Published online Mar 18, 2023. doi: 10.5312/wjo.v14.i3.136
Peer-review started: July 9, 2022
First decision: September 26, 2022
Revised: October 23, 2022
Accepted: January 31, 2023
Article in press: January 31, 2023
Published online: March 18, 2023
Processing time: 250 Days and 15.3 Hours
The distinction between foot and ankle wound healing complications as opposed to infection is crucial for the appropriate and efficacious allocation of antibiotic therapy. Multiple reports have focused on the diagnostic accuracy of different inflammatory markers, however, mainly in the diabetic population.
To evaluate the diagnostic accuracy of white cell count (WCC) and C-reactive protein (CRP) as diagnostic tools for this distinction in the non-diabetic cohort.
Data was reviewed from a prospectively maintained Infectious Diseases Unit database of 216 patients admitted at Leicester University Hospitals–United Kingdom with musculoskeletal infections over the period between July 2014 and February 2020 (68 mo). All patients with confirmed diagnosis of diabetes were excluded while only those with confirmed microbiological or clinical diagnosis of foot or ankle infection were included in our study. For the included patients, we retrospectively retrieved the inflammatory markers (WCCs and CRP) at the time of presentation. Values of CRP 0-10 mg/L and WCC 4.0-11.0 × 109/L were considered normal.
After exclusion of patients with confirmed diabetes, 25 patients with confirmed foot or ankle infections were included. All infections were confirmed microbiologically with positive intra-operative culture results. 7 (28%) patients with osteomyelitis (OM) of the foot, 11 (44%) with OM of the ankle, 5 (20%) with ankle septic arthritis and 2 (8%) patients with post-surgical wound infection were identified. Previous bony surgery was identified in 13 (52%) patients, either a corrective osteotomy or an open reduction and internal fixation for a foot or ankle fracture with the infection developing on top of the existing metalwork. 21 (84%) patients did have raised inflammatory markers while 4 (16%) patients failed to mount an inflammatory response even with subsequent debridement and removal of metal work. CRP sensitivity was 84%, while WCC sensitivity was only 28%.
CRP has a relatively good sensitivity in the diagnosis of foot and ankle infections in non-diabetic patients, whereas WCC is a poor inflammatory marker in the detection of such cases. In presence of clinically high level of suspicion of foot or ankle infection, a normal CRP should not rule out the diagnosis of OM.
Core Tip: Distinction between foot and ankle wound healing complications as opposed to infection is crucial for appropriate and efficacious allocation of antibiotic therapy. Multiple reports have focused on diagnostic accuracy of different inflammatory markers, however, mainly in the diabetic population. Our aim was to evaluate the diagnostic accuracy of white cell count and C-reactive protein as diagnostic tools for this distinction in the non-diabetic cohort.
- Citation: Ahmed AH, Ahmed S, Barakat A, Mangwani J, White H. Inflammatory response in confirmed non-diabetic foot and ankle infections: A case series with normal inflammatory markers. World J Orthop 2023; 14(3): 136-145
- URL: https://www.wjgnet.com/2218-5836/full/v14/i3/136.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i3.136
Early stages of infection are difficult to discern from non-infected wound healing complications which warrants a different course of management and appropriate allocation of antibiotic treatment. Antibiotic treatment for non-infected wound dehiscence would kill commensal flora and may impair healing as well as possibly leading to an ensued infection with emergence of multi-drug resistance[1,2]. Conversely, delayed diagnosis of infection will lead to potentially avoidable complications which might culminate in amputation. It is therefore of paramount importance to assess strategies for differentiating non-infected from infected wounds at an early stage to begin advanced testing and treatment in high-risk patients.
Most of the literature addressing osteomyelitis (OM) of the foot and ankle focuses on patients with diabetes mellitus (DM) owing to the significantly higher rates of infection in this cohort of patients. It was established by a retrospective review on 1000 foot and ankle orthopedic surgical related infections that diabetic patients were five times more likely to experience a severe infection requiring hospitalization compared with non-diabetic patients[3]. That being said, it was further affirmed by another retrospective review on 1465 consecutive foot and ankle surgical cases that it was more specifically complicated diabetes (in terms of peripheral neuropathy and foot ulceration) that was incriminated in this significantly higher rate of infection rather than diabetes itself[4].
Diagnosis of foot and ankle OM relies on a thorough clinical examination and history taking further validated with laboratory evaluation, microbiological assessment, and diagnostic imaging. As previously mentioned, complicated diabetes adds significantly to the risk of post-operative infections and should be excluded through examination for peripheral neuropathy and ankle brachial index or other vascular examination if warranted. Plain radiographs are the initial imaging modalities to be considered and can be 67% specific and 60% sensitive for OM[5]. In equivocal cases, an advanced imaging such as a magnetic resonance imaging (MRI) especially new functional MRI modalities, including Dixon imaging, diffusion-weighted imaging and dynamic contrast-enhanced MRI or even Bone scans, such as the white blood cell labelled Indium-111 or Sulphur colloid marrow scan, may prove beneficial in distinguishing infections from other non-infective etiologies such as Charcot’s arthropathy or non-infected wound healing complications[6].
In terms of laboratory workup, acute phase reactants such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white cell count (WCC) as well as less commonly utilised surrogates for infection such as serum albumin levels, pro-calcitonin (PCT) and interleukin (IL)-6 have all been described for surgical foot and ankle infections[7].
Increased serum inflammatory markers such as CRP and ESR have been used for the diagnosis of OM with a sensitivity and specificity of > 0.70[8,9]. From the immunological perspective, a raised CRP heralds a mounting response to tumour necrosis factor-α-, IL-6- and IL-1-mediated insult. CRP should be interpreted carefully as it is routinely elevated postoperatively peaking at the second to third post-operative day and plummeting back to normal within three weeks. Therefore, any second peak in CRP level after the third postoperative day may be a sign of infection[10]. Another consideration is that CRP levels might not be elevated in a subset of patients with low virulent pathogens specifically coagulase negative Staphylococcus as well as fungal infections. This was established by diagnostic studies on shoulder and hip prosthetic joint infections showing low sensitivity for serum CRP when low-virulent organisms such as Propionibacterium acnes, coagulase negative Staphylococci and Enterococcus faecalis when compared to Streptococcal and Staphylococcal highly virulent culture diagnosis[11-14]. Leucocytosis may or may not be present and should not be used as an absolute indicator of OM. In the acute stage, elevation of the WCC may be seen. However, this condition is not true in all patients, in immunocompromised individuals, the normality of systemic temperature and WCC may be misleading in the face of an infection, making other diagnostic modalities essential[15,16].
Our study aimed to assess the diagnostic accuracy of simple and readily available inflammatory markers such as WCC and CRP as an aid to making this distinction in confirming suspected foot and ankle infections in the non-diabetic population thereby reducing morbidity and associated healthcare costs.
A prospectively managed database for all patients discharged home on intravenous antibiotics through the University Hospitals of Leicester NHS Trust Outpatient Parenteral Antimicrobial Therapy (OPAT) service was interrogated, to identify patients who had been treated for musculoskeletal infections. The period of inclusion was from July 1, 2014 (inception of the database) until February 28, 2020 over a period of 68 mo. Patient with infections at sites other than foot and/or ankle and those with preceding confirmed diagnosis of DM were excluded. We subsequently retrieved the inflammatory markers for included patients at the time of presentation and during the perioperative period. Values of CRP 0-10 mg/L and WCC 4.0-11.0 × 109/L were considered normal.
The diagnosis was based on the clinical picture and confirmed by imaging and laboratory investigations. All patients presented with pain, stiffness, swelling, and erythema of the affected area. Plain radiographs were the first imaging modality requested in the investigation work-up. If no radiographic evidence was present, but clinical suspicion was high, other modalities of diagnostic studies were considered i.e., ultrasonography, computed tomography and/or MRI. Laboratory investigations included WCC, CRP and blood cultures. ESR, procalcitonin and IL-6 were not routinely assessed in our hospital. Wound swabs were requested for infected surgical wounds, ulcers, or sinuses. Arthrocentesis with microscopic examination, gram staining, culture and sensitivity was performed for patients with septic arthritis. Intra-operative tissue and bone samples from OM patients acquired at the time of surgical debridement were sent to the microbiology laboratory to confirm the diagnosis, identify the causative organism and to tailor the antibiotic regimen. At least 5 samples were taken for each patient for microbiological and histological analysis.
A total of 216 patients were identified. Only 37 patients were identified as having foot and/or ankle infection, of those 12 had a diagnosis of DM. After exclusion of those with a confirmed diagnosis of DM at the time of the diagnosis or infections other than foot or ankle, 25 patients remained. The mean age at presentation was 48 years (range = 26–74) and 14 (56%) were males while 11 (44%) were females.
Of those 25 patients, 11 (44%) were admitted for foot OM, 7 (28%) for ankle OM, 5 (20%) patients with septic arthritis of the ankle joint, and 2 (8%) cases were diagnosed of having surgical site infection (SSI). A history of previous bony surgery, whether an elective osteotomy for deformity correction or fracture fixation was identified in 13 patients (52%) while 12 patients (48%) had non-surgical infections. The clinical summary of these patients is shown in Table 1.
| Diagnosis | No | Surgery related | Not surgery related |
| OM foot | 7 | 1 | 6 |
| OM ankle | 11 | 10 | 1 |
| Septic arthritis ankle | 5 | 0 | 5 |
| Postsurgical wound infection | 2 | 2 | 0 |
Of those 21 patients (84%) showed raised inflammatory markers at the time of presentation. CRP was elevated in 21 patients with a sensitivity of 84% (range = 13–417, median = 108), whereas WCC was raised only in 7 patients with a poor sensitivity of 28% (range = 10.8–20, median = 16.5). Four patients (16%) did not mount an inflammatory response: 2 with foot OM and 2 with ankle OM. All of them showed normal inflammatory markers at the time of presentation and during the perioperative period. None of these patients had a history of DM and all had normal blood glucose levels (BGL) at the time of presentation (random BGL < 11.1 mmol/L). All these patients had a history of previous bony surgery, either a corrective osteotomy (25%, n = 1) or an open reduction and internal fixation (ORIF) (75%, n = 3) for a foot or ankle fracture with the infection developing on top of the existing metalwork. Methicillin sensitive Staphylococcus aureus (MSSA) was isolated in all 4 patients. Even after implant removal and subsequent debridement for those patients, their inflammatory markers remained normal and their response to treatment was monitored clinically by successful control of local signs of infection. No significant systemic illness was noted in any of those patients and no immunosuppressive aetiology was identified. Recurrence of infection was noted in only 1 patient of those 4 (25%). The demographics of these 4 patients, their clinical and microbiological data are shown in Table 2. A more detailed history for each of those 4 presentations is described below.
| No. | Diagnosis | Procedure | Gender | Age (years) | Surgery-infection interval (months) | Isolated organism |
| Case 1 | OM 1st metatarsal | SCARF and Akin osteotomy for hallux valgus | Female | 46 | 22 | MSSA and corynebacterium |
| Case 2 | OM distal fibula | ORIF lateral malleolus fracture | Female | 54 | 5 | MSSA and Coagulase-ve Staphylococcus |
| Case 3 | OM ankle | ORIF medial malleolus fracture | Female | 56 | 5 | MSSA |
| Case 4 | OM 5th metatarsal | ORIF 5th metatarsal fracture | Male | 48 | 2 | MSSA |
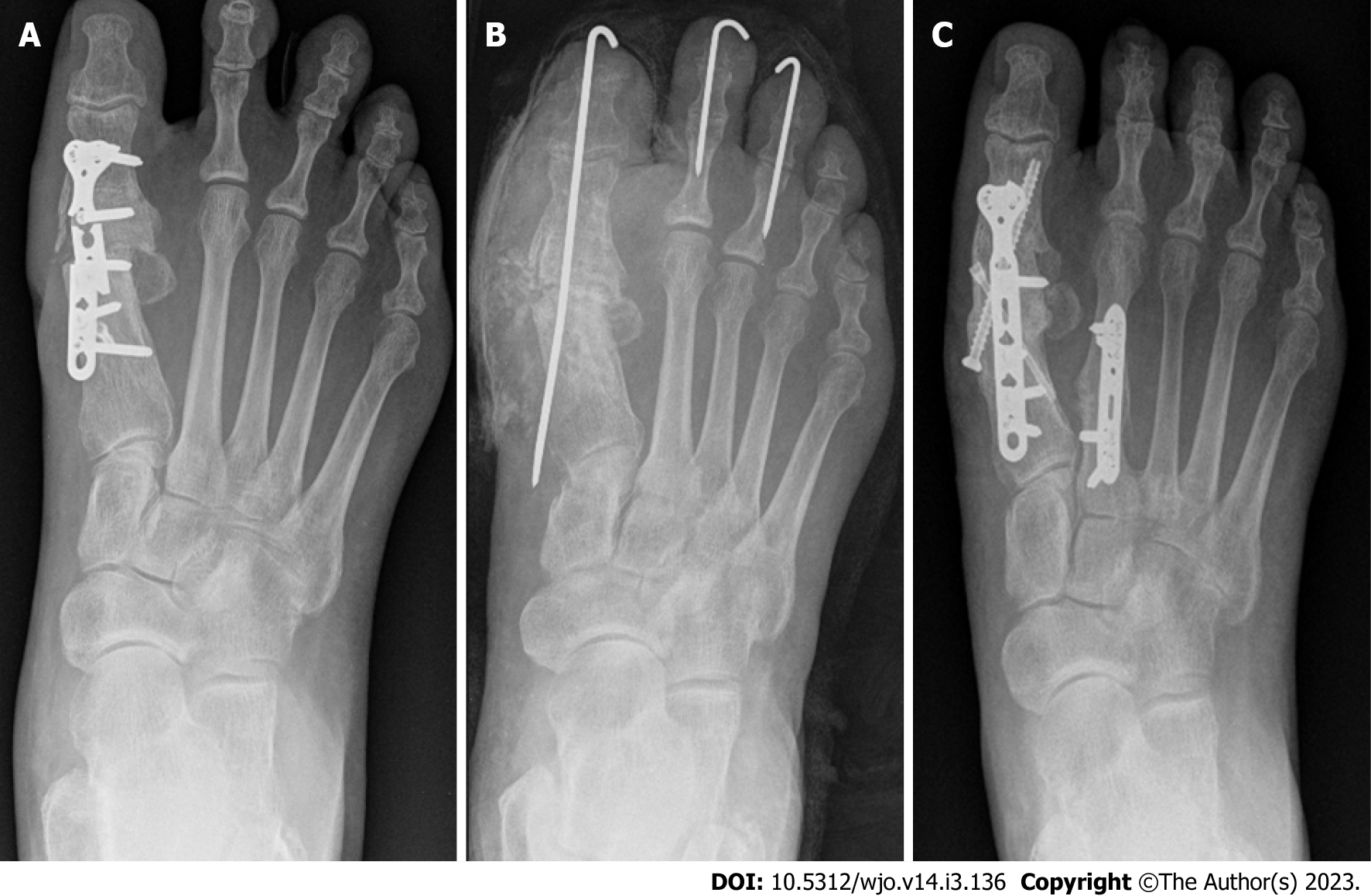
Case 1: A 46-year-old female patient was admitted for debridement of a-4-week-old ulceration on right big toe, complicated by septic 1st metatarsophalangeal joint on a background history of arthrodesis 2 years prior with uneventful postoperative period. She had normal inflammatory markers with CRP and WCC values of 7 and 6.9 respectively. MSSA and Corynebacterium were isolated from all surgical specimens. Post debridement and implant removal, postoperative inflammatory markers remained within normal range, with CRP of < 5 and WCC of 7.7. One year later, she was admitted with recurrent OM, and underwent multiple procedures with the aim of infection eradication and achieving union (Figure 1). Throughout all these procedures, CRP and WCC remained within the normal range.
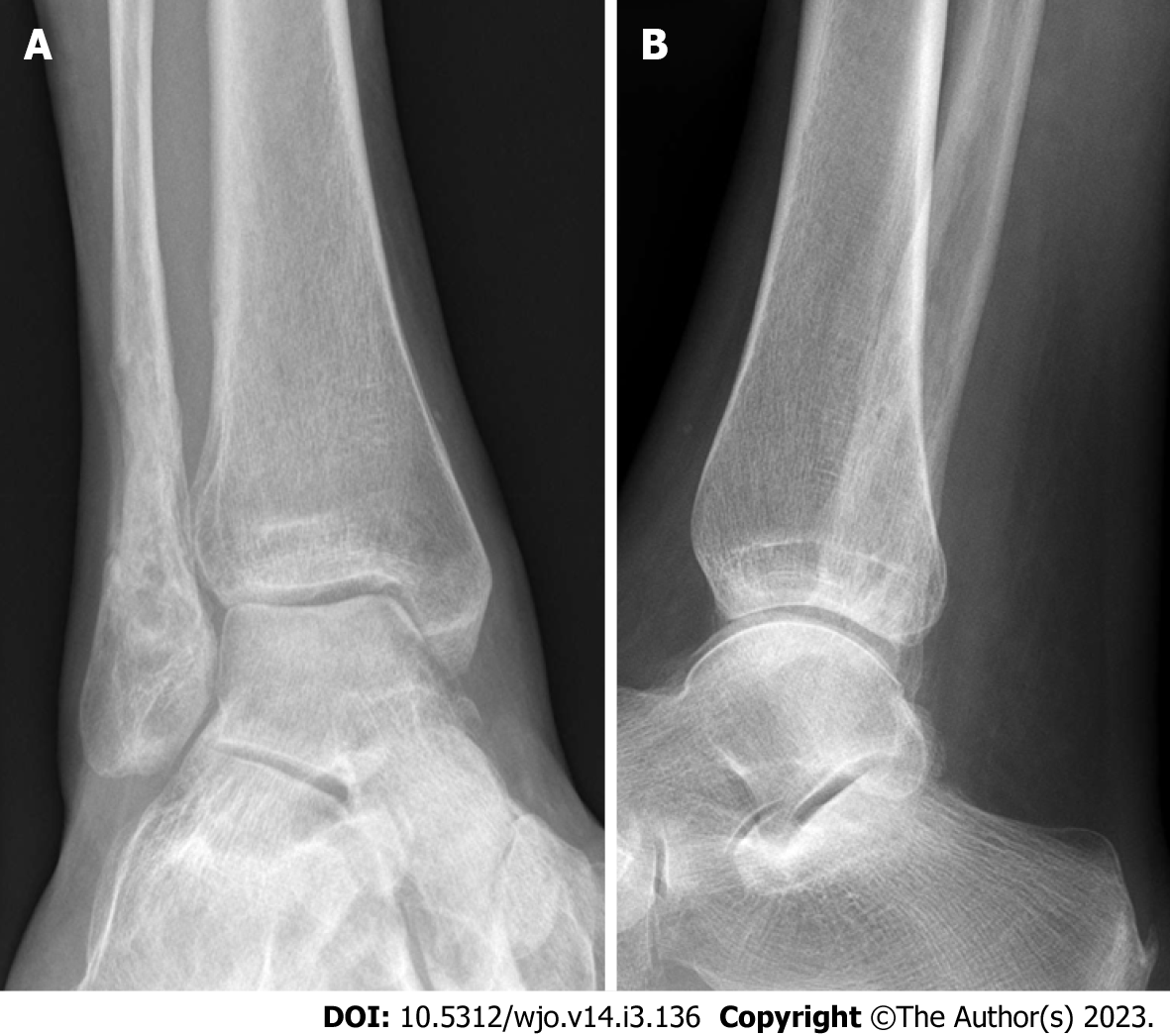
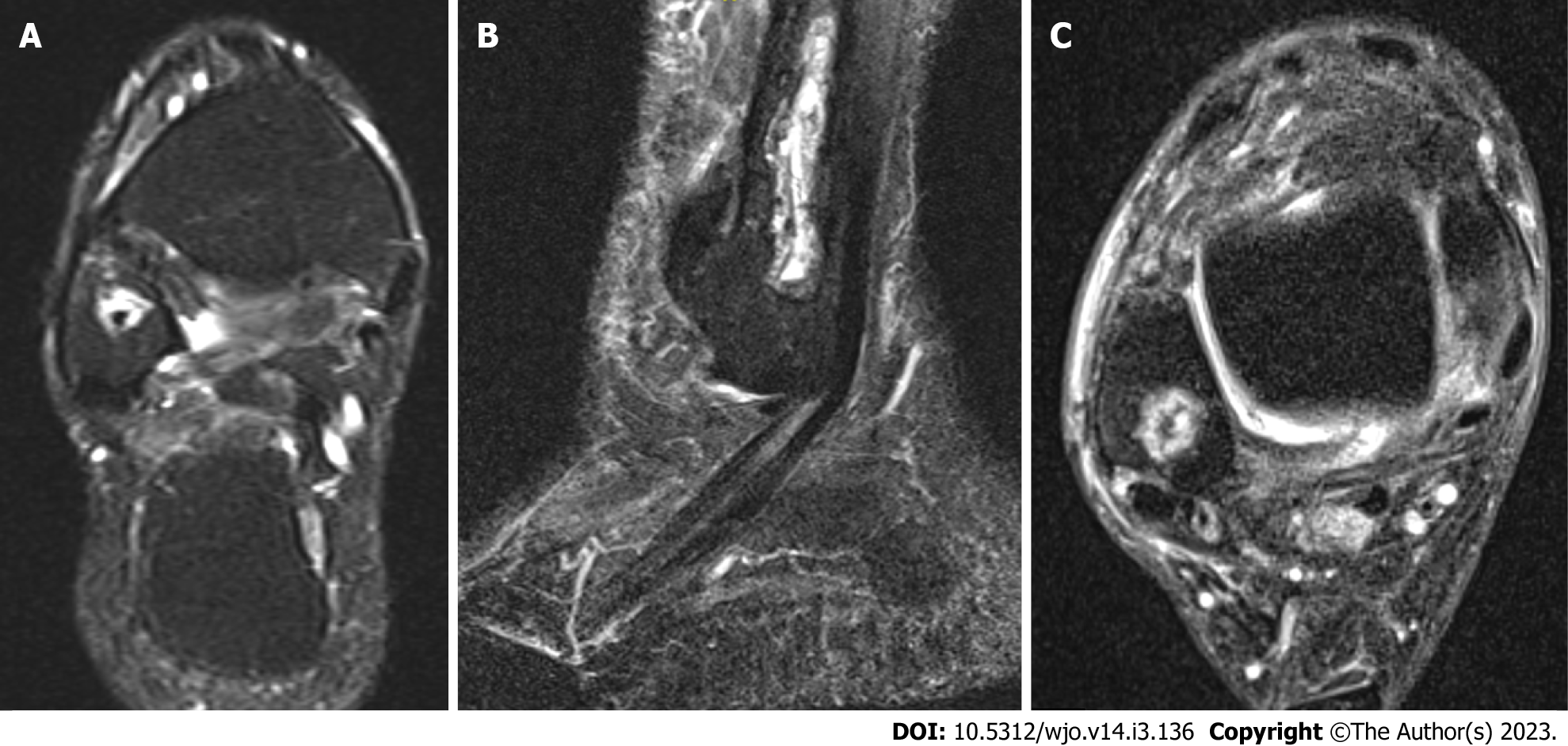
Case 2: A 54-year-old female patient diagnosed with OM of the right distal fibula 5 mo following ORIF for closed Weber-B lateral malleolus fracture. This infection did not mount an inflammatory response with normal values of CRP and WCC at time of presentation (CRP < 5 and WCC 6.3), and even after metalwork removal and debridement (CRP < 5 and WCC 5.4). The diagnosis was based on clinical findings, radiographs, and MRI scans (Figures 2 and 3). Tissue specimens grew MSSA and Coagulase –ve Staphylococcus.
Case 3: A 56-year-old female patient presented with infected left medial malleolus metal work and OM 5 mo after the index procedure. Pus was draining from the medial wound on presentation; however, with normal inflammatory markers (CRP 6, WCC 6.6). She was admitted for IV antibiotics with repeated inflammatory markers 3 d later still within the normal range (CRP 6 and WCC 4.3). Removal of all metalwork was done a week later with tissue and pus samples growing MSSA.
Case 4: A 48–year-old male patient presented with OM of right 5th metatarsal 2 mo following a closed fracture to the right 5th metatarsal bone managed with ORIF. On presentation, his WCC and CRP were normal with values of 8.4 and < 5 respectively. The clinical diagnosis was confirmed by MRI showing sinus tract extending from the head of the fifth metatarsal to the skin. He underwent washout and debridement with excision of the distal right 5th metatarsal followed by 5th ray amputation. Intra-operative bone and tissue samples grew MSSA. His CRP and WCC results were within the normal values from the date of presentation till the date of his last operation.
SSI following ankle surgery is one of the most common complications usually with substantial sequalae on both the patient such as permanent disability and eventually amputation if not addressed promptly, and the healthcare costs with estimated increase more than 300% for subsequent procedures[17-19].
Despite that surgical foot and ankle infections in diabetic patients is significantly higher than in their non-diabetic counterparts, OM in non-diabetics is not uncommon[2,3]. The population-based study by Kremers et al[20] reported the incidence of OM during a 41-year period; there was a 15% incidence of OM of the foot in patients without diabetes. Similarly, Haji Zaine et al[21] reported an 18.8% incidence of OM among non-diabetic patients. Although advanced imaging such as MRI and leucocyte-labelled bone scans can provide high sensitivity and specificity in diagnosing these infections, they are expensive and might not be readily available in some centres[22,23] That warranted better understanding of the reliability and sensitivity of different readily available surrogate markers for infection in that population for early diagnosis and mitigation of associated healthcare costs.
A readily available and cost-effective laboratory tests for OM diagnosis are WCCs and CRP[24]. In the presence of infection, bone marrow accelerates white blood cells production with resultant increases in WCC which is used to signify severity of infection[25]. CRP is an acute phase reactant produced by hepatocytes that increases significantly in concentration in response to infection particularly bacterial infections[26,27].
In our case series, we found that CRP > 10 mg/L had a sensitivity of 84% in the diagnosis of non-diabetic foot and ankle infections, whereas WCC had a poor sensitivity of 28% in the diagnosis of such cases. Other authors have shown similar high sensitivity of CRP diagnosis of OM. Fleischer et al[28] showed in their diagnostic study that CRP > 32 mg/L has a sensitivity of 0.85 and specificity of 0.65 for the diagnosis of OM. In another retrospective cohort study on 102 surgical foot and ankle infections, it was shown that CRP had a sensitivity of 71% in differentiating between superficial wound infection and OM[29].
In our cohort, a small subset of confirmed infections (16%, n = 4) did not seem to mount any inflammatory systemic reaction with resultant normal CRP and WCC levels. In their review article, Harris et al[30] concluded that for patients with risk factors for OM or a clinically high level of suspicion, values of ESR < 30 mm/h or CRP < 10 mg/L should not rule out the diagnosis of OM, especially in patients with puncture wounds or foot ulcers/infections. This finding has been corroborated by our results with 16% of radiologically/microbiologically confirmed infections having a CRP < 10 mg/L on presentation. In a study by Armstrong and colleagues[31], 54% of the patients with acute OM presented with normal WCC. Our series reported even higher percentage with 72% of confirmed infections presenting with normal WCC. Thus, we recommend corroborating the results with different radiological investigations as well as also considering other emerging biochemical markers for diagnosis of infection.
Those other biochemical markers are increasingly being embedded in the diagnostic panel of investigations. PCT was found in a meta-analysis to be more sensitive than CRP for differentiating bacterial from non-infective causes of inflammation (88% vs 75%)[32]. In another study on 93 diabetic foot ulcers, a CRP cut-off value of 17 mg/dL was found to be the single most sensitive marker for confirming infection with sensitivity 0.727, specificity 1.000, positive predictive value 1.000, and negative predictive value 0.793 while total leucocytic neutrophil count was found to be non-predictive[33]. Moreover, combining CRP with PCT yielded higher diagnostic accuracy than solely relying on only one parameter. Serum IL-6 has been described as a more sensitive marker of acute periprosthetic infection particularly in hips and knees with high accuracy, sensitivity, and specificity (97%, 100% and 95% respectively) but has not been specifically investigated in foot and ankle infections[34-36]. Measurement of bacterial load with a critical level of bacteria ≥ 104 to 106 colony-forming units per g of tissue has been also described to objectively confirm an infective aetiology[37,38].
The retrospective nature of our study has inherent limitations. It may not have included all patients with foot and ankle infections, since not all patients may have been discharged on OPAT and therefore would not have been captured by the database. The proportion of deep infections preceded by a superficial infection was not recorded however eventually all deep infections in our series were identified and reported. Our data relied on different biochemical markers but no attempt to identify a cut-off value or to quantify bacterial load was done. We agree that bacterial load is a reliable indicator of infection in acute infections but has been shown to be less reliable in early subacute or chronic infections as well as in healing wounds[39,40].
Our case series also lacked the assessment of the diagnostic sensitivity of other inflammatory markers for the diagnosis of foot and ankle infections e.g., ESR, PCT and IL-6 as they are not routinely performed in our hospital. Further evaluation of these biochemical markers is recommended.
In conclusion, CRP has good sensitivity in the diagnosis these non-diabetic infections, whereas WCC is a poor inflammatory marker in the detection of such cases and should not be used as an absolute indicator of OM. In a subset of patients, relying on these inflammatory markers solely can delay diagnosis as they can be normal, and no inflammatory response mounted. We recommend incorporating other inflammatory markers such as PCT, IL-6 and bacterial load as well as radiological diagnosis when there is a high index of suspicion despite negative CRP and WCC in this subset of patients. A noteworthy finding in our study is that CRP is a readily available, cost-effective, and reliable indicator for ankle and foot infections, directing appropriate antibiotic therapy for those likely to benefit from it.
Non-diabetic foot and ankle infections are not uncommon. Despite this, there is a paucity of the literature investigating the diagnostic accuracy of different inflammatory markers in the diagnosis of these infections as opposed to the diabetic population.
Defining the reliability of inflammatory markers in the diagnosis of non-diabetic foot and ankle infections can aid in early diagnosis and mitigate associated healthcare costs for delayed treatments.
Our aim was to define the reliability of the commonly utilized inflammatory markers such as white cell count (WCC) and C-reactive protein (CRP) in the diagnosis of non-diabetic foot and ankle infections as well as to highlight the shortcomings of those markers in a small subset of patients with normal inflammatory markers despite a microbiologically confirmed diagnosis of infection.
This was a retrospective cohort study looking into microbiologically confirmed foot and ankle infections in the non-diabetic population presenting to our hospital (University Hospitals Leicester-United Kingdom) over the period of 6 years (2014-2020).
A total of 25 non-diabetic patients with confirmed foot or ankle infections were identified. Previous bony surgery was identified in 13 (52%) patients. Inflammatory markers were raised in 21 (84%) patients while 4 (16%) patients did not mount an inflammatory response even with subsequent surgical procedures. CRP sensitivity was shown to be 84%, while WCC sensitivity was only 28%.
CRP had a relatively good sensitivity whereas WCC is a poor inflammatory marker in the detection of non-diabetic foot and ankle infections. In a subset of non-diabetic foot and ankle infections, inflammatory markers will not be raised, and a normal CRP should not rule out the diagnosis of osteomyelitis. In these cases where a high level of suspicion persists despite normal CRP, further advanced radiological and laboratory investigations should be performed.
Further evaluation of different inflammatory markers in the non-diabetic foot and ankle infections (erythrocyte sedimentation rate, pro-calcitonin and interleukin-6) could improve diagnostic accuracy and avoid more expensive investigative procedures.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: British Orthopedic Association, No. 38006.
Specialty type: Orthopedics
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Papotto G, Italy; Rezus E, Romania S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Edmonds M, Foster A. The use of antibiotics in the diabetic foot. Am J Surg. 2004;187:25S-28S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Yan X, Song JF, Zhang L, Li X. Analysis of risk factors for multidrug-resistant organisms in diabetic foot infection. BMC Endocr Disord. 2022;22:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Wukich DK, Lowery NJ, McMillen RL, Frykberg RG. Postoperative infection rates in foot and ankle surgery: a comparison of patients with and without diabetes mellitus. J Bone Joint Surg Am. 2010;92:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Wukich DK, McMillen RL, Lowery NJ, Frykberg RG. Surgical site infections after foot and ankle surgery: a comparison of patients with and without diabetes. Diabetes Care. 2011;34:2211-2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Termaat MF, Raijmakers PG, Scholten HJ, Bakker FC, Patka P, Haarman HJ. The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87:2464-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Palestro CJ, Mehta HH, Patel M, Freeman SJ, Harrington WN, Tomas MB, Marwin SE. Marrow versus infection in the Charcot joint: indium-111 leukocyte and technetium-99m sulfur colloid scintigraphy. J Nucl Med. 1998;39:346-350. [PubMed] |
| 7. | Akinci B, Yener S, Yesil S, Yapar N, Kucukyavas Y, Bayraktar F. Acute phase reactants predict the risk of amputation in diabetic foot infection. J Am Podiatr Med Assoc. 2011;101:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Michail M, Jude E, Liaskos C, Karamagiolis S, Makrilakis K, Dimitroulis D, Michail O, Tentolouris N. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Lavery LA, Ahn J, Ryan EC, Bhavan K, Oz OK, La Fontaine J, Wukich DK. What are the Optimal Cutoff Values for ESR and CRP to Diagnose Osteomyelitis in Patients with Diabetes-related Foot Infections? Clin Orthop Relat Res. 2019;477:1594-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Larsson S, Thelander U, Friberg S. C-reactive protein (CRP) levels after elective orthopedic surgery. Clin Orthop Relat Res. 1992;237-242. [PubMed] |
| 11. | Akgün D, Müller M, Perka C, Winkler T. The serum level of C-reactive protein alone cannot be used for the diagnosis of prosthetic joint infections, especially in those caused by organisms of low virulence. Bone Joint J. 2018;100-B:1482-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Akgün D, Wiethölter M, Siegert P, Danzinger V, Minkus M, Braun KF, Moroder P. The role of serum C-reactive protein in the diagnosis of periprosthetic shoulder infection. Arch Orthop Trauma Surg. 2022;142:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Deirmengian CA, Citrano PA, Gulati S, Kazarian ER, Stave JW, Kardos KW. The C-Reactive Protein May Not Detect Infections Caused by Less-Virulent Organisms. J Arthroplasty. 2016;31:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Unter Ecker N, Suero EM, Gehrke T, Haasper C, Zahar A, Lausmann C, Hawi N, Citak M. Serum C-reactive protein relationship in high- versus low-virulence pathogens in the diagnosis of periprosthetic joint infection. J Med Microbiol. 2019;68:910-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Chen K, Balloch R. Management of calcaneal osteomyelitis. Clin Podiatr Med Surg. 2010;27:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Rabjohn L, Roberts K, Troiano M, Schoenhaus H. Diagnostic and prognostic value of erythrocyte sedimentation rate in contiguous osteomyelitis of the foot and ankle. J Foot Ankle Surg. 2007;46:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, Steckelberg J, Osmon D. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2010;92:2102-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 296] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 18. | de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 829] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 19. | Woods TJ, Tesfay F, Speck P, Kaambwa B. Economic evaluations considering costs and outcomes of diabetic foot ulcer infections: A systematic review. PLoS One. 2020;15:e0232395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Kremers HM, Nwojo ME, Ransom JE, Wood-Wentz CM, Melton LJ 3rd, Huddleston PM 3rd. Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J Bone Joint Surg Am. 2015;97:837-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 289] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 21. | Haji Zaine N, Burns J, Vicaretti M, Fletcher JP, Begg L, Hitos K. Characteristics of diabetic foot ulcers in Western Sydney, Australia. J Foot Ankle Res. 2014;7:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, Kono S, Lavery LA, Malone M, van Asten SA, Urbančič-Rovan V, Peters EJG; International Working Group on the Diabetic Foot (IWGDF). Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 364] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 23. | Cheng Q, Lazzarini PA, Gibb M, Derhy PH, Kinnear EM, Burn E, Graves N, Norman RE. A cost-effectiveness analysis of optimal care for diabetic foot ulcers in Australia. Int Wound J. 2017;14:616-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Barakat A, Schilling WHK, Sharma S, Guryel E, Freeman R. Chronic osteomyelitis: a review on current concepts and trends in treatment. Orthop Trauma. 2019;33:181-187. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | King W, Toler K, Woodell-May J. Role of White Blood Cells in Blood- and Bone Marrow-Based Autologous Therapies. Biomed Res Int. 2018;2018:6510842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Samsudin I, Vasikaran SD. Clinical Utility and Measurement of Procalcitonin. Clin Biochem Rev. 2017;38:59-68. [PubMed] |
| 27. | Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018;9:754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 1707] [Article Influence: 243.9] [Reference Citation Analysis (0)] |
| 28. | Fleischer AE, Didyk AA, Woods JB, Burns SE, Wrobel JS, Armstrong DG. Combined clinical and laboratory testing improves diagnostic accuracy for osteomyelitis in the diabetic foot. J Foot Ankle Surg. 2009;48:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Ryan EC, Ahn J, Wukich DK, Kim PJ, La Fontaine J, Lavery LA. Diagnostic Utility of Erythrocyte Sedimentation Rate and C-Reactive Protein in Osteomyelitis of the Foot in Persons Without Diabetes. J Foot Ankle Surg. 2019;58:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Harris JC, Caesar DH, Davison C, Phibbs R, Than MP. How useful are laboratory investigations in the emergency department evaluation of possible osteomyelitis? Emerg Med Australas. 2011;23:317-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Armstrong DG, Lavery LA, Sariaya M, Ashry H. Leukocytosis is a poor indicator of acute osteomyelitis of the foot in diabetes mellitus. J Foot Ankle Surg. 1996;35:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1129] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 33. | Jeandrot A, Richard JL, Combescure C, Jourdan N, Finge S, Rodier M, Corbeau P, Sotto A, Lavigne JP. Serum procalcitonin and C-reactive protein concentrations to distinguish mildly infected from non-infected diabetic foot ulcers: a pilot study. Diabetologia. 2008;51:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Di Cesare PE, Chang E, Preston CF, Liu CJ. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg Am. 2005;87:1921-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Alrashidi Y, Galhoum AE, Wiewiorski M, Herrera-Pérez M, Hsu RY, Barg A, Valderrabano V. How To Diagnose and Treat Infection in Total Ankle Arthroplasty. Foot Ankle Clin. 2017;22:405-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Maniar RN, Navaneedhan G, Ranvir S, Maniar AR, Dhiman A, Agrawal A. What Is the Normal Trajectory of Interleukin-6 and C-reactive Protein in the Hours and Days Immediately After TKA? Clin Orthop Relat Res. 2019;477:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1312] [Cited by in RCA: 1184] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 38. | Ponraj DS, Falstie-Jensen T, Jørgensen NP, Ravn C, Brüggemann H, Lange J. Diagnosis of orthopaedic-implant-associated infections caused by slow-growing Gram-positive anaerobic bacteria - a clinical perspective. J Bone Jt Infect. 2021;6:367-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Serena T, Robson MC, Cooper DM, Ignatius J; on behalf of the Human Genome Sciences Clinical Trial Group. Lack of reliability of clinical/visual assessment of chronic wound infection: the incidence of biopsy-proven infection in venous leg ulcers. Wounds. 2006;18:197-202. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Senneville É, Lipsky BA, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, Kono S, Lavery LA, Malone M, van Asten SA, Urbančič-Rovan V, Peters EJG. Diagnosis of infection in the foot in diabetes: a systematic review. Diabetes Metab Res Rev. 2020;36 Suppl 1:e3281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |