abdominal surgery or trauma. Its presentation is non-specific and physical examination is usually unremarkable until the mass reaches large dimensions. Laboratory examinations are within normal limits. Computed tomography scan is essential for the diagnosis, since it can show the typical “zonal patterns” of the calcifications. Histopathology can differentiate MO from infections and malignancies. However, histology could be misinterpreted for fibromatosis or sarcoma, thus leading to improper cure. The treatment may be complex and should be based on patients’ symptoms. Most patients can be treated conservatively and surgical procedures should be reserved for selected patients since repetitive surgery promotes further and more aggressive calcifications.
- Citation: Carbone G, Andreasi V, De Nardi P. Intra-abdominal myositis ossificans - a clinically challenging disease: A case report. World J Orthop 2023; 14(5): 362-368
- URL: https://www.wjgnet.com/2218-5836/full/v14/i5/362.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i5.362
Myositis ossificans (MO) is a rare and benign connective tissue disorder characterized by non-neoplastic heterotopic ossification in the context of soft tissues. MO tends to be secondary to trauma. It is mostly found in adolescents or young adults and the lesions are predominantly located in the large skeletal muscles of the arms and thighs. This form, also known as post-traumatic MO (PTMO), is usually self-limiting thus not requiring specific surgical treatment. However, the clinical, radiological, and histological presentation of MO is often non-specific and can mimic a soft tissue malignancy, resulting in misdiagnosis and inappropriate management. We herein report a very rare case of intra-abdominal MO (IMO) and review the main features of this rarely encountered, but clinically demanding condition.
A 69-year-old man presented at the surgical clinic with a painless abdominal lump, located in the left iliac fossa. The patient did not refer any systemic symptom.
The mass had slowly increased in size over the last couple of months.
His past medical history included an appendectomy during his childhood. Four years earlier a car accident occurred resulting in abdominal trauma and left iliac bone fracture.
The patient denied any family or personal history of malignancies.
Clinical examination revealed a non-tender, firm, oval-shaped abdominal mass with smooth surface in the left lower quadrant.
Laboratory findings were within normal range.
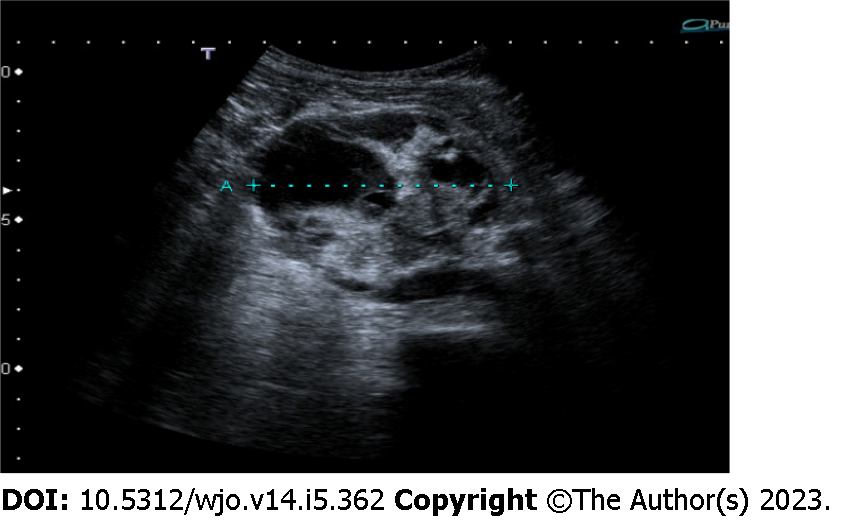
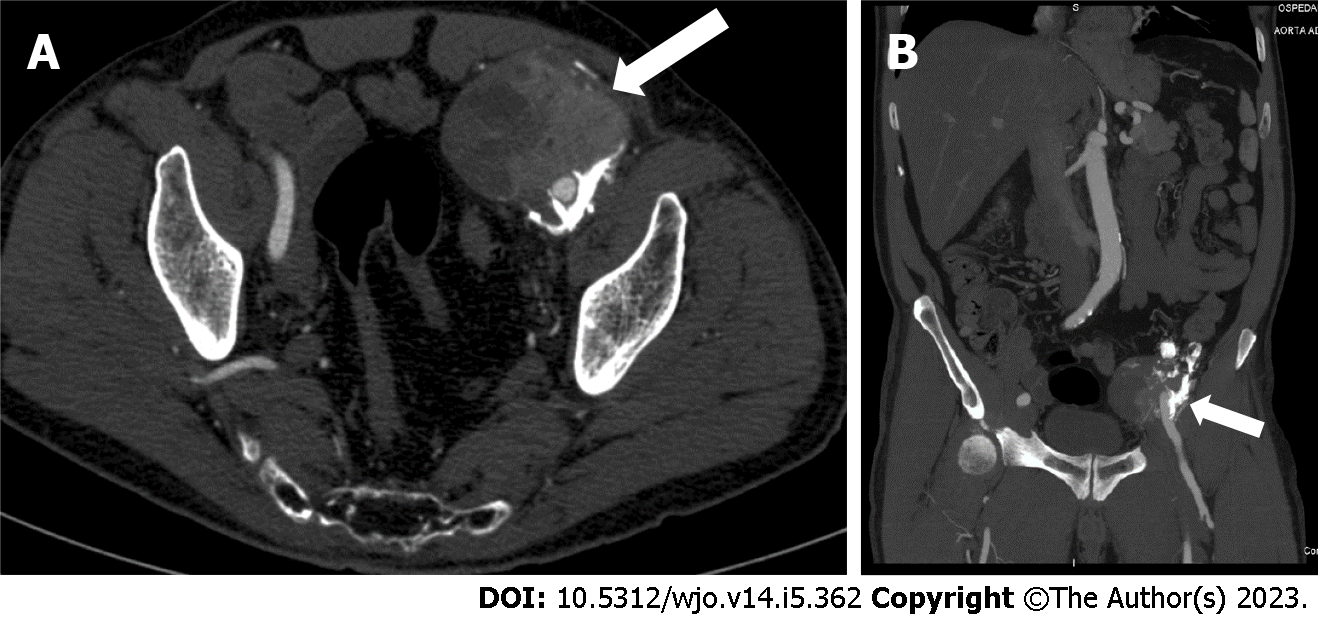
An abdominal ultrasound showed a 7-cm ovoidal lesion with a mixed echo structure, diffuse calcifications and increased vascular density at the color-Doppler control (Figure 1). A computed tomography (CT) scan was obtained with evidence of an 8 cm × 6.5 cm × 7 cm, highly inhomogeneous mass composed by multiple calcifications at the periphery (Figure 2).
The imaging features were strongly suspicious for a malignancy originating from mesenchymal cells. Based on these findings, a surgical exploration was performed.
A bulky tumor with dense adhesions to the left iliac bone was found and the intraoperative histological examination was consistent with osteosarcoma. A radical excision of the mass was then carried out.
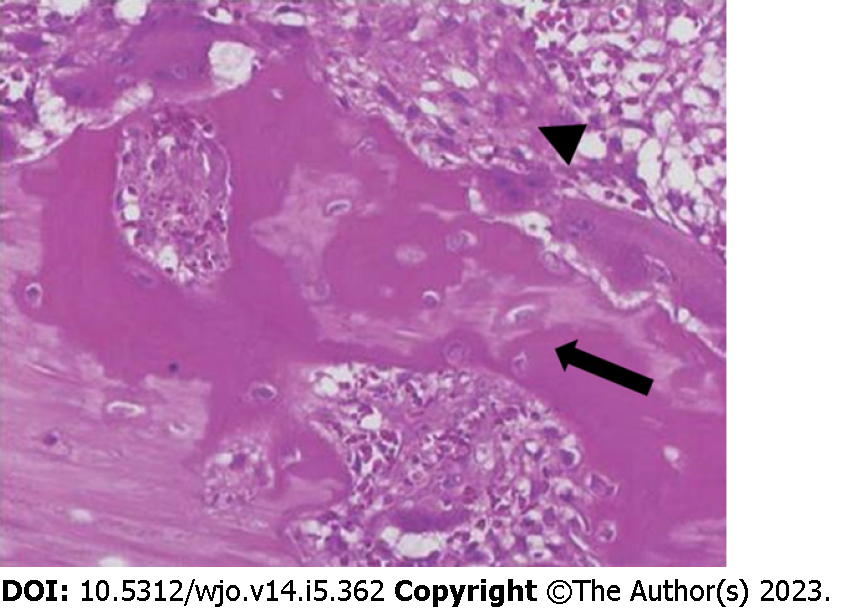
Gross examination of the surgical specimen revealed a partially capsulated, 8.5 cm × 8 cm × 6 cm mass with hemorrhagic and calcified areas. The lesion presented trabecular architecture characterized by a central area consisting of fiber cells and by a peripheral area consisting of osteoblasts, producing osteoid and bone matrix. Histopathological findings were compatible with IMO (Figure 3).
The post-operative course was complicated by a chylous fistula that was managed conservatively. Additionally, a complete thrombosis of the left femoral vein was diagnosed. The patient was then discharged with anticoagulant therapy.
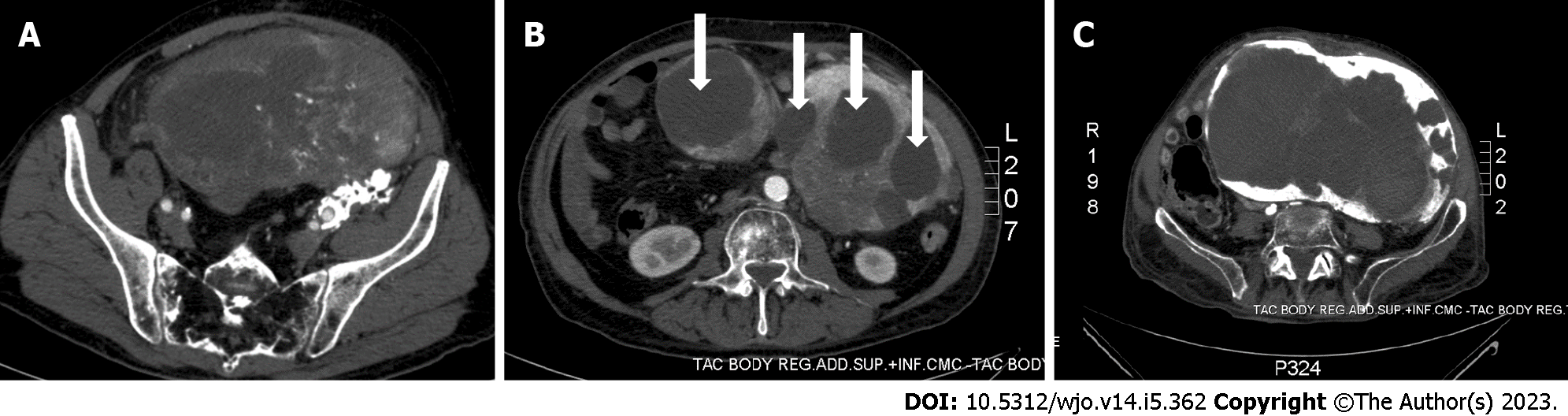
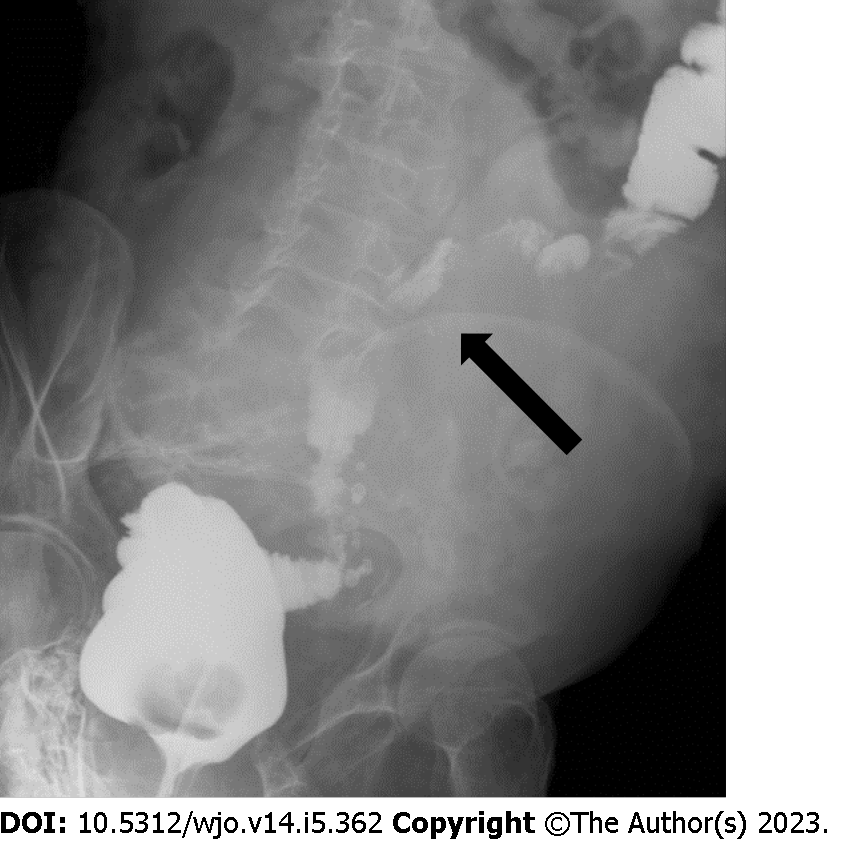
Five months later, the patient returned to the Emergency Department complaining rapidly worsening abdominal pain, nausea, and vomiting. The radiological examinations showed disease recurrence with an inhomogeneous pelvic mass measuring approximately 20 cm in diameter and causing compression of the sigmoid colon (Figures 4A and 5). Because of the bowel obstruction, an explorative laparotomy was planned. At surgery, a hard, hyper vascular lesion strongly adherent to the anterior abdominal wall and to the deep posterior planes was found. Thus, due to the high risk of bleeding, only a biopsy of the mass was performed and the histopathological diagnosis confirmed an IMO. During the postoperative course the patient developed a hemorrhagic shock caused by multiple intralesional bleedings (Figure 4B). An angiography, with embolization of vessels originating from the left iliac artery, was performed with cessation of the bleeding. The lesion, however, continued to grow rapidly, infiltrating the aortic plane and both ureters (Figure 4C). Eventually, the patient dramatically died eight months after the first diagnosis.
MO is an infrequent and non-malignant disease distinguished by abnormal heterotopic bone formation within soft tissues[1]. Two different clinical entities of MO have been reported in the literature: Progressive or genetic MO and acquired MO[2,3].
Progressive MO (PMO), also known as Munchmeyer disease, is a rare genetic autosomic dominant disorder characterized by congenital malformation of big toes and progressive heterotopic ossification of connective tissue. It usually begins in the spine and proceeds in a proximal to distal, cranial to caudal or axial to appendicular direction[4]. PMO normally develops in the first decade of life and the evidence of malformed great toes may lead to the correct diagnosis[5].
Acquired MO can be classified into neurogenic (associated with paraplegia) and non-neurogenic; the latter group can be divided into post-traumatic and idiopathic/pseudo malignant[2,3]. The case described in our report was reasonably a PTMO and, in the present article, we will discuss this clinical entity, which represents the most frequent form of MO, accounting for almost 70% of cases[6].
PTMO usually presents within the second and third decade of life, but lesions can arise at any age, as showed in our patient. PTMO typically originates in the context of the large skeletal muscles of the limbs. Quadriceps and brachialis muscles are the two most affected regions because of the high risk of traumas and injuries. However, PTMO can occur in almost every site of the body and even cases of PTMO in the hand have been described in the literature[7].
Abdominal PTMO mostly arises from a midline surgical incision or a trauma site[8], while IMO is an extremely rare condition associated with neoplasms of gastrointestinal tract or previous intra-abdominal surgery[9]. In the few IMO cases reported in literature[10,11], lesions developed inside the mesentery or the omentum after a surgical procedure and tended to grow rapidly, often resulting in bowel obstruction. Interestingly, in our patient the IMO lesion was located next to the previously fractured iliac bone and the mechanical trauma was likely the primum movens for the development of the lesion. The following surgical stress was probably responsible for disease recurrence and especially for the rapid growth evolution.
The clinical presentation of PTMO is highly variable, depending also on the site of origin. In case of PTMO of the limbs, it may range from an incidental finding to a rapidly enlarging painful mass with reduced range of movement. Otherwise, as mentioned before, patients with IMO can present with bowel obstruction. In some cases, clinical symptoms can mimic those of an aggressive malignant sarcoma, representing a diagnostic challenge[12,13].
The diagnosis of PTMO depends strictly on radiological and histological features since symptoms can be vague and clinical history is often silent (the only relevant element may be a trauma in the affected area). CT is the preferred imaging technique for PTMO as it best demonstrates the typical “zonal pattern”. This feature is represented by a low-attenuating center with a well-circumscribed, high-attenuating, peripheral rim of calcification. CT may also show the classical “string sign”, a thin radiolucent cleft which separates MO from adjacent bone. In our case, imaging revealed an inhomogeneous and hyper vascularized lesion with calcifications located only in one third of the circumference. These features were highly suspicious for a malignant lesion[14,15].
The “zone phenomenon” is also observed on histological tissue samples with a well-organized mature lamellar bone at the periphery, an intermediate osteoid region, and a central immature non-ossified fibroblastic focus[16]. The evidence of that histological feature makes MO diagnosis certain, since malignant neoplasms as osteosarcomas usually have a disorderly growth with a “reverse zoning effect”. However, in some cases, a biopsy from the central zone that represents the least organized portion can be histologically suggestive of malignancies, leading to incorrect diagnoses[17]. This is what happened in our case, with an intraoperative histological exam positive for osteosarcoma that led to a surgical excision. Immunohistochemistry is not strictly essential for a correct diagnosis, but it helps to differentiate MO from malignant lesions, especially in atypical cases. MO samples generally include a large number of osteoblasts, which are positive for the marker SATB2. On other hand, osteosarcomas may be characterized by MDM2 and CDK4 overexpression[18,19]. The surgical specimen of our patient demonstrated organized mature lamellar bone at the periphery of the lesion, with a great amount of SATB2 positive cells, thus confirming MO diagnosis.
The management of PTMO is controversial and depends on the severity of patient's symptoms. PTMO is generally self-limiting and lesions often decreases in volume within two years. For this reason, most patients can be treated conservatively with nonsteroidal anti-inflammatory drugs, acetic acid therapy, iontophoresis treatment, magnesium therapy, and etidronate disodium[20,21]. In case of severe and persistent symptoms, surgery is the definitive treatment even if it is associated with local recurrence[22,23]. Particularly, IMO tends to recur and, since this condition is clearly exacerbated by surgery, surgical procedures should be reserved for selected patient[11]. The highest risk of recurrence is reached when surgical excision is performed within 6 mo of previous trauma. Therefore, it is preferable to perform a surgical procedure at least six months after the trauma when the lesion is completely mature and ossified[24]. As discussed before, we decided to proceed with a surgical excision based on pre-operative and intra-operative data and the disease recurrence was dramatically extraordinary in term of growing time. To the best of our knowledge, such aggressive behavior was only described in the literature for genetic forms of MO[25] that usually present in the first decade of life and have a life expectancy of forty years[26].
MO is an uncommon and benign condition that can develop in any part of the body. The diagnosis may be difficult even with multiple imaging modalities, especially when MO develops in atypical locations. Biopsy is the gold standard, but in some case it can be misleading. Surgical treatment should be reserved for symptomatic patients because of the risk of recurrence. The present case emphasizes the challenges and pitfalls a clinician has to face to ensure a correct diagnosis and an appropriate management of this disease.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bottari A, Italy; Freund O, Israel; Kai K, Japan S-Editor: Zhao S L-Editor: A P-Editor: Zhao S
| 1. | McCarthy EF, Sundaram M. Heterotopic ossification: a review. Skeletal Radiol. 2005;34:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Edwards DS, Clasper JC. Heterotopic ossification: a systematic review. J R Army Med Corps. 2015;161:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Meyers C, Lisiecki J, Miller S, Levin A, Fayad L, Ding C, Sono T, McCarthy E, Levi B, James AW. Heterotopic Ossification: A Comprehensive Review. JBMR Plus. 2019;3:e10172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 310] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 4. | Sheth SB, Santhosh Kumar SN, Sabhlok S, Singh M. Munchmeyer's disease-a rare case report and review of literature. Dentomaxillofac Radiol. 2014;43:20140022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Kartal-Kaess M, Shore EM, Xu M, Schwering L, Uhl M, Korinthenberg R, Niemeyer C, Kaplan FS, Lauten M. Fibrodysplasia ossificans progressiva (FOP): watch the great toes! Eur J Pediatr. 2010;169:1417-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Saad A, Azzopardi C, Patel A, Davies AM, Botchu R. Myositis ossificans revisited - The largest reported case series. J Clin Orthop Trauma. 2021;17:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Al-Qattan MM, Al-Fahdil L, Al-Shammari HM, Joarder AI. Management of Myositis Ossificans of the Hand: A Case Report and a Review of the Literature. J Hand Surg Am. 2017;42:576.e1-576.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Jung EJ, Lee YJ, Park ST, Ha WS, Choi SK, Hong SC, Jeong CY, Joo YT, Na JB, Ko GH. Myositis ossificans of the abdominal rectus muscle: report of a case. Surg Today. 2006;36:619-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Bovo G, Romano F, Perego E, Franciosi C, Buffa R, Uggeri F. Heterotopic mesenteric ossification ("intraabdominal myositis ossificans''): a case report. Int J Surg Pathol. 2004;12:407-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Zamolyi RQ, Souza P, Nascimento AG, Unni KK. Intraabdominal myositis ossificans: a report of 9 new cases. Int J Surg Pathol. 2006;14:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Wilson JD, Montague CJ, Salcuni P, Bordi C, Rosai J. Heterotopic mesenteric ossification ('intraabdominal myositis ossificans'): report of five cases. Am J Surg Pathol. 1999;23:1464-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Cortellazzo Wiel L, Trevisan M, Murru FM, Rabusin M, Barbi E. Myositis ossificans mimicking sarcoma: a not so rare bioptic diagnostic pitfall. Ital J Pediatr. 2020;46:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Ragunanthan N, Sugavanam C. Pseudomalignant myositis ossificans mimicking osteosarcoma: a case report. J Orthop Surg (Hong Kong). 2006;14:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Parikh J, Hyare H, Saifuddin A. The imaging features of post-traumatic myositis ossificans, with emphasis on MRI. Clin Radiol. 2002;57:1058-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Tyler P, Saifuddin A. The imaging of myositis ossificans. Semin Musculoskelet Radiol. 2010;14:201-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Cocks M, Mohan A, Meyers CA, Ding C, Levi B, McCarthy E, James AW. Vascular patterning in human heterotopic ossification. Hum Pathol. 2017;63:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Luczyńska E, Kasperkiewicz H, Domalik A, Cwierz A, Bobek-Billewicz B. Myositis ossificans mimicking sarcoma, the importance of diagnostic imaging - case report. Pol J Radiol. 2014;79:228-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Yoshida A, Ushiku T, Motoi T, Shibata T, Beppu Y, Fukayama M, Tsuda H. Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimics. Mod Pathol. 2010;23:1279-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Savvidou O, Papakonstantinou O, Lakiotaki E, Melissaridou D, Korkolopoulou P, Papagelopoulos PJ. Post-traumatic myositis ossificans: a benign lesion that simulates malignant bone and soft tissue tumours. EFORT Open Rev. 2021;6:572-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Moore KD, Goss K, Anglen JO. Indomethacin versus radiation therapy for prophylaxis against heterotopic ossification in acetabular fractures: a randomised, prospective study. J Bone Joint Surg Br. 1998;80:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Wieder DL. Treatment of traumatic myositis ossificans with acetic acid iontophoresis. Phys Ther. 1992;72:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Orava S, Sinikumpu JJ, Sarimo J, Lempainen L, Mann G, Hetsroni I. Surgical excision of symptomatic mature posttraumatic myositis ossificans: characteristics and outcomes in 32 athletes. Knee Surg Sports Traumatol Arthrosc. 2017;25:3961-3968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Pavey GJ, Polfer EM, Nappo KE, Tintle SM, Forsberg JA, Potter BK. What Risk Factors Predict Recurrence of Heterotopic Ossification After Excision in Combat-related Amputations? Clin Orthop Relat Res. 2015;473:2814-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Conner GA, Duffy M. Myositis ossificans: a case report of multiple recurrences following third molar extractions and review of the literature. J Oral Maxillofac Surg. 2009;67:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | de Ruiter RD, Smilde BJ, Pals G, Bravenboer N, Knaus P, Schoenmaker T, Botman E, Sánchez-Duffhues G, Pacifici M, Pignolo RJ, Shore EM, van Egmond M, Van Oosterwyck H, Kaplan FS, Hsiao EC, Yu PB, Bocciardi R, De Cunto CL, Longo Ribeiro Delai P, de Vries TJ, Hilderbrandt S, Jaspers RT, Keen R, Koolwijk P, Morhart R, Netelenbos JC, Rustemeyer T, Scott C, Stockklausner C, Ten Dijke P, Triffit J, Ventura F, Ravazzolo R, Micha D, Eekhoff EMW. Fibrodysplasia Ossificans Progressiva: What Have We Achieved and Where Are We Now? Front Endocrinol (Lausanne). 2021;12:732728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Kaplan FS, Zasloff MA, Kitterman JA, Shore EM, Hong CC, Rocke DM. Early mortality and cardiorespiratory failure in patients with fibrodysplasia ossificans progressiva. J Bone Joint Surg Am. 2010;92:686-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |