Published online Mar 18, 2023. doi: 10.5312/wjo.v14.i3.123
Peer-review started: August 25, 2022
First decision: December 26, 2022
Revised: January 1, 2023
Accepted: February 15, 2023
Article in press: February 15, 2023
Published online: March 18, 2023
Processing time: 203 Days and 16.2 Hours
Perioperative surgical home (PSH) is a novel patient-centric surgical system developed by American Society of Anesthesiologist to improve outcomes and patient satisfaction. PSH has proven success in large urban health centers by reducing surgery cancellation, operating room time, length of stay (LOS), and readmission rates. Yet, only limited studies have assessed the impact of PSH on surgical outcomes in rural areas.
To evaluate the newly implemented PSH system at a community hospital by comparing the surgical outcomes using a longitudinal case-control study.
The research study was conducted at an 83-bed, licensed level-III trauma rural community hospital. A total of 3096 TJR procedures were collected retrospectively between January 2016 and December 2021 and were categorized as PSH and non-PSH cohorts (n = 2305). To evaluate the importance of PSH in the rural surgical system, a case-control study was performed to compare TJR surgical outcomes (LOS, discharge disposition, and 90-d readmission) of the PSH cohort against two control cohorts [Control-1 PSH (C1-PSH) (n = 1413) and Control-2 PSH (C2-PSH) (n = 892)]. Statistical tests including Chi-square test or Fischer’s exact test were performed for categorical variables and Mann-Whitney test or Student’s t-test were performed for continuous variables. The general linear models (Poisson regression and binomial logistic regression) were performed to fit adjusted models.
The LOS was significantly shorter in PSH cohort compared to two control cohorts (median PSH = 34 h, C1-PSH = 53 h, C2-PSH = 35 h) (P value < 0.05). Similarly, the PSH cohort had lower percentages of discharges to other facilities (PSH = 3.5%, C1-PSH = 15.5%, C2-PSH = 6.7%) (P value < 0.05). There was no statistical difference observed in 90-d readmission between control and PSH cohorts. However, the PSH implementation reduced the 90-d readmission percentage (PSH = 4.7%, C1-PSH = 6.1%, C2-PSH = 3.6%) lower than the national average 30-d readmission percentage which is 5.5%. The PSH system was effectively established at the rural community hospital with the help of team-based coordinated multi-disciplinary clinicians or physician co-management. The elements of PSH including preoperative assessment, patient education and optimization, and longitudinal digital engagement were vital for improving the TJR surgical outcomes at the community hospital.
Implementation of the PSH system in a rural community hospital reduced LOS, increased direct-to-home discharge, and reduced 90-d readmission percentages.
Core Tip: The study evaluated the newly implemented perioperative surgical home (PSH) at a rural community hospital using a case-control design. With limited supporting microsystems, team-based physician co-management was vital to establish the PSH system and following protocols including preoperative assessment, patient education, and longitudinal digital engagement. The surgical outcomes - length of stay, discharge disposition, and 90-d readmission - were compared between the PSH cohort and the control cohorts. The results from this study highlighted the effectiveness of PSH in improving total joint replacement surgical outcomes, especially for high-risk patients who are older and have one or more medical complications.
- Citation: Sridhar S, Mouat-Hunter A, McCrory B. Rural implementation of the perioperative surgical home: A case-control study. World J Orthop 2023; 14(3): 123-135
- URL: https://www.wjgnet.com/2218-5836/full/v14/i3/123.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i3.123
The demand for orthopedic surgeries including total joint replacement (TJR), which are primarily performed on hips, knees, and shoulders, are drastically increasing each year[1]. Yet, delivering quality surgical care to large volumes of TJR patients is a challenge to many hospitals, specifically those hospitals located in rural areas[2,3]. Rural and frontier health systems have siloed perioperative care that is spread across many disciplines and institutions, which contributes to inadequate communication, high cost, poor care continuity, and preventable complications[4]. On average, TJR patients are 65 years or older, and have one or more health conditions (e.g., comorbidities). Due to generally higher risk of surgery in these populations, there is a 1% to 50% chance of adverse events in TJR surgeries including major cardiac incidents, healthcare-acquired conditions, extended length of stay (LOS), readmission to inpatient facilities, improper pain management, and side effects[4,5].
To improve surgical outcomes and patient experience, the perioperative surgical home (PSH) model of care was created by the leaders within American Society of Anesthesiologists (ASA)[6,7]. Compared to a traditional surgical system, the PSH is a coordinated interdisciplinary team providing all surgical care to patients from the preoperative phase (30 d before surgery) to recovery phase (90 d after surgery) (Figure 1)[7-11].
The components of PSH also included patient-centered coordination programs and enhanced recovery after surgery[12,13]. The implementation of PSH in larger healthcare systems and academic medical institutions has shown promising results in surgical outcomes, especially in orthopedic procedures[9]. For example, Qiu et al[14] and Alvis et al[15] observed that the PSH cohort had a day shorter LOS than the control cohort when examining hip and knee procedures. Kim et al[16] analyzed 1194 TJR procedures and found that the PSH cohort had higher discharges to home by 8.1% compared to the non-PSH cohort. The authors also noticed the surgical cost in the non-PSH cohort was 14.9% greater cost than the PSH cohort. Yajnik et al[17] retrospectively analyzed 40 knee procedures and demonstrated that the PSH cohort experienced optimized post-surgical pain management with less consumption of opioids than the non-PSH cohort. Likewise, past researchers found that PSH contributed to improved surgical outcomes including, lower readmission rates, faster postoperative recovery, improved operational efficiency, and higher patient satisfaction[15,18-21].
Despite these successes, some researchers found no change in surgical outcomes with PSH in similar size urban health centers. For example, Vetter et al[22] and Powell et al[23] found no significant difference in LOS after implementing PSH for orthopedic surgeries. Qui et al[14] and Vetter et al[22] found no difference in readmission rates using the PSH system. In terms of surgery cost, Leahy et al[24] found there was no significant reduction for pediatric patients. These examples exhibit that there is no standard PSH program to achieve a standardized surgical outcome[9]. These PSH studies were performed in urban healthcare systems and academic-affiliated medical centers. To authors’ knowledge only our pilot study has explored PSH systems in rural or frontier healthcare service area[4]. This current study addresses this gap by assessing TJR outcomes at a rural hospital with a newly implemented PSH system using a case-control study design.
Surgical care inequality is greater within rural community hospitals due to limited resources, socioeconomic differences, and poor access to healthcare[25-27]. Compared to urban hospitals, rural surgical outcomes have higher odds of in-hospital mortality and higher hospitalization cost[28]. One of the reasons for this is many rural patients are uninsured, older, and have one or more medical complications[29,30]. Rural hospitals in the United States can often be overwhelmed by the growing demand for TJR surgeries and factors such as poor coordination among clinicians, lack of patient education, poor patient care transitions, limited patient engagement (pre-operative and post-operative), and inconsistent/non-standardized care delivery affect rural orthopedic surgical care negatively[1,5].
A newly implemented PSH at a community hospital in rural Montana was created to address the factors mentioned above, which have plagued the rural orthopedic surgery system. With limited resources and supporting microsystems, the PSH was successfully initiated with the help of strong team-based coordination amongst clinicians. The PSH multi-disciplinary team consisted of the patient’s selected surgeon, anesthesiologist, hospitalist, physician assistant, registered nurse, and the patient’s primary care manager. This collaboration focused on improving surgical care and enhancing patient engagement perioperatively. Effective communication between clinicians was established for communal decision-making for patient-centric - “physician co-management”[31].
This research study’s primary objective was to evaluate the newly implemented PSH system at a local rural, community hospital by comparing TJR surgical outcomes using a longitudinal case-control study design. Based on our preliminary study[32], it was hypothesized that the implementation of the PSH in the rural community hospital would positively impact patients’ TJR outcomes (i.e., shorter LOS, reduced readmissions, and increased rate of home discharge) across three distinct cohorts for the case-control design.
The PSH clinic affiliated with the local community hospital began seeing TJR patients in November 2018. The hospital was an 83-bed, licensed level-III trauma center primarily serving three counties. However, based on initial analyses, the hospital was serving patients from more than 10 surrounding counties covering 9000 square miles and approximately 136000 residents. The research team (health systems engineers and clinicians) retrospectively collected and analyzed all TJR data from January 2016 to December 2021. The observational timeframes were reviewed, and three distinct cohorts were determined for the case-control study design.
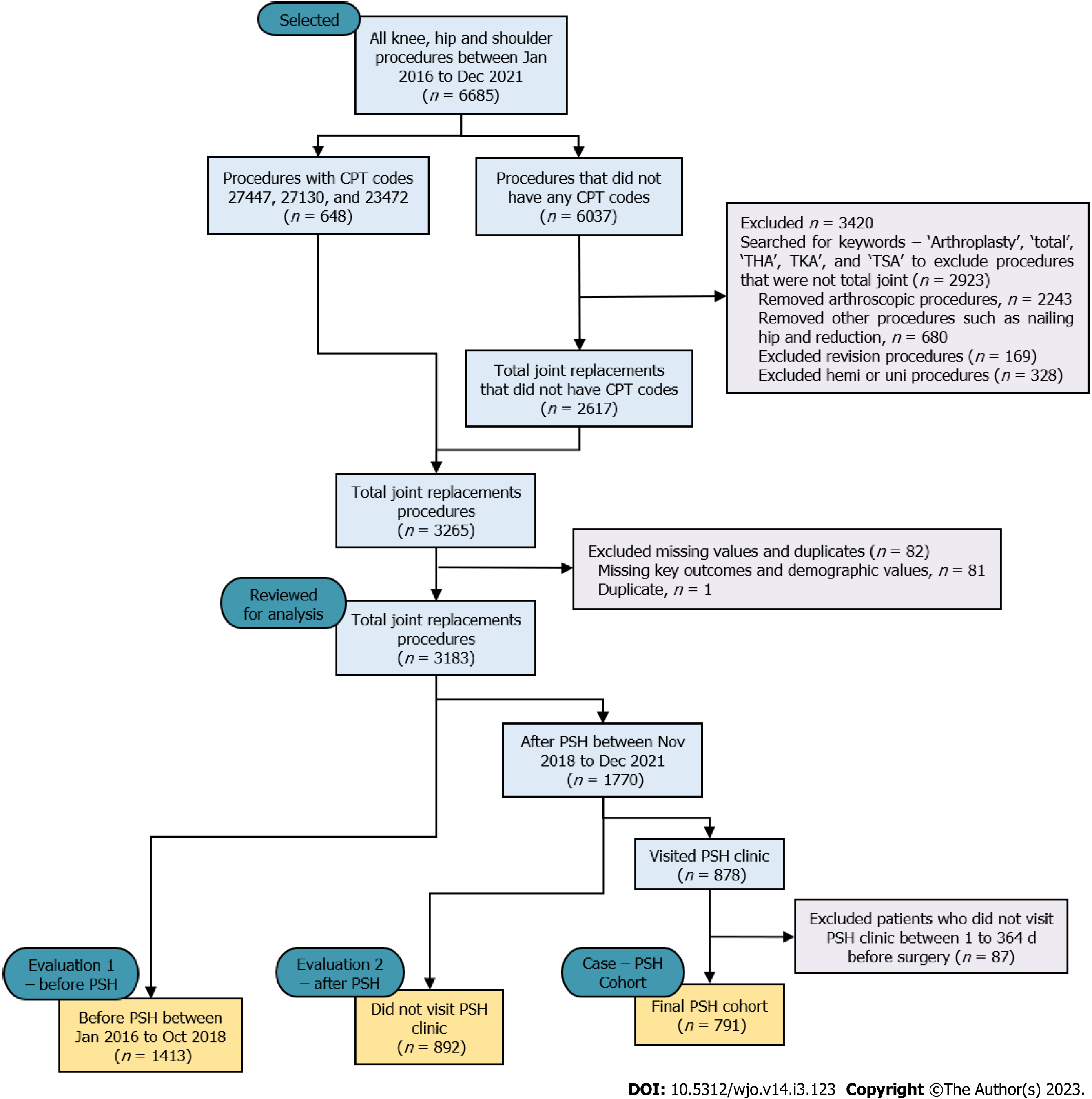
Data were extracted from the electronic medical record for a total of 6685 orthopedic procedures that were performed on knees, hips, and shoulders between January 2016 and December 2021 (Figure 2). Six hundred and forty-eight (n = 648) procedures were included that had CPT codes - 27447 (total knee), 27130 (total hip), and 23472 (total shoulder). The remaining 6037 did not have CPT codes and were filtered for TJR procedures by searching for keywords ‘arthroplasty’, ‘total’, ‘THA’ (i.e., Total Hip Arthroplasty), ‘TKA’ (i.e., Total Knee Arthroplasty), and ‘TSA’ (i.e., Total Shoulder Arthroplasty). During this filtering process, a total of 3420 procedures were excluded because they were identified as non-TJR procedures, (e.g., arthroscopic procedures, reductions, nailing hip). A total of 82 TJR procedures were also excluded from the analysis because they were either duplicate records (n = 1) or missing key outcomes and demographic values (n = 81) of the patients.
A total of 3183 TJR procedures were considered for the analysis and were categorized into: the PSH cohort (case) and non-PSH cohort (control). The PSH pathway begins with visiting PSH clinic for preoperative assessment. Most patients visited the PSH clinic between 30 to 60 d before surgery for their preoperative assessment. Very few medically complicated patients needed more time for optimization and postponed their surgery 6-9 mo (not more than a year) after their preoperative assessment. Therefore, the inclusion criteria for the PSH cohort (n = 791) included if the patient visited the PSH clinic for optimization between 1 and 364 d before surgery. Those patients who visited the PSH clinic but failed to meet the inclusion criteria (i.e., visited the PSH clinic a year before their surgery or after their surgery), were excluded from the analysis (n = 87). The inclusion criteria for the non-PSH cohort included the patients who did not visit the PSH clinic during their surgical process at all. The non-PSH cohort was further subcategorized based on the timeframes: Control-1 PSH (C1-PSH) cohort (before PSH implementation between January 2016 and October 2018, n = 1413) and Control-2 PSH (C2-PSH) cohort (after PSH implementation between November 2018 and December 2021, n = 892).
The study utilized two control cohorts to evaluate the importance of the PSH system in two timeframes - before and after PSH implementation. In the first evaluation, the PSH cohort was compared with C1-PSH cohort. In the second evaluation, the PSH cohort was compared with C2-PSH cohort. The baseline characteristics were compared with variables including patient age, gender, body mass index (BMI), ASA score (Class 1, 2, 3, or 4), procedure type (THA, TKA, and TSA) and insurance type (private or public payer). These variables were included in the baseline characteristics and in the analysis, as they were found to be potential confounders at PSH implemented hospitals with the surgical outcomes LOS, discharge disposition, and 90-d readmission[14,16,24,33,34].
Either the Fischer’s exact test or Chi-square test for association were used to compare the categorical variables between non-PSH and PSH cohorts. The continuous variables between two cohorts were analyzed using the Mann-Whitney test or Student’s t test, as appropriate. The LOS was found to be right skewed and was not normally distributed using the Shapiro-Wilk test (P value < 0.01). Therefore, a Poisson regression was performed to fit an adjusted model[14,35]. For dichotomous variables, i.e., discharge disposition and 90-d readmission, the binomial logistic regression was used to fit an adjusted model. All data handling, visualization, and statistical analyses were performed using R (V4.0.3, Vienna, Austria). The statistical analyses were performed with an alpha (α) value of 0.05. All data were encrypted and were accessed only by the authors and clinicians working at the hospital.
There were no significant differences observed in the baseline characteristics for the variables gender, BMI, and procedure type (P value > 0.05) (Table 1). However, a difference was observed between cohorts for the variables age, ASA class, and insurance type (P value < 0.05). On average, patients in the PSH cohort were two years older than in the C1-PSH cohort. The PSH cohort also included more medically complex patients with a higher proportion of ASA class 3 (42%) compared to the C1-PSH cohort (36%). For insurance, there were more public insurance payers in the PSH cohort (82%) compared to the C1-PSH cohort (71%).
| Characteristics | C1-PSH (n = 1413) | PSH (n = 791) | P value |
| mean (SD) [min, max] or n (%) | mean (SD) [min, max] or n (%) | ||
| Age | 67.3 (10) [18, 95] | 69.2 (8.6) [31, 90] | < 0.011 |
| Gender | 0.192 | ||
| Male | 629 (45) | 379 (47.5) | |
| BMI | 29.7 (6.2) [17.3, 68.5] | 29 (6.09) [14.67, 55.3] | 0.861 |
| ASA | 0.0093 | ||
| Class 1 | 67 (4.7) | 20 (2.5) | |
| Class 2 | 817 (57.8) | 434 (54.9) | |
| Class 3 | 517 (36.6) | 332 (42) | |
| Class 4 | 12 (0.8) | 5 (0.6) | |
| Procedure | 0.082 | ||
| THA | 489 (35) | 311 (39.3) | |
| TKA | 686 (49) | 356 (45) | |
| TSA | 238 (17) | 124 (15.7) | |
| Insurance | < 0.012 | ||
| Private | 415 (29) | 145 (18) | |
| Public | 998 (71) | 646 (82) |
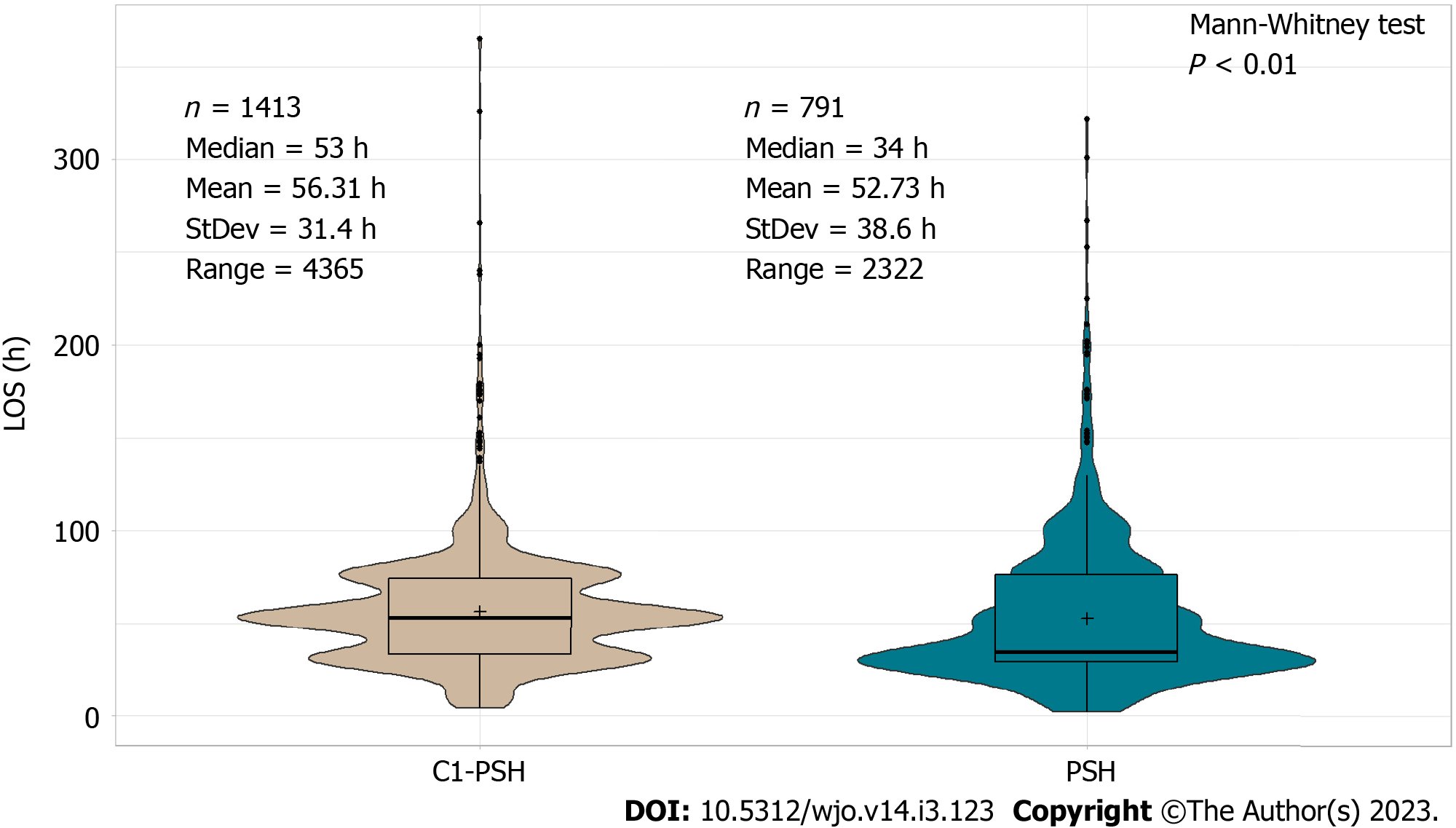
The LOS was lower in the PSH cohort compared to the C1-PSH cohort (median 34 vs 53 h, P value < 0.01) (Figure 3). Based on the Poisson regression results, the PSH clinic had a positive effect on LOS (P value < 0.01). On average, the LOS was 10% shorter in the PSH cohort compared to the C1-PSH cohort (Table 1). Other variables that also had a significant effect on patients’ LOS were age, gender, BMI, procedure type, and insurance type (P value < 0.05) (Supplementary Table 1).
Discharge disposition was classified into two types: patient discharged to home or discharged to other facilities such as skilled nurse facilities, inpatient rehabilitation facilities, or other hospitals’ swing beds. Discharge disposition was significantly different between the PSH and C1-PSH cohort (χ2 = 72, P value < 0.01) (Figure 4). The unadjusted odds for the PSH cohort discharged to other facilities was 80% lower than the C1-PSH cohort (P value < 0.01) (Table 2). Using logistic regression, the adjusted odds for the PSH cohort discharged to other facilities were 91% lower than the C1-PSH cohort (P value < 0.01) (Table 2). Age, gender, procedure type, insurance type, and LOS were also associated with the patient’s discharge type (P < 0.05) (Supplementary Table 1).
Readmission was categorized by if a patient was readmitted to any inpatient within 90 d post-surgery or not. The Chi-square test had no strong evidence for a difference in the readmission rates between the PSH and C1-PSH cohort (χ2 = 1.65, P = 0.2) (Figure 4). The unadjusted odds for the PSH cohort readmitted after surgery was 24% lower than the C1-PSH cohort (P = 0.17) (Table 2). The adjusted odds for the PSH cohort readmitted after surgery was 28% lower than the C1-PSH cohort (P = 0.17) (Table 2). In this adjusted analysis, no variable had a significant effect on patient readmission (Supplementary Table 1).
Except for variables ASA and procedure type, there was no significant difference observed between cohorts in the baseline characteristics (P = 0.046) (Table 3). Similar to evaluation 1, the PSH cohort had more medically complex patients with a higher proportion of ASA class 3 (42%) compared to the C2-PSH cohort (36%). For procedure types, there were more knee procedures in the PSH cohort and there were more hip and shoulder procedures in the C2-PSH cohort.
| Characteristics | C2-PSH (n = 892) | PSH (n = 791) | P value |
| mean (SD) [min, max] or n (%) | mean (SD) [min, max] or n (%) | ||
| Age | 69 (8.5) [37, 98] | 69.2 (8.6) [31, 90] | 0.21 |
| Gender | 0.522 | ||
| Male | 439 (49.2) | 376 (47.5) | |
| BMI | 30 (6.2) [17, 57.8] | 30 (6.1) [14.67, 55.3] | 0.761 |
| ASA | 0.0463 | ||
| Class 1 | 24 (2.7) | 16 (3.4) | |
| Class 2 | 546 (61.2) | 434 (54.9) | |
| Class 3 | 319 (35.8) | 332 (42) | |
| Class 4 | 3 (0.3) | 5 (0.6) | |
| Procedure | < 0.012 | ||
| THA | 294 (33) | 311 (39.3) | |
| TKA | 355 (39.8) | 356 (45) | |
| TSA | 243 (27.2) | 124 (15.7) | |
| Insurance | 0.42 | ||
| Private | 179 (20) | 145 (18) | |
| Public | 713 (80) | 646 (82) |
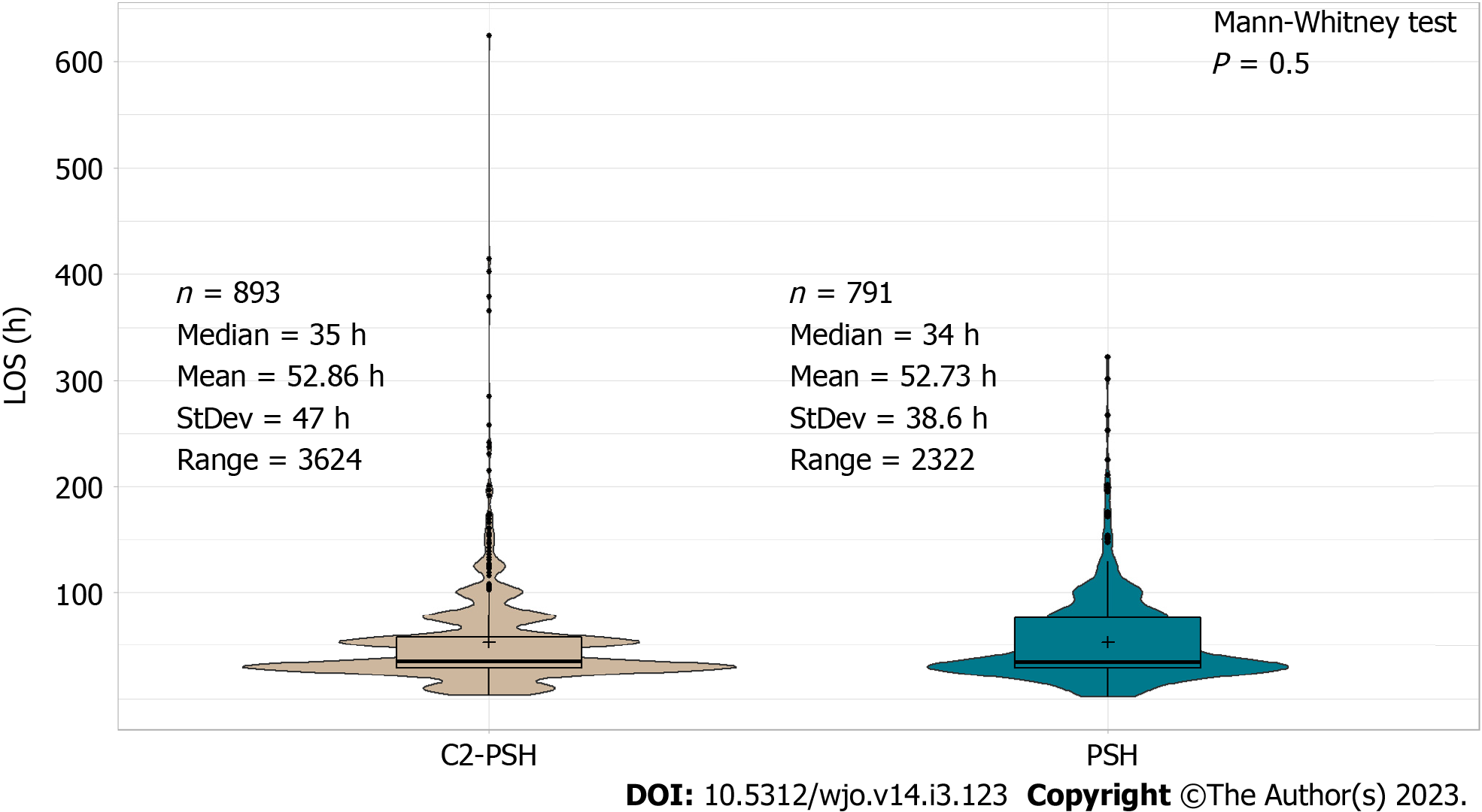
There was no significant difference between LOS in the PSH cohort and C2-PSH cohort in the unadjusted analysis (median 34 vs 35 h, P = 0.5) (Figure 5). However, in the adjusted analysis using Poisson regression, the LOS was found to be lower in the PSH cohort compared to the C2-PSH cohort (P value < 0.01). On average, the LOS was 10% shorter in the PSH cohort compared to the C2-PSH cohort (Table 4). Other variables that also had significant effect on patients’ LOS were age, gender, BMI, procedure type, and insurance type (P value < 0.05) (Supplementary Table 2).
Similar to evaluation 1, the discharge disposition was found to be significantly different between the PSH and C2-PSH cohorts (χ2 = 8, P value < 0.01) (Figure 6). The unadjusted odds for the PSH cohort discharged to other facilities was 49% lower than the C2-PSH cohort (P value < 0.01) (Table 4). Using logistic regression, the adjusted odds for the PSH cohort discharged to other facilities was 62% lower than the C2-PSH cohort (P value < 0.01) (Table 4). Age, gender, procedure type, insurance type, and LOS were also associated with patient discharge type (P value < 0.05) (Supplementary Table 2).
Similar to evaluation 1, the Chi-square test had no strong evidence for a difference in the readmission rates between the PSH and C2-PSH cohort (χ2 = 1, P = 0.31) (Figure 6). However, atypical results were observed in the unadjusted analysis, where the odds of the PSH cohort readmitted after surgery was 31% higher than the C2-PSH cohort (P = 0.26) (Table 4). Atypical results were also observed in the adjusted analysis, where the odds for the PSH cohort readmitted after surgery was 29% higher than the C2-PSH cohort (P = 0.26) (Table 4). In the adjusted analysis, no variable had a significant effect on patient readmission (P value > 0.05) (Supplementary Table 2).
This study evaluated the importance of PSH at a rural community hospital by comparing the PSH cohort with two control cohorts. In the first evaluation, the PSH cohort was compared with the C1-PSH cohort and for the second evaluation, the PSH cohort was compared with the C2-PSH cohort. The C1-PSH cohort included patients who had TJR surgeries before the PSH was implemented. The C2-PSH cohort consists of patients, who had TJR surgeries after PSH was implemented but did not visit the PSH clinic or followed the PSH-pathway.
In both evaluations, the LOS was shorter in the PSH cohort compared to the control cohorts (median PSH = 34 h, C1-PSH = 53 h, C2-PSH = 35 h)[14,33,34]. Although there was no statistical difference in LOS between the PSH and the C2-PSH cohort in the unadjusted analysis, the LOS was significantly shorter in the PSH cohort (10% shorter) in the adjusted analysis. This is because the PSH cohort had older and more medically complicated patients than the control cohorts. Therefore, when adjusted for the variables age, BMI, ASA, etc., the PSH had a significant effect in reducing LOS. Correspondingly, the PSH cohort had lower percentage of discharges to other facilities compared to the control cohorts (PSH = 3.5%, C1-PSH = 15.5%, C2-PSH = 6.7%).
There was no statistical significance in adjusted and unadjusted analysis for 90-d readmission. The readmission percentage was lower in the PSH cohort (4.7%) than the C1-PSH cohort (6.1%). Conversely, the PSH cohort (4.7%) had slightly higher percentage of 90-d readmission than the C2-PSH cohort (3.6%). Despite a marginal increase in the PSH cohort, the 90-d readmission percentage was still lower than the national average 30-d readmission which is 5.5%[36-38]. Past studies also demonstrated similar results where despite no statistical significance, the implementation of PSH helped to lower the readmission rates after surgery[33,38,39].
Akin to other studies of urban health systems[6,9], implementing PSH at a community hospital helped to improve the TJR surgical outcomes. With only limited resources and siloed supporting microsystems, physician co-management was vital to effectively establish the PSH system at the rural community hospital. The PSH preoperative process utilized patient assessment and patient education approximately 30 d before surgery. The assessment helped clinicians identify patients with high-risk factors such as diabetes, high or low blood pressure, sleep apnea, obesity, and heart or respiratory complications[7,40]. Based on these risks, the patients were ‘optimized’ and received treatment to improve the overall care by minimizing existing conditions or controlling undiagnosed conditions. In addition, the total joint education class hosted by the PSH clinicians educated patients on how to prepare for surgery, manage pain, plan for postoperative discharge, and reach clinicians for postoperative assistance[6,41]. Finally, a digital platform was initiated in the recovery phase to improve patient-clinician coordination and communication after surgery. The digital platform was used to engage and assess longitudinal patient-reported outcomes (post and pre-surgical pain, satisfaction, sleep, etc.) from 30 d preoperative to 90 d postoperative. These factors were conducive to improving patient satisfaction, shortening the LOS, increasing discharge to home, and reducing readmission after the surgery[6,24,42].
The PSH clinic majorly saw patients who were high risk (older, high ASA score, high BMI, one or more medical complications such as diabetes, hypertension), which left the C2-PSH cohort with low to medium-risk patients. This explained why the C2-PSH had improved surgical outcomes for LOS, discharge disposition, and 90-readmission compared to the C1-PSH cohort. The PSH system was effective in optimizing medically complicated patients, delivering similar or improved surgical outcomes compared to the C2-PSH cohort. The results from this study support that more patients (especially high and medium risk) should follow the PSH pathway for an effective and improved surgical experience.
Unlike the majority of the PSH studies that were performed at hospitals or health institutions located in metropolitical areas, this research examined the dissemination of PSH system and its effectiveness at a community hospital located in a micro-statistical area (population between 10000 to 50000). According to the United States Census Bureau, 27.2 million people (8.4% of the United States population) live in micro-statistical areas encompassing 660 counties[43]. Compared to metropolitical areas, patients living in micro-statistical areas are often prone to experiencing health equity issues and access to health services, including surgical care[44]. This study contributes to improving surgical outcomes using PSH system for community hospitals that are specifically located in micro-statistical areas. The authors envision that these study results will immensely help researchers and clinicians who are working to enhance surgical care in states similar to Montana demographics and social factors, including Alaska, Idaho, Wyoming, North Dakota, and South Dakota.
The limitations of this study include being a retrospective which may contain data collection biases that could alter the results and key findings[45]. Instead, a prospective clinical trial study can minimize these biases and provide better evidence-based results[46]. Second, this study was performed at a community hospital located in a rural micro-statistical area (with a population greater than 10000). The results from this study may not be generalizable to more rural places (e.g., with a population of less than 5000).
To the author’s knowledge, this study is first of its kind to evaluate the effectiveness of a PSH in a rural surgical system using a case-control study design. Implementing PSH at a community hospital was primarily successful because of patient-centric physician co-management to ensure continuity of care across all perioperative surgical phases. The PSH elements including preoperative assessment, patient education, and longitudinal digital engagement were imperative for improving the TJR surgical outcomes at the community hospital. Future research should include analysis of outcomes including same-day surgery cancellation, surgical cost, postoperative recovery measures, and postoperative opioid consumption. Other future research should also include advanced analytics and predictive modeling such as machine learning and deep learning to predict patient risk and improve the performance of surgical systems at rural and frontier hospitals[47].
With increasing demand for total joint replacement (TJR) procedures, delivering quality surgical care is a challenge to many hospitals, specifically those hospitals located in rural areas. The perioperative surgical home (PSH) developed by American Society of Anesthesiologists has proven successful in large urban health centers by reducing surgery cancellation, operating room time, length of stay (LOS), and readmission rates. Yet, only limited studies have assessed the impact of PSH on surgical outcomes in rural areas.
Compared to urban hospitals, rural hospitals in the United States can often be overwhelmed by the growing demand for TJR surgeries and factors such as poor coordination among clinicians, lack of patient education, poor patient care transition, and inconsistent care delivery that affect rural orthopedic surgical care negatively. A new PSH system was implemented at a community hospital located in rural Montana to address these issues, which have plagued the rural orthopedic surgery system.
The objective of this research was to evaluate the newly implemented PSH system at a local rural, community hospital by comparing TJR surgical outcomes using a longitudinal case-control study.
A case-control study was performed to compare the PSH and non-PSH cohorts of TJR surgical outcomes performed at a rural community hospital. Statistical tests including the Chi-square test or Fischer's exact test were performed to compare the categorical variables between non-PSH and PSH cohorts. Similarly, for continuous variables, student's t test or Mann-Whitney test was performed, as appropriate. The adjusted analysis was performed using general linear models; Poisson regression for the LOS, and binomial logistic regression for discharge disposition and 90-d readmission.
The LOS was shorter in PSH cohort compared to the control cohorts [median PSH = 34 h, Control-1 PSH (C1-PSH) = 53 h, Control-2 PSH (C2-PSH) = 35 h]. Correspondingly, the PSH cohort had a lower percentage of discharges to other facilities than the control cohorts (PSH = 3.5%, C1-PSH = 15.5%, C2-PSH = 6.7%). No statistically significant difference was observed in 90-d readmission between PSH and control cohorts. However, the implementation of PSH helped to lower the readmission rates after surgery.
Implementing PSH at a community hospital helped to improve the TJR surgical outcomes. The patient-centric physician co-management to ensure continuity of care across all perioperative surgical phases was vital for establishing PSH system at a rural community hospital. The PSH elements including preoperative assessment, patient education, and longitudinal digital engagement were imperative for improving patient satisfaction, shortening the LOS, increasing discharge to home, and reducing readmission after the surgery.
This study contributes to improving surgical outcomes using PSH system for community hospitals that are specifically located in micro-statistical areas. The authors envision that these study results will immensely help researchers and clinicians who are working to enhance surgical care in states similar to Montana demographics and social factors, including Alaska, Idaho, Wyoming, North Dakota, and South Dakota. In the long term, this research will contribute to reducing socio-economic and socio-demographic differences in delivering high-quality surgical care to patients in the United States.
We would like thank Dr. Nicole Carnegie and Dr. Andrew Hoegh from statistics department at Montana State University for their expertise and guidance in statistical analyses. Also, this research would not have been possible without the support from the local communities, clinic, hospital patients, and their families.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bugaj AM, Poland; Giacomelli L, Italy S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Singh JA, Yu S, Chen L, Cleveland JD. Rates of Total Joint Replacement in the United States: Future Projections to 2020-2040 Using the National Inpatient Sample. J Rheumatol. 2019;46:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 719] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 2. | Dowsey MM, Petterwood J, Lisik JP, Gunn J, Choong PF. Prospective analysis of rural-urban differences in demographic patterns and outcomes following total joint replacement. Aust J Rural Health. 2014;22:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Jeschke E, Citak M, Günster C, Matthias Halder A, Heller KD, Malzahn J, Niethard FU, Schräder P, Zacher J, Gehrke T. Are TKAs Performed in High-volume Hospitals Less Likely to Undergo Revision Than TKAs Performed in Low-volume Hospitals? Clin Orthop Relat Res. 2017;475:2669-2674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | McCrory B, Hoge JA, Whiteley R, Wiley JB, Sridhar S, Ma J. Outcomes Following Initial Perioperative Surgical Home Integration at a Rural Community Hospital. Proceedings of the Proceedings of the Human Factors and Ergonomics Society Annual Meeting; 2019. SAGE Publications Sage CA: Los Angeles, CA: 683-687. [DOI] [Full Text] |
| 5. | Lese A, Sraj S. Rural Orthopedics: Providing Orthopedic Care in Rural Communities. Orthopedics. 2019;42:e350-e355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Kash B, Cline K, Menser T, Zhang Y. The perioperative surgical home (PSH): a comprehensive literature review for the American Society of Anesthesiologists. Schaumburg (IL): The Society 2014. [RCA] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Vetter TR, Goeddel LA, Boudreaux AM, Hunt TR, Jones KA, Pittet JF. The Perioperative Surgical Home: how can it make the case so everyone wins? BMC Anesthesiol. 2013;13:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Al-Shammari L, Douglas D, Gunaratnam G, Jones C. Perioperative medicine: a new model of care? Br J Hosp Med (Lond). 2017;78:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Cline KM, Clement V, Rock-Klotz J, Kash BA, Steel C, Miller TR. Improving the cost, quality, and safety of perioperative care: A systematic review of the literature on implementation of the perioperative surgical home. J Clin Anesth. 2020;63:109760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Desebbe O, Lanz T, Kain Z, Cannesson M. The perioperative surgical home: An innovative, patient-centred and cost-effective perioperative care model. Anaesth Crit Care Pain Med. 2016;35:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Kain ZN, Vakharia S, Garson L, Engwall S, Schwarzkopf R, Gupta R, Cannesson M. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118:1126-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 12. | Cannesson M, Kain Z. Enhanced recovery after surgery versus perioperative surgical home: is it all in the name? Anesth Analg. 2014;118:901-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Nicolescu TO. Perioperative Surgical Home. Meeting tomorrow's challenges. Rom J Anaesth Intensive Care. 2016;23:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Qiu C, Cannesson M, Morkos A, Nguyen VT, LaPlace D, Trivedi NS, Khachatourians A, Rinehart J, Kain ZN. Practice and Outcomes of the Perioperative Surgical Home in a California Integrated Delivery System. Anesth Analg. 2016;123:597-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Alvis BD, King AB, Pandharipande PP, Weavind LM, Avila K, Leisy PJ, Ajmal M, McHugh M, Keegan KA, Baker DA, Walia A, Hughes CG. Creation and Execution of a Novel Anesthesia Perioperative Care Service at a Veterans Affairs Hospital. Anesth Analg. 2017;125:1526-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Kim KY, Anoushiravani AA, Chen KK, Li R, Bosco JA, Slover JD, Iorio R. Perioperative Orthopedic Surgical Home: Optimizing Total Joint Arthroplasty Candidates and Preventing Readmission. J Arthroplasty. 2019;34:S91-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Yajnik M, Hill JN, Hunter OO, Howard SK, Kim TE, Harrison TK, Mariano ER. Patient education and engagement in postoperative pain management decreases opioid use following knee replacement surgery. Patient Educ Couns. 2019;102:383-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Kim E, Lee B, Cucchiaro G. Perioperative Surgical Home: Evaluation of a New Protocol Focused on a Multidisciplinary Approach to Manage Children Undergoing Posterior Spinal Fusion Operation. Anesth Analg. 2017;125:812-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Qiu C, Rinehart J, Nguyen VT, Cannesson M, Morkos A, LaPlace D, Trivedi NS, Mercado PD, Kain ZN. An Ambulatory Surgery Perioperative Surgical Home in Kaiser Permanente Settings: Practice and Outcomes. Anesth Analg. 2017;124:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Raman VT, Tumin D, Uffman J, Thung AK, Burrier C, Jatana KR, Elmaraghy C, Tobias JD. Implementation of a perioperative surgical home protocol for pediatric patients presenting for adenoidectomy. Int J Pediatr Otorhinolaryngol. 2017;101:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Chimento GF, Thomas LC. The Perioperative Surgical Home: Improving the Value and Quality of Care in Total Joint Replacement. Curr Rev Musculoskelet Med. 2017;10:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Vetter TR, Barman J, Hunter JM Jr, Jones KA, Pittet JF. The Effect of Implementation of Preoperative and Postoperative Care Elements of a Perioperative Surgical Home Model on Outcomes in Patients Undergoing Hip Arthroplasty or Knee Arthroplasty. Anesth Analg. 2017;124:1450-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Powell AC, Thearle MS, Cusick M, Sanderson DJ, Van Lew H, Lee C, Kieran JA. Early results of a surgeon-led, perioperative surgical home. J Surg Res. 2017;211:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Leahy I, Johnson C, Staffa SJ, Rahbar R, Ferrari LR. Implementing a Pediatric Perioperative Surgical Home Integrated Care Coordination Pathway for Laryngeal Cleft Repair. Anesth Analg. 2019;129:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Kaufman BG, Thomas SR, Randolph RK, Perry JR, Thompson KW, Holmes GM, Pink GH. The Rising Rate of Rural Hospital Closures. J Rural Health. 2016;32:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 26. | Nakayama DK, Hughes TG. Issues that face rural surgery in the United States. J Am Coll Surg. 2014;219:814-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Weichel D. Orthopedic surgery in rural American hospitals: a survey of rural hospital administrators. J Rural Health. 2012;28:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Chaudhary MA, Shah AA, Zogg CK, Changoor N, Chao G, Nitzschke S, Havens JM, Haider AH. Differences in rural and urban outcomes: a national inspection of emergency general surgery patients. J Surg Res. 2017;218:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Gruca TS, Pyo TH, Nelson GC. Improving Rural Access to Orthopaedic Care Through Visiting Consultant Clinics. J Bone Joint Surg Am. 2016;98:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Snyder JE, Jensen M, Nguyen NX, Filice CE, Joynt KE. Defining Rurality in Medicare Administrative Data. Med Care. 2017;55:e164-e169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Norful AA, de Jacq K, Carlino R, Poghosyan L. Nurse Practitioner-Physician Comanagement: A Theoretical Model to Alleviate Primary Care Strain. Ann Fam Med. 2018;16:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Sridhar S, Carnegie N, Mouat-Hunter A, McCrory B. A Rural Community Hospital’s Perioperative Surgical Home Model Compared to Traditional Surgical Systems. Proceedings of the International Symposium on Human Factors and Ergonomics in Health Care. 2022;11:140-144. [DOI] [Full Text] |
| 33. | Alvis BD, Amsler RG, Leisy PJ, Feng X, Shotwell MS, Pandharipande PP, Ajmal M, McHugh M, Walia A, Hughes CG. Effects of an anesthesia perioperative surgical home for total knee and hip arthroplasty at a Veterans Affairs Hospital: a quality improvement before-and-after cohort study. Can J Anaesth. 2021;68:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Duplantier N, Briski D, Ochsner JL, Meyer M, Stanga D, Chimento GF. The Financial Impact of a Multidisciplinary Preoperative Risk Stratification Program for Joint Arthroplasty. J Arthroplasty. 2015;30:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Carter EM, Potts HW. Predicting length of stay from an electronic patient record system: a primary total knee replacement example. BMC Med Inform Decis Mak. 2014;14:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Ramkumar PN, Chu CT, Harris JD, Athiviraham A, Harrington MA, White DL, Berger DH, Naik AD, Li LT. Causes and Rates of Unplanned Readmissions After Elective Primary Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. Am J Orthop (Belle Mead NJ). 2015;44:397-405. [PubMed] |
| 37. | Chand MR, Meiyappan A, Villa JM, Kanwar S, Sabesan VJ, Gilot G. Ninety-day readmission following shoulder arthroplasty. J Shoulder Elb Arthroplast. 2018;2:2471549218810016. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Alem N, Rinehart J, Lee B, Merrill D, Sobhanie S, Ahn K, Schwarzkopf R, Cannesson M, Kain Z. A case management report: a collaborative perioperative surgical home paradigm and the reduction of total joint arthroplasty readmissions. Perioper Med (Lond). 2016;5:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Couch CA, Coleman KL, Fellner AN, Guend H. A Comparison of Re-admission and Emergency Department Visits in a Colorectal Surgical Home versus Traditional Perioperative Care. Research Square. 2022;. [DOI] [Full Text] |
| 40. | Duncan MJ. Perioperative Surgical Home, Fixing a Fragmented Process to Improve Quality of Care. Mo Med. 2019;116:53-57. [PubMed] |
| 41. | Yoon RS, Nellans KW, Geller JA, Kim AD, Jacobs MR, Macaulay W. Patient education before hip or knee arthroplasty lowers length of stay. J Arthroplasty. 2010;25:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 42. | Lesher AP, Gavrilova Y, Ruggiero KJ, Evans HL. Surgery and the Smartphone: Can Technology Improve Equitable Access to Surgical Care? J Surg Res. 2021;263:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Toerien D. Orderliness in tourism enterprise dynamics in United States micropolitan statistical areas. Sustainability. 2021;13:6180. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Novak NL, Baquero B, Askelson NM, Diers L, Dunn B, Haines H, Afifi R, Parker EA. Health Equity in Midsize Rural Communities: Challenges and Opportunities in a Changing Rural America. Am J Public Health. 2020;110:1342-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Hess DR. Retrospective studies and chart reviews. Respir Care. 2004;49:1171-1174. [PubMed] |
| 46. | Euser AM, Zoccali C, Jager KJ, Dekker FW. Cohort studies: prospective versus retrospective. Nephron Clin Pract. 2009;113:c214-c217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 47. | Sridhar S, Whitaker B, Mouat-Hunter A, McCrory B. Predicting Length of Stay using machine learning for total joint replacements performed at a rural community hospital. PLoS One. 2022;17:e0277479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Reference Citation Analysis (0)] |