Published online May 18, 2023. doi: 10.5312/wjo.v14.i5.340
Peer-review started: January 23, 2023
First decision: January 31, 2023
Revised: February 14, 2023
Accepted: March 27, 2023
Article in press: March 27, 2023
Published online: May 18, 2023
Processing time: 115 Days and 13.3 Hours
Transmission of severe acute respiratory syndrome coronavirus 2 can occur during aerosol generating procedures. Several steps in spinal fusion may aerosolize blood but little data exists to quantify the risk this may confer upon surgeons. Aerosolized particles containing infectious coronavirus are typically 0.5-8.0 μm.
To measure the generation of aerosols during spinal fusion using a handheld optical particle sizer (OPS).
We quantified airborne particle counts during five posterior spinal instrumentation and fusions (9/22/2020-10/15/2020) using an OPS near the surgical field. Data were analyzed by 3 particle size groups: 0.3-0.5 μm/m3, 1.0-5.0 μm/m3, and 10.0 μm/m3. We used hierarchical logistic regression to model the odds of a spike in aerosolized particle counts based on the step in progress. A spike was defined as a > 3 standard deviation increase from average baseline levels.
Upon univariate analysis, bovie (P < 0.0001), high speed pneumatic burring (P = 0.009), and ultrasonic bone scalpel (P = 0.002) were associated with increased 0.3-0.5 μm/m3 particle counts relative to baseline. Bovie (P < 0.0001) and burring (P < 0.0001) were also associated with increased 1-5 μm/m3 and 10 μm/m3 particle counts. Pedicle drilling was not associated with increased particle counts in any of the size ranges measured. Our logistic regression model demonstrated that bovie (OR = 10.2, P < 0.001), burring (OR = 10.9, P < 0.001), and bone scalpel (OR = 5.9, P < 0.001) had higher odds of a spike in 0.3-0.5 μm/m3 particle counts. Bovie (OR = 2.6, P < 0.001), burring (OR = 5.8, P < 0.001), and bone scalpel (OR = 4.3, P = 0.005) had higher odds of a spike in 1-5 μm/m3 particle counts. Bovie (OR = 0.3, P < 0.001) and drilling (OR = 0.2, P = 0.011) had significantly lower odds of a spike in 10 μm/m3 particle counts relative to baseline.
Several steps in spinal fusion are associated with increased airborne particle counts in the aerosol size range. Further research is warranted to determine if such particles have the potential to contain infectious viruses. Previous research has shown that electrocautery smoke may be an inhalation hazard for surgeons but here we show that usage of the bone scalpel and high-speed burr also have the potential to aerosolize blood.
Core Tip: In this study we use a handheld optical particle sizer to measure the generation of aerosols during surgical steps in spinal fusion because of the risk this may confer upon surgeons in regards to the airborne transmission of severe acute respiratory syndrome coronavirus 2. Several steps in spinal fusion, specifically the bone scalpel and high-speed burr, were found to be associated with increased airborne particle counts in the aerosol size range.
- Citation: Langner JL, Pham NS, Richey A, Oquendo Y, Mehta S, Vorhies JS. Spinal fusion is an aerosol generating procedure. World J Orthop 2023; 14(5): 340-347
- URL: https://www.wjgnet.com/2218-5836/full/v14/i5/340.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i5.340
The World Health Organization has warned that airborne transmission of severe acute respiratory syndrome coronavirus 2 (coronavirus disease 2019, COVID-19) can occur during medical procedures that generate aerosols, especially those involving the airway[1]. Instrumentation of the upper and lower airways is often considered high risk and some research has indicated that these procedures are aerosol-generating procedures (AGPs)[2]. Certain tools used in orthopaedic surgery have previously been shown to generate blood containing aerosols less than 5 μm in diameter[3]. Despite this potential hazard, there are no previous reports describing the risk of aerosolization of body fluids during spinal fusion.
The naked COVID-19 virus is 0.06-0.14 μm in diameter but aerosolized particles containing infectious virus are typically 0.5-8.0 μm. Particles greater than 5 μm are generally considered droplets whereas those less than 5 μm represent aerosols that remain suspended for periods of time and travel significant distances, though there is some controversy over these definitions[4]. In a 2010 study researchers found that electrocautery, bone saws, reamers and drills appear to produce aerosols[5]. Other authors have raised the theoretical concern that aerosolized blood during spine surgery could put members of the surgical team at risk for exposure to infectious viral particles, but there is little in vivo data to help quantify this risk[6].
The current guidelines for personal protective equipment during orthopedic surgery vary by institution but generally providers are advised to wear N95 masks during intubation and other procedures considered to be aerosol generating[7-9]. However, controversy remains over which procedures or components of procedures should be considered AGPs, leaving policy makers with a lack of evidence to guide decisions related to infection control. Here we present a pilot study using handheld optical particle sizers (OPSs) to evaluate the potential for various surgical steps during spinal fusion to aerosolize blood and other body fluids. We hypothesize that surgical steps of interest, mainly bovie electrocautery, burring, drilling, and harmonic bone scalpel, will significantly increase airborne aerosol particle counts during spinal fusion.
We quantified airborne particle counts throughout the course of five posterior spinal instrumentation and fusions (9/22/2020-10/15/2020). The University Institutional Review Board (IRB No. 58206) granted an IRB waiver with a determination that no human subjects were involved in this study. This pilot study was designed as a quality improvement assessment for our institution.
An Aerotrak Handheld Airborne Particle Counter (Model 9306-V2) from TSI Incorporated (Shoreview, Minnesota) was used as the OPS. Before each procedure, a Zero Check was performed in the operating room on the OPS by attaching the high efficiency particulate air (HEPA) zero filter assembly to the inlet nozzle and running a two-minute purge. This step was repeated until one or less particles of any size were counted. The zero filter was then removed and the Stainless-Steel Isokinetic inlet was attached for sampling. The OPS was secured to a hanger to ensure consistent sampling height and distance to the patient and stabilized with a sandbag (Figure 1A).
A sterile bag was placed over the OPS and hanger. The vented aspects of the OPS used for sampling remained uncovered (Figure 1B).
Because this study is primarily evaluating the risk to the surgical team, the OPS was positioned next to the surgical staff hanging over the central operating area at a comparable height and distance to the wound as the surgeon’s face.
Throughout the procedure, the OPS took continuous 30-s samples and reported the sum of particles sizes 0.3 μm/m3, 0.5 μm/m3, 1.0 μm/m3, 3.0 μm/m3, 5.0 μm/m3, and 10.0 μm/m3 in each 1.4 L sample. Based on the usage of high-speed power tools and shear forces, the following surgical steps were identified for study: Bovie, burring, drilling, and bone scalpel. As the OPS sampled, researchers recorded the start and end times of each surgical step of interest, the approximate distance from the wound/central operating area to the particle sensor, and the maximum number of people in the operating room at any point during the sampling period (excluding the patient). The operating rooms used for sampling in this study have an airflow of 25 air exchanges per hour with laminar flow ceilings that create an air curtain about four feet on each side of the patient. There were two low wall returns in each room that used a recirculated system with HEPA filtration.
The data was downloaded using the TSI TrakPro Lite Secure 3.1 software in an XML format and analyzed in RStudio version 1.1.456 using a two-sided level of significance of 0.05. Each 30 s sample was labeled with the surgical step of interest that was occurring. If there was more than one step occurring during a 30 s sample, this was labeled as multiple steps. Each 30 s sample labeled with a surgical step of interest was compared to baseline samples when there were no steps of interest occurring. In addition, the 30 s samples before and after a sample with a step of interest occurring were removed from the analysis to reduce the potential of biasing the data with leftover aerosols.
Multivariable hierarchical logistic regression models were used to model the odds of a spike in particle counts, accounting for within-surgery variability and autocorrelated errors. Surgical steps, the sensor distance, and the maximum number of people in the operating room were included in these models. A spike in particle counts was defined as a greater than 3 standard deviations increase in particles from baseline levels. Data were analyzed by grouping the particle sizes into 0.3-0.5 μm/m3, 1.0-5.0 μm/m3, and 10.0 μm/m3. This division in particle sizes was not alterable and based on the internal settings of the OPS. To adjust for potential confounding, we used a regression model to control for the variables of sensor distance to the surgical field and the maximum number of people in the room during sampling.
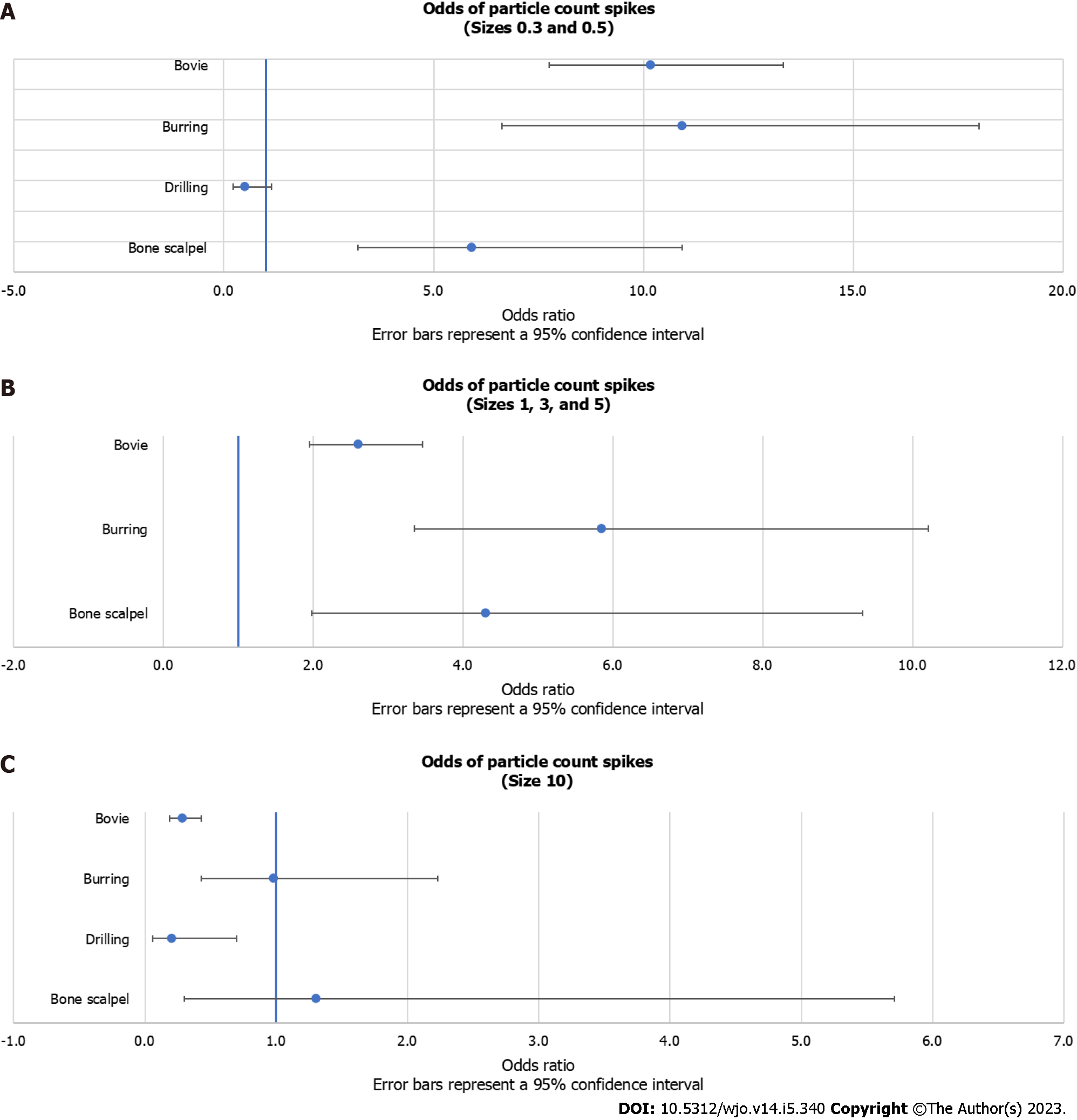
The logistic regression analysis revealed that bovie (OR = 10.2; 95%CI: 7.8, 13.3; P < 0.001), burring (OR = 10.9; 95%CI: 6.6, 18.0; P < 0.001), and bone scalpel (OR = 5.9; 95%CI: 3.2, 10.9; P < 0.001) had significantly higher odds of a spike in 0.3-0.5 μm/m3 particle counts (“spike” being defined as a greater than 3 standard deviation increase from average baseline levels) (Table 1). Drilling was not significantly associated with a spike in 0.3-0.5 μm/m3 particle counts (OR = 0.5; 95%CI: 0.2, 1.1; P = 0.099).
| Variable | OR | Lower 95% | Upper 95% | Z score | P value |
| Bovie | 10.20 | 7.80 | 13.30 | 16.80 | < 0.001 |
| Burring | 10.90 | 6.60 | 18.00 | 9.40 | < 0.001 |
| Drilling | 0.50 | 0.20 | 1.10 | -1.70 | 0.099 |
| Bone scalpel | 5.90 | 3.20 | 10.90 | 5.70 | < 0.001 |
| Max in room | 1.40 | 0.90 | 2.00 | 1.60 | 0.116 |
| Sensor distance | 0.03 | 0.01 | 0.10 | -4.70 | < 0.001 |
Bovie (OR = 2.6; 95%CI: 2.0, 3.5; P < 0.001), burring (OR = 5.8; 95%CI: 3.4, 10.2; P < 0.001), and bone scalpel (OR = 4.3; 95%CI: 2.0, 9.3; P = 0.005) had significantly higher odds of a spike in 1-5 μm/m3 particle counts (Table 2). Drilling was excluded in this analysis because no spikes in the 1-5 μm/m3 particle size range were recorded during drilling.
| Variable | OR | Lower 95% | Upper 95% | Z score | P value |
| Bovie | 2.60 | 2.00 | 3.5 | 6.5 | < 0.001 |
| Burring | 5.80 | 3.40 | 10.2 | 6.2 | < 0.001 |
| Bone scalpel | 4.30 | 2.00 | 9.3 | 3.7 | 0.005 |
| Max in room | 1.40 | 0.90 | 2.4 | 1.3 | 0.179 |
| Sensor distance | 0.05 | 0.01 | 0.4 | -2.9 | 0.004 |
Bovie (OR = 0.3; 95%CI: 0.2, 0.4; P < 0.001) and drilling (OR = 0.2; 95%CI: 0.1, 0.7; P = 0.011) had significantly lower odds of a spike in 10 μm/m3 particle counts (Table 3). Burring (OR = 1.0; 95%CI: 0.4, 2.2; P = 0.962) and the bone scalpel (OR = 1.3; 95%CI: 0.3, 5.7; P = 0.724) were not significantly associated with a spike in 10 μm/m3 particle counts. All logistic regression results are visually presented as forest plots (Figure 2).
| Variable | OR | Lower 95% | Upper 95% | Z score | P value |
| Bovie | 0.3 | 0.2 | 0.4 | -6.0 | < 0.001 |
| Burring | 1.0 | 0.4 | 2.2 | 0.0 | 0.962 |
| Drilling | 0.2 | 0.1 | 0.7 | -2.5 | 0.011 |
| Bone scalpel | 1.3 | 0.3 | 5.7 | 0.4 | 0.724 |
| Max in room | 1.7 | 0.9 | 3.5 | 1.6 | 0.111 |
| Sensor distance | 0.02 | 0.001 | 0.2 | -3.0 | 0.003 |
In addition, using generalized least squares (GLS) regression models, we compared average particle counts found within a certain step to the baseline defined before and after the steps. The results from this analysis are highly consistent with those of the logistic regression. The bovie (P < 0.001), burring (P = 0.009), and bone scalpel (P = 0.002) were associated with an increase in the average 0.3-0.5 μm/m3 particle counts relative to baseline, while drilling was not (P = 0.323). Bovie (P < 0.001) and burring (P < 0.001) were associated with an increase in the average 1-5 μm/m3 particle counts, while drilling (P = 0.748) and bone scalpel (P = 0.110) were not. Bovie (P = 0.032) was associated with a decrease in the average 10 μm/m3 particle counts, while burring (P < 0.001) was associated with an increase in the average 10 μm/m3 particle counts. Drilling (P = 0.403) and the bone scalpel (P = 0.638) were not associated with changes in the average 10 μm/m3 particle counts.
Handheld OPSs are common tools used to quantify particles present in an air sample. OPSs have previously been employed in the healthcare setting to quantify aerosol generation during medical and surgical procedures[10-14]. In this study we use an OPS to demonstrate that several steps in spinal fusion are associated with increased airborne particle counts in the aerosol size range. Specifically, bovie, burring, and the bone scalpel showed consistently higher odds of spikes, while drilling did not. Although bovie and drilling had lower odds of a spike in 10 μm/m3 particle counts, this was likely due to the large number of 10 μm/m3 particle counts at baseline, which may have influenced our ability to recognize a spike in this range.
It is well established that the bovie produces large clouds of aerosolized particles[9]. This study has confirmed this finding and given validity to our methodology of identifying aerosol producing surgical steps. Previous research has shown that electrocautery smoke may be an inhalation hazard for surgeons but here we show that usage of the high speed burr and bone scalpel have the potential to aerosolize blood and other body substances[11,15]. Of note, burring confers less heat than bovie and bone scalpel, potentially making the aerosol particles it produces a larger risk if they are carrying infectious agents. These steps generate an abundance of particles in a size range that has been established to carry infectious particles. Multiple lines of evidence have confirmed that viral particles are detectable in the blood, but further research is warranted to determine if such particles have the potential to contain infectious viruses. Furthermore, if aerosolized blood has the potential to transmit the COVID-19 virus further research would be needed to determine if and whether the blood aerosolized through the surgical techniques described here is an infectious hazard or if particles are heated up to the point that they are no longer infectious[12,16-18].
The coronavirus disease 2019 (COVID-19) pandemic has raised awareness of aerosol generation during medical procedures as an occupational hazard. Several authors have speculated that certain steps during spinal fusion have the potential to generate aerosols, however there is a dearth of data to quantify this risk. Publishing the type of data, we present here is critical to help hospitals create evidence-based workplace safety policies. As such, we believe that the findings presented here will be of interest to the readership of your journal and will hopefully inform future research and clinical care.
Several steps in spinal fusion are associated with increased airborne particle counts in the aerosol size range. Further research is warranted to determine if such particles have the potential to contain infectious viruses.
Upon univariate analysis, bovie (P < 0.0001), high speed pneumatic burring (P = 0.009), and ultrasonic bone scalpel (P = 0.002) were associated with increased 0.3-0.5 μm/m3 particle counts relative to baseline. Bovie (P < 0.0001) and burring (P < 0.0001) were also associated with increased 1-5 μm/m3 and 10 μm/m3 particle counts. Our logistic regression model demonstrated that bovie (OR = 10.2, P < 0.001), burring (OR = 10.9, P < 0.001), and bone scalpel (OR = 5.9, P < 0.001) had higher odds of a spike in 0.3-0.5 μm/m3 particle counts. Bovie (OR = 2.6, P < 0.001), burring (OR = 5.8, P < 0.001), and bone scalpel (OR = 4.3, P = 0.005) had higher odds of a spike in 1-5 μm/m3 particle counts.
We quantified airborne particle counts during five posterior spinal instrumentation and fusions (9/22/2020-10/15/2020) using an optical particle sizer (OPS) near the surgical field. Data were analyzed by 3 particle size groups: 0.3-0.5 μm/m3, 1.0-5.0 μm/m3, and 10.0 μm/m3. We used hierarchical logistic regression to model the odds of a spike in aerosolized particle counts based on the step in progress.
In this study we use a handheld OPS to measure the generation of aerosols during surgical steps in spinal fusion because of the risk this may confer upon surgeons in regards to the airborne transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Several steps in spinal fusion may aerosolize blood but little data exists to quantify the risk this may confer upon surgeons.
Transmission of SARS-CoV-2 can occur during aerosol generating procedures. Several steps in spinal fusion may aerosolize blood but little data exists to quantify the risk this may confer upon surgeons.
The authors would like to thank Dr. Julius Bishop and Dr. Michael Gardner for their contributions of medical and scientific knowledge for the completion of this research project.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Active Fellow of the Scoliosis Research Society (SRS); American Orthopaedic Association (AOA); Emerging Leaders; Fellow of the American Academy of Orthopaedic Surgeons (AAOS); Candidate Fellow of the Scoliosis Research Society (SRS); Active Member of the Pediatric Orthopaedic Society of North America (POSNA); Active Member of the Ruth Jackson Orthopaedic Society (RJOS); Active Member of the J. Robert Gladden Orthopaedic Society.
Specialty type: Orthopedics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Solanki SL, India; Zhu F, China S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY
| 1. | World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions. July 9, 2020. [cited 1 March 2023]. Available from: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions. |
| 2. | Thompson KA, Pappachan JV, Bennett AM, Mittal H, Macken S, Dove BK, Nguyen-Van-Tam JS, Copley VR, O'Brien S, Hoffman P, Parks S, Bentley A, Isalska B, Thomson G; EASE Study Consortium. Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic--the risk of aerosol generation during medical procedures. PLoS One. 2013;8:e56278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Jewett DL, Heinsohn P, Bennett C, Rosen A, Neuilly C. Blood-containing aerosols generated by surgical techniques: a possible infectious hazard. Am Ind Hyg Assoc J. 1992;53:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657-1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 628] [Cited by in RCA: 523] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 5. | Yeh HC, Turner RS, Jones RK, Muggenburg BA, Lundgren DL, Smith JP. Characterization of Aerosols Produced during Surgical Procedures in Hospitals. Aerosol Sci Technol. 2010;22:; 151-161. [DOI] [Full Text] |
| 6. | Shah S, Gadiya A, Patel MS, Shafafy M. Coronavirus Disease 2019 Transmission: Blood Viremia and Aerosol Generation from Spinal Surgery. Is There an Increased Risk to the Surgical Team? Asian Spine J. 2020;14:702-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Farrell S, Schaeffer EK, Mulpuri K. Recommendations for the Care of Pediatric Orthopaedic Patients During the COVID-19 Pandemic. J Am Acad Orthop Surg. 2020;28:e477-e486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Raghavan R, Middleton PR, Mehdi A. Minimising aerosol generation during orthopaedic surgical procedures- Current practice to protect theatre staff during Covid-19 pandemic. J Clin Orthop Trauma. 2020;11:506-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Baldock TE, Bolam SM, Gao R, Zhu MF, Rosenfeldt MPJ, Young SW, Munro JT, Monk AP. Infection prevention measures for orthopaedic departments during the COVID-2019 pandemic: a review of current evidence. Bone Jt Open. 2020;1:74-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Rameau A, Lee M, Enver N, Sulica L. Is Office Laryngoscopy an Aerosol-Generating Procedure? Laryngoscope. 2020;130:2637-2642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Liu N, Filipp N, Wood KB. The utility of local smoke evacuation in reducing surgical smoke exposure in spine surgery: a prospective self-controlled study. Spine J. 2020;20:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Kabariti R, Green N, Turner R. Drill splatter in orthopaedic procedures and its importance during the COVID-19 pandemic: an experimental study. Bone Jt Open. 2021;2:752-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Putzer D, Dammerer D, Huber C, Boschert H, Thaler M, Nogler M. Aerosol morphology and particle size distribution in orthopaedic bone machining: a laboratory worst-case contamination simulation. Is high-speed bone machining potentially harmful by pollution and quality schemes and what measures could be taken for prevention? Int Orthop. 2022;46:1647-1655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Putzer D, Coraça-Huber D, Huber C, Boschert H, Thaler M, Nogler M. The spatial distribution of aerosols in high-speed bone burring with external irrigation. J Microbiol Methods. 2021;184:106205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Limchantra IV, Fong Y, Melstrom KA. Surgical Smoke Exposure in Operating Room Personnel: A Review. JAMA Surg. 2019;154:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 2658] [Article Influence: 531.6] [Reference Citation Analysis (0)] |
| 17. | Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, Wang YY, Xiao GF, Yan B, Shi ZL, Zhou P. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1192] [Cited by in RCA: 1219] [Article Influence: 243.8] [Reference Citation Analysis (0)] |
| 18. | Chen W, Lan Y, Yuan X, Deng X, Li Y, Cai X, Li L, He R, Tan Y, Gao M, Tang G, Zhao L, Wang J, Fan Q, Wen C, Tong Y, Tang Y, Hu F, Li F, Tang X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9:469-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |