Published online Dec 18, 2023. doi: 10.5312/wjo.v14.i12.878
Peer-review started: June 20, 2023
First decision: August 30, 2023
Revised: September 12, 2023
Accepted: November 8, 2023
Article in press: November 8, 2023
Published online: December 18, 2023
Processing time: 177 Days and 18.2 Hours
Lumbar disc herniation and non-specific low back pain are common conditions that seriously affect patients’ health-related quality of life (HRQoL). Although empirical evidence has demonstrated that novel Thermobalancing therapy and Dr Allen’s Device can relieve chronic low back pain, there have been no randomised controlled trials for these indications.
To evaluate the efficacy of Dr Allen’s Device in lumbar disc herniation (LDH) and non-specific low back pain (NSLBP).
A randomised clinical trial was conducted investigating 55 patients with chronic low back pain due to LDH (n = 28) or NSLBP (n = 27), out of which 15 were randomly assigned to the control group and 40 were assigned to the treatment group. The intervention was treatment with Dr Allen’s Device for 3 mo. Changes in HRQoL were assessed using the Numerical Pain Rating Scale and the Japanese Orthopedic Association Back Pain Questionnaire.
Thermobalancing therapy with Dr Allen’s Device showed a significant reduction in pain in the treatment group (P < 0.001), with no recorded adverse effects. Both pain assessment scales showed a significant improvement in patients’ perception of pain indicating improvement in HRQoL.
The out-of-hospital use of Thermobalancing therapy with Dr Allen’s Device for Low Back Treatment relieves chronic low back pain significantly and without adverse effects, improves the level of activity and HRQoL among patients with LDH and NSLBP. This study demonstrates the importance of this safe first-line therapy that can be used for effective at-home management of chronic low back pain.
Core Tip: This prospective randomised controlled trial assessed the efficacy and safety of novel Thermobalancing therapy and wearable Dr Allen’s Device for chronic low back pain. Patients with non-specific low back pain and lumbar disc herniation were assessed by the Numerical Pain Rating Scale, and the Japanese Orthopedic Association Back Pain Evaluation Questionnaire. Research results showed a significant reduction in pain and the improvement in patients’ health-related quality of life in the treatment group. No adverse effects were observed. Therefore, Thermobalancing therapy with Dr Allen’s Device can be recommended for the at-home management of chronic low back pain.
- Citation: Allen S, Rashid A, Adjani A, Akram M, Khan FS, Sherwani R, Khalil MT. Efficacy and safety of thermobalancing therapy with Dr Allen’s Device for chronic low back pain: A randomised controlled trial. World J Orthop 2023; 14(12): 878-888
- URL: https://www.wjgnet.com/2218-5836/full/v14/i12/878.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i12.878
Non-specific low back pain (NSLBP) is the leading cause of disability worldwide[1]. Of the numerous causes of low back pain, lumbar disc herniation (LDH) is a major contributor accounting for around 9% of chronic low back pain (CLBP) in the population[2]. The main symptom of LDH and NSLBP is chronic pain in the lower spine area that can impact the quality of life and heavily burden individuals, their families, and society[3].
Common treatments for NSLBP and LDH include pain management with acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), opioids and anticonvulsants, steroid injections, and spinal surgeries. Oral medications for pain control can be effective but they only provide short term symptomatic relief and are not without adverse effects. NSAIDs, acetaminophen and anticonvulsants can cause serious side effects and can lead to health problems, such as gastrointestinal complications and cardiovascular events[4,5]. Opioid use in people with chronic pain can cause constipation, nausea, itching, dizziness and can even result in opioid dependence. Opioid overdose is becoming a major public health problem[6,7]. Epidural “around the spinal cord” steroid injections aimed at relieving low back pain can lead to irreversible complications and should therefore be used as the last resort[8]. Lower back surgeries may provide pain relief more rapidly than conservative therapy but carry more risk and the outcomes at one year are similar using both these modalities. Moreover, surgery can still be an option after conservative therapy[9].
The above adverse effects of the available treatment options for low back pain limit their use and highlight the need for a conservative, safe and effective mode of treatment.
Over the last decade, Thermobalancing therapy and non-invasive Dr Allen’s Devices were used in patients for effective and safe out-of-hospital management of chronic diseases affecting different organs. This novel treatment was patented as “Therapeutic device and method”[10].
The use of Thermobalancing therapy with Dr Allen’s Device as a monotherapy in men over 55 years of age with benign prostatic hyperplasia (BPH) showed improvement of clinical symptoms, including lower urinary tract symptoms[11,12]. Evidence also suggests that Thermobalancing therapy is effective in the treatment of chronic prostatitis/chronic pelvic pain syndrome[13] and kidney stone disease (KSD) without renal colic[14].
Although empirical evidence has also demonstrated that this novel treatment option can relieve chronic low back pain in cases of lumbar disc herniation and non-specific causes, there have been no randomised control trials for these indications.
This is the first clinical study that attempts to demonstrate the effectiveness and safety of Dr Allen’s Device for Low Back Treatment in patients with chronic low back pain due to LDH or NSLBP, as well as the ability of this therapy to improve health-related quality of life (HRQoL).
Our study was a prospective, randomised, interventional, parallel group study conducted to demonstrate the efficacy of Dr Allen’s Device for low back treatment in the management of chronic low back pain. The trial was registered at the Iranian Registry of Clinical Trials. The study was conducted in accordance with the Declaration of Helsinki. It was approved by the Ethics Review Committee.
Patients aged 18-60 years having low back pain for at least 12 wk were recruited at a hospital and an outpatient clinic for the trial after initial radiological evaluation. For every patient with LDH, one patient with NSLBP was recruited to maintain a 1:1 ratio of the conditions. Participants were excluded if they had severe co-morbidities, such as cancer, heart failure or infection.
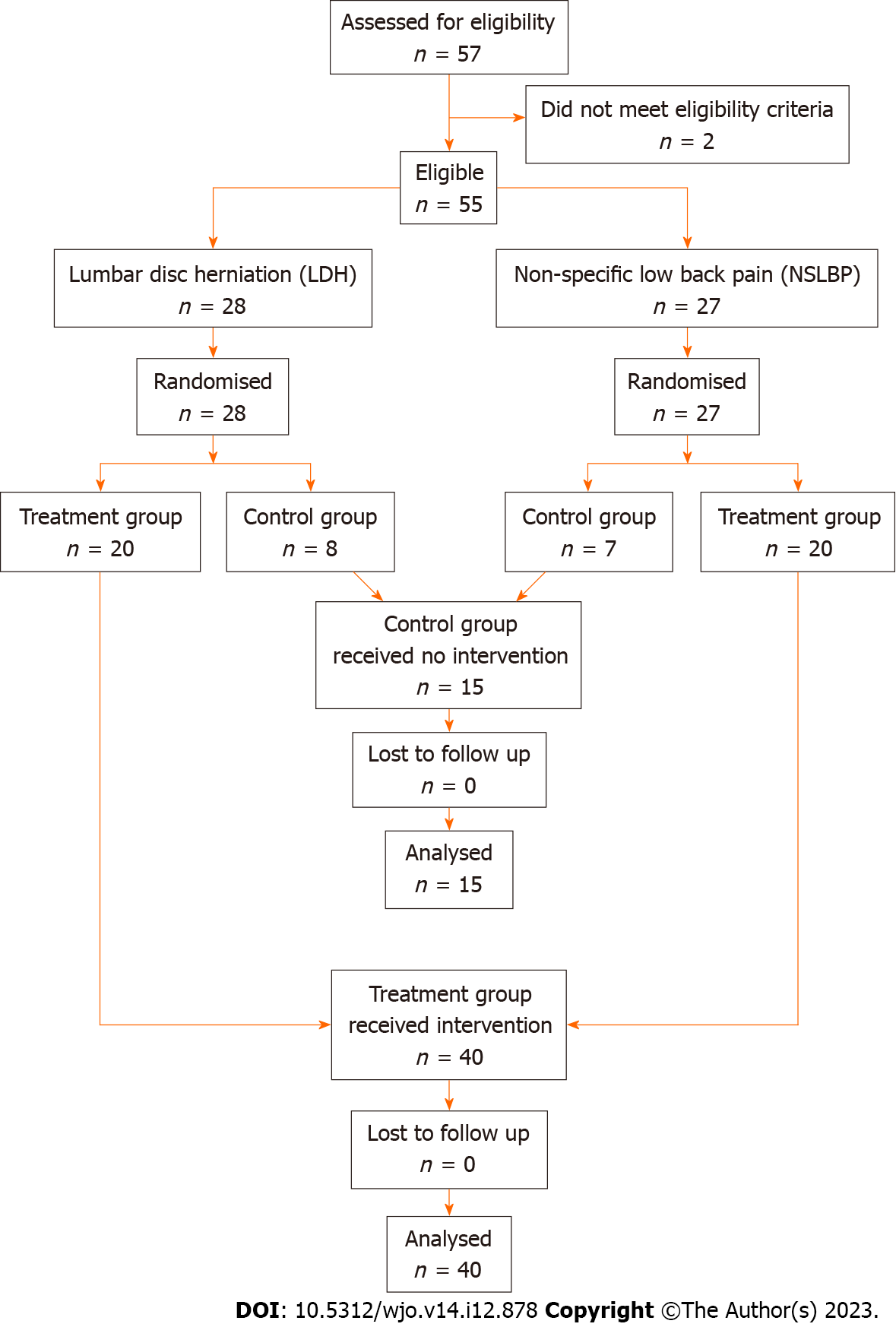
A total of 55 male and female patients with low back pain met the eligibility criteria and were recruited for the study. The patients were then randomised into treatment and control groups. This resulted in two groups: A treatment group of 40 patients with chronic low back pain, consisting of 20 patients with NSLBP and 20 patients with LDH, and a control group of 15 patients consisting of 7 patients with NSLBP and 8 patients with LDH. Randomisation was performed using simple randomisation procedures and computer-generated random numbers. No blinding was done due to the nature of the intervention.
Patient satisfaction was considered an adequate representation of treatment efficacy and was assessed at the beginning of the study and after treatment using two well-established self-administered questionnaires: Numerical Pain Rating Scale (NPRS)[15] and The Japanese Orthopedic Association Back Pain Evaluation Questionnaire (JOABPEQ)[16]. The NPRS scores were obtained at the beginning of the study and after 1 and 3 mo of using Dr Allen’s Device for low back treatment, and the JOABPEQ scores were obtained at the beginning and end of therapy.
A total of 55 patients with chronic low back pain were recruited for the clinical trial after receiving a detailed explanation of the clinical trial and the possible benefits of thermobalancing therapy, and after giving informed consent. They were divided into two groups, those with LDH and those with NSLBP, based on their aetiology identified by radiological imaging. The ratio of patients in these groups was 1:1 as an equal number of patients with LDH (n = 28) and NSLBP (n = 27) were recruited. Although NSLBP is more common on average in the general population, the type of pain in LDH tends to be more severe, and such patients present more frequently to the hospital compared to patients with NSLBP.
Each group (LDH, NSLBP) was randomised separately using a 3:1 ratio for ethical reasons as the control group was not going to receive the intervention. It was decided that a larger treatment group would allow for a better understanding of adverse effects if any. Patients with LDH were randomised into a treatment group of 20 patients and a control group of 8 patients and patients with NSLBP were randomised into a treatment group of 20 patients and a control group of 7 patients. The final treatment group consisted of 40 patients and the control group consisted of 15 patients.
The intervention was Thermobalancing therapy with Dr Allen’s Device for Low Back Pain Treatment used as a monotherapy for a 3-mo period. The patients in the treatment group used Dr Allen’s Device for low back pain treatment as a monotherapy for 3 mo. Patients in the control group did not receive any intervention.
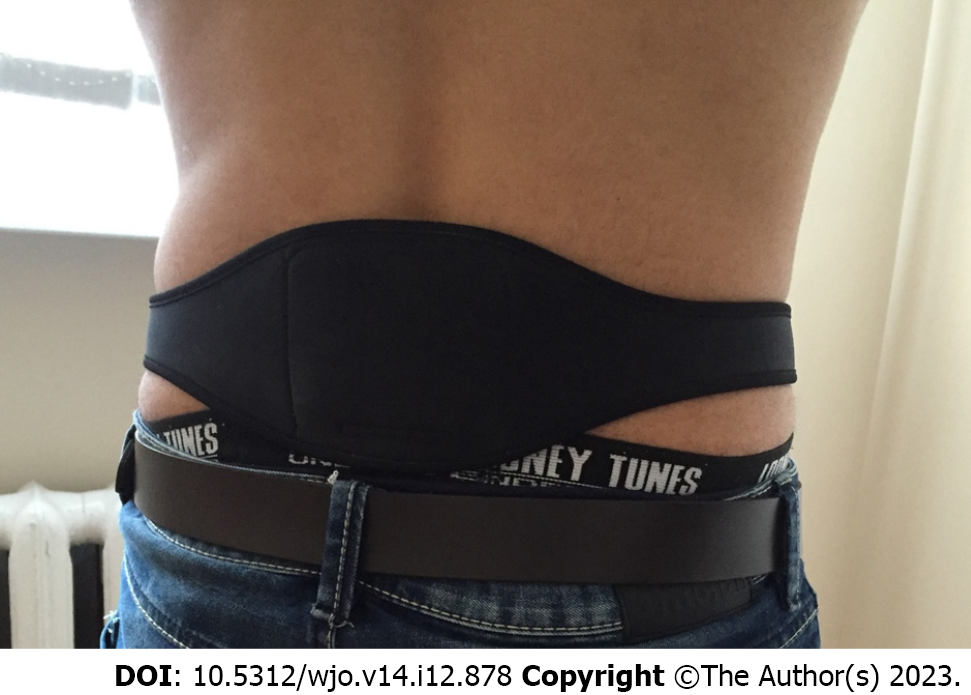
Dr Allen’s Device is a Class I medical device and registered with the British Medicines and Healthcare Products Regulatory Agency (MHRA). Thermobalancing therapy with Dr Allen’s Device can be used as an out-of-hospital treatment by patients at home. Dr Allen’s Device applies a thermoelement, made from a special wax-based material, topically to the skin or worn over close-fitting underwear over the affected organs. In people with lower back pain, the thermoelement is applied to the back over the sore area (Figure 1). This thermoelement accumulates the naturally emitted body heat, warms up, directs heat to the region of pain, and maintains the optimal level of heat in the region of pain over time. Wearing and using Dr Allen’s Device for Low Back Pain Treatment is comfortable.
The NPRS and the JOABPEQ were used to assess the effectiveness of treatment. The NPRS is an 11-point pain rating scale that can be used to estimate the degree of pain experienced by the patient. The scale ranges from 0 (no pain) to 10 (worst imaginable pain)[17]. A study about the responsiveness of NPRS has found that a 2-point improvement represents meaningful change[18].
The JOABPEQ has 25 questions which evaluate 5 functional domains with scores ranging from 0-100. According to a study, a 20 point improvement in the functional domains of JOABPEQ represents meaningful change[19].
In our study, the change in the NPRS score and the functional domains of JOABPEQ assessed before the intervention and during the follow up period was considered as the primary endpoint.
Sample size was calculated using G*Power software. A total of 54 participants (40 participants in treatment group and 14 participants in control group) were required to conduct a two tailed study with a type I error of 5% and a power of 80%. We had 15 patients in the control group.
The collected data was organised in Microsoft Excel and statistical analysis of the results was performed using the Data Analysis module of Microsoft Excel 2010. Paired sample t-tests were used for the comparison of parametric data. The non-parametric data was assessed using the Mann-Whitney U test. A P value < 0.05 was considered statistically significant.
A total of 55 patients with chronic low back pain either due to NSLBP (n = 27) or LDH (n = 28) identified by radiological assessment participated in the study. After randomisation, the treatment group consisted of 40 patients, 20 with LDH and 20 with NSLBP, and the control group consisted of 15 patients, 8 with LDH and 7 with NSLBP, as shown in the patient allocation flow diagram (Figure 2).
Mean age of the patients was 41.07 ± 8.05 years. The overall male to female ratio was 29:26. Correlation analysis between demographic variables and the NPRS and JOABPEQ scores and patient characteristics are detailed in Table 1. Pearson’s product-moment correlation was used for continuous variables and point-biserial correlation was used for dichotomous variables.
| Characteristic | Treatment group | Correlation coefficient (r) | Control group | Correlation coefficient (r) |
| Number of patients (n) | 40 | 15 | ||
| Age (mean ± SD) | 42.2 ± 8.49 | -0.1124a; 0.099b | 38.06 ± 5.98 | 0.4919a; 0.045b |
| Gender (M:F) | 18:22 | 0.2353a; -0.08b | 11:4 | 0.1183a; -0.34b |
| NSLBP:LDH | 1:1 | -0.131a; 0.126b | 7:8 | 0.1396a; 0.172b |
| NPRS – how would you rate your pain right now? | 6.48 ± 1.63 | 8.07 ± 1.16 | ||
| JOABPEQ – low back pain | 8.9 ± 14.38 | 10.40 ± 12.66 |
For initial evaluation, patients were presented with the NPRS scale and the JOABPEQ. Instructions about how to answer the questions were provided and the clinicians ensured that the patients understood the questions adequately in order to obtain the most accurate responses. Details of low back pain intensity experienced by the patients was obtained using the NPRS scale as answers to four questions, namely, “How would you rate your pain RIGHT NOW”, “USUAL level of pain during the last week”, “BEST level of pain during the last week” and “WORST level of pain during the last week”. The patients scored the intensity of their pain from 0 to 10.
The JOABPEQ questionnaire required answers to 25 separate questions which were then evaluated to scores ranging from 0 to 100 for five different functional domains.
The NPRS scale was presented to the patients again at the end of one month, and the NPRS scale and the JOABPEQ were presented to the patients again at the end of three months of treatment and their responses were compared with the initial assessment.
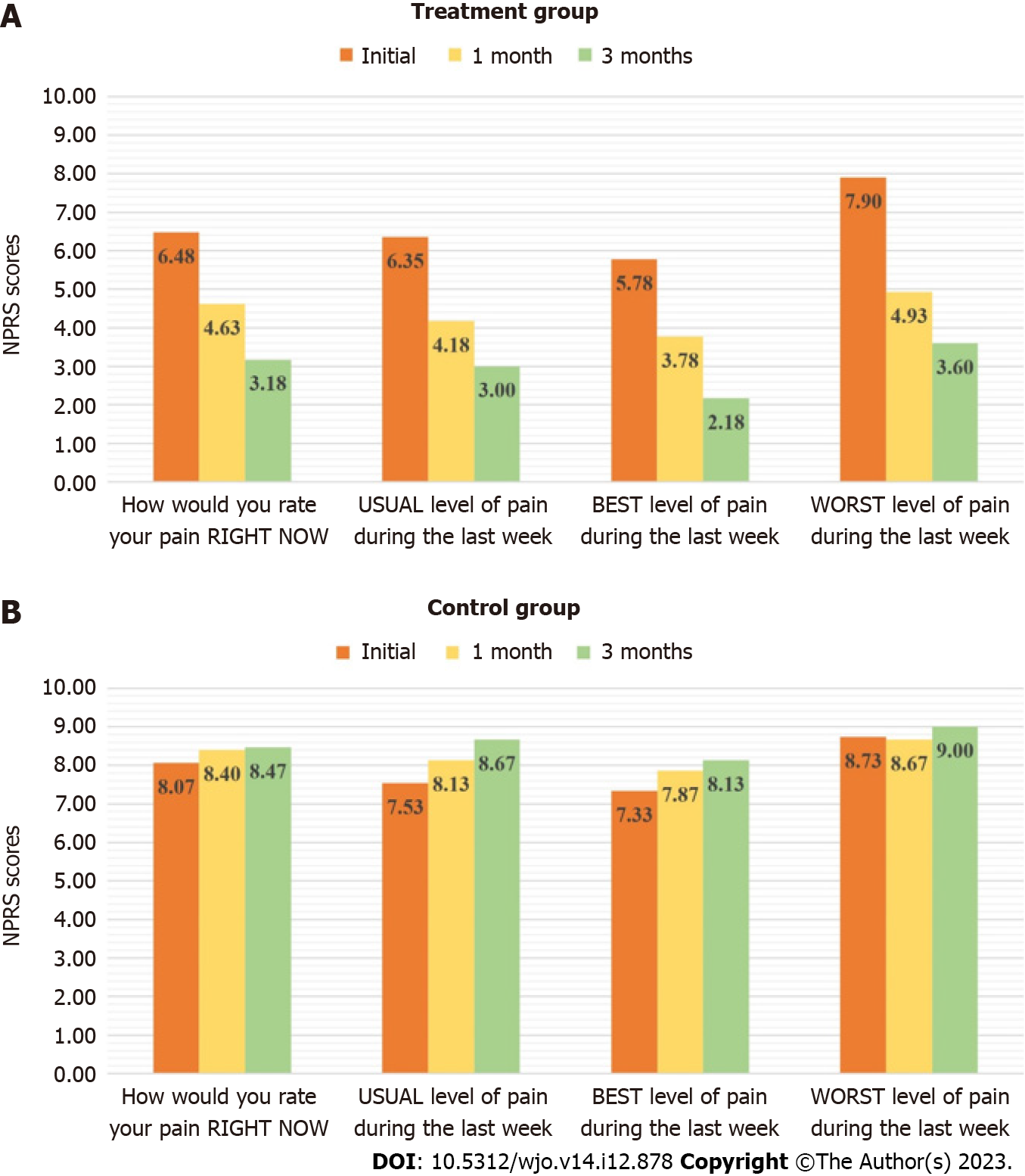
A 2-point improvement in the different parameters of the NPRS scale was considered significant. Out of the 40 patients in the treatment group, 82.5%, 77.5%, 75% and 75% showed significant improvement in the “How would you rate your pain RIGHT NOW”, “USUAL level of pain during the last week”, “BEST level of pain during the last week” and the “WORST level of pain during the last week” scores respectively. A total of 7 patients had unchanged scores and 2 patients experienced increased pain intensity.
In the control group, none of the patients showed any significant improvement. Out of the 15 patients in the control group, 26.67%, 40%, 40% and 13.3% showed significant increase in the “How would you rate your pain RIGHT NOW”, “USUAL level of pain during the last week”, “BEST level of pain during the last week” and “WORST level of pain during the last week” scores indicating increase in pain intensity. Scores remained unchanged in 12 patients.
On statistical analysis, the comparison of the mean initial and final pain scores in the treatment group was found to be statistically significant across all the parameters of the NPRS scale, while no statistically significant change was observed in the control group. The comparison is presented in Figure 3 and in Table 2.
| NPRS parameters | Initial | 1 mo (mean ± SD) | 3 mo | P value | ||
| Treatment group | How would you rate your pain RIGHT NOW | 6.48 ± 1.63 | 4.63 ± 1.00 | 3.18 ± 1.87 | < 0.001 | |
| P value | < 0.001a | < 0.001b | ||||
| USUAL level of pain during the last week | 6.35 ± 1.23 | 4.18 ± 0.96 | 3.0 ± 1.43 | < 0.001 | ||
| P value | < 0.001a | < 0.001b | ||||
| BEST level of pain during the last week | 5.78 ± 1.25 | 3.78 ± 0.97 | 2.18 ± 1.62 | < 0.001 | ||
| P value | < 0.001a | < 0.001b | ||||
| WORST level of pain during the last week | 7.9 ± 1.50 | 4.93 ± 1.10 | 3.6 ± 2.01 | < 0.001 | ||
| P value | < 0.001a | < 0.001b | ||||
| Control group | How would you rate your pain RIGHT NOW | 8.07 ± 1.16 | 8.4 ± 0.63 | 8.47 ± 0.64 | > 0.05 | |
| P value | > 0.05a | > 0.05b | ||||
| USUAL level of pain during the last week | 7.53 ± 0.92 | 8.13 ± 0.52 | 8.67 ± 0.49 | > 0.05 | ||
| P value | > 0.05a | > 0.05b | ||||
| BEST level of pain during the last week | 7.33 ± 0.98 | 7.87 ± 0.64 | 8.13 ± 0.74 | > 0.05 | ||
| P value | > 0.05a | > 0.05b | ||||
| WORST level of pain during the last week | 8.73 ± 1.10 | 8.67 ± 0.49 | 9.00 ± 0.38 | > 0.05 | ||
| P value | > 0.05a | > 0.05b | ||||
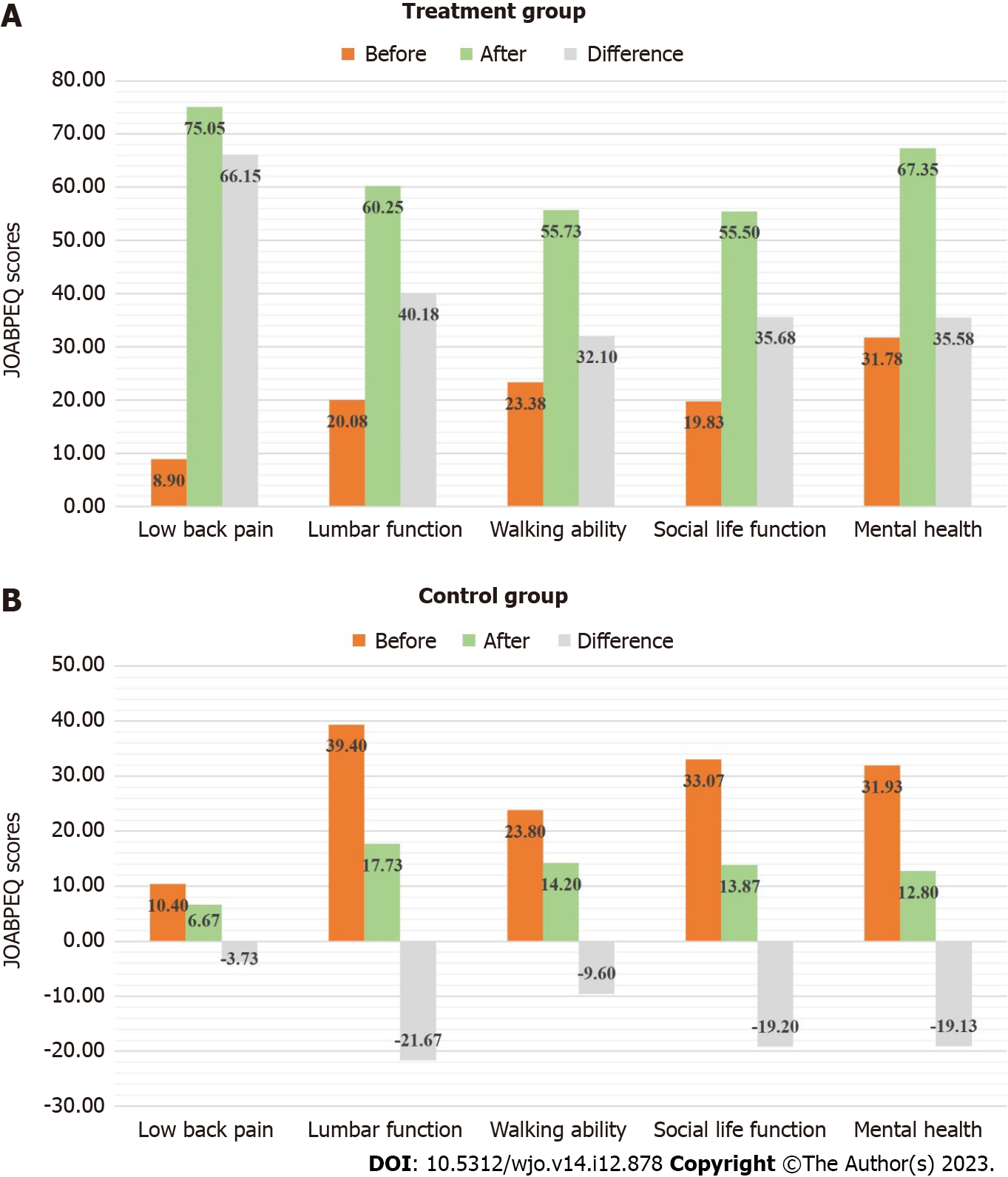
The scores of the five domains of JOABPEQ evaluated at the beginning of the study were compared with those evaluated at the end of 3 months. In the treatment group, all 40 patients showed an improvement indicated by an increase in 20 points in the low back pain domain. A 72.5% improvement was observed in the lumbar function domain and 10% of the patients showed worsening. Walking ability was improved in 75% of the patients and worsened in 10% of the patients. Social life function was improved in 75% of the patients of the treatment group and worsened in 5%. Mental health was improved in 77.5% of patients, with no patients showing worsening.
In the control group, a very small proportion of patients, i.e., 13.3%, 6.7%, 20% and 6.7% of the 15 patients experienced an improvement in low back pain, lumbar function, walking ability and social life domains respectively. No improvement was observed in the mental health domain. Most patients experienced low scores across all domains.
A comparison of the mean difference between the scores of the five functional domains before and after thermobalancing therapy showed a statistically significant improvement across all domains in the treatment group. No significant improvement was observed in the control group, but a statistically significant worsening of lumbar function and mental health were seen further emphasising the need for effective treatment. This data is presented in Figure 4 and in Table 3.
| JOABPEQ functional domains | Initial evaluation | Final evaluation | Difference | P value | |
| Treatment group | Low back pain | 8.9 ± 14.38 | 75.05 ± 20.97 | 66.15 | < 0.001 |
| Lumbar function | 20.08 ± 18.24 | 60.25 ± 18.38 | 40.17 | < 0.001 | |
| Walking ability | 23.38 ± 19.09 | 55.73 ± 21.07 | 32.35 | < 0.001 | |
| Social life function | 19.83 ± 14.02 | 55.50 ± 17.37 | 35.67 | < 0.001 | |
| Mental health | 31.78 ± 20.85 | 67.35 ± 13.83 | 35.57 | < 0.001 | |
| Control group | Low back pain | 10.40 ± 12.66 | 6.67 ± 13.13 | -3.73 | 0.29 |
| Lumbar function | 39.40 ± 25.57 | 17.73 ± 14.69 | -21.67 | 0.01 | |
| Walking ability | 23.80 ± 30.96 | 14.20 ± 16.01 | -9.6 | 0.69 | |
| Social life function | 33.07 ± 24.10 | 13.87 ± 9.24 | -19.2 | 0.01 | |
| Mental health | 31.93 ± 19.76 | 12.80 ± 6.83 | -19.13 | < 0.001 |
The changes in the NPRS and the JOABPEQ scores before and after Thermobalancing therapy with Dr Allen’s Device show significant improvement in pain perception in all patients in the treatment group.
The use of Dr Allen’s Device for low back pain treatment did not cause any adverse events in the participating individuals. This observation confirms that thermobalancing therapy and Dr Allen’s Device are safe.
The aim of this study was to determine the effect of Thermobalancing therapy on CLBP, defined as pain that continues for 12 wk or longer, and to assess the general improvement in the HRQoL in male and female patients when used as monotherapy compared with patients who received no treatment. The objective of this study was not to compare one treatment with another but to show the efficacy of the prolonged use of the intervention on the treatment group and, as expected, the final data showed a significant improvement in pain in the treatment group and no change in the control group indicating that the intervention was efficacious.
A difference in baseline NPRS scores of the treatment and control groups was observed. This can be attributed to the difference in the sizes of the treatment (n = 40) and control (n = 15) groups. Since this difference in baseline values occurred despite efficient randomisation, we consider the data valid.
The results of our study show that Thermobalancing therapy is safe and reduces the intensity of back pain, consequently improving the level of activity among patients with LDH and NSLBP. The results showed that there was significant difference in the improvement of daily activities between the treatment and control groups with the treatment group showing a striking functional improvement. However, the difference in effectiveness between the NSLBP treatment group and the LDH treatment group was not significant, implying that Dr Allen’s Device was equally effective in improving the symptoms and functional activity in chronic low back pain due to lumbar disc herniation or other non-specific causes.
These findings allow to suggest that Dr Allen’s Device can be used as the first-line therapy for chronic low back pain.
Low back pain can occur due to a variety of causes, and, in most instances, the cause is multifactorial. The pain can be caused due to a pathology in either the soft tissues, the vertebrae, the joints, intervertebral discs, or neurovascular structures. The numerous treatment modalities available for low back pain include conservative treatments like self-care, psychological care, physical rehabilitation and symptomatic care, and surgical procedures in refractory cases where the pain is not amenable to any conservative option. But most treatment modalities are singular in their effectiveness and, therefore, are not effective when more than one cause of the pain concurrently exist[20].
Dr Allen’s Device for low back treatment is a novel, conservative option in the treatment of chronic pain in the lower spine. It is easy to use with no identified adverse effects in the short or long term. It is a comfortable, wearable device which allows long term patient compliance. As observed in our study, thermobalancing therapy and Dr Allen’s Device provide pain relief in chronic low back pain due to intervertebral disc related causes as well as other causes, thereby making it a holistic treatment option for this condition.
Low back pain is more common in females than in males and its prevalence increases with age. A study evaluated physical and mental health indicators to assess the quality of life in patients with low back pain and found that irrespective of sociodemographic conditions, and the presence of other comorbidities or causes of chronic pain, low back pain can significantly lower quality of life[21].
The NPRS scale and the JOABPEQ are indicators of pain intensity and quality of life respectively. Both these indicators have been found to be reliable, responsive, and valid as indicators of pain and functional abilities. The combined use of NPRS scale and JOABPEQ as done in our study have been found to be the most appropriate tools to assess pain as well as quality of life in patients with low back pain[22].
The strengths of our study are the combined use of a pain intensity scale and a quality-of-life questionnaire enabling us to better understand the benefits of Thermobalancing therapy on individual well-being.
Back pain is associated with physical, psychological, emotional, social changes, and even inappropriate exercise[23,24]. However, a survey found no significant association between LBP and psychological stress[25]. Health systems must prioritise policies that empower doctors and consumers to make informed choices, encourage clinicians to provide appropriate care to those who need it most and provide financial support for evidence-based non-pharmacological treatment[26].
Dr Allen’s Device is a valuable medical innovation for the at-home use[27]. Its patented design accumulates the naturally emitted body heat and spreads it to the affected organs and areas of the body to which it is applied[28]. This out-of-hospital treatment targets the cause of pain at the capillary level. It improves blood circulation locally, that reduces inflammation and pressure in the affected tissue and, consequently, relieves chronic pain and other clinical symptoms[29,30].
The limitation of our study is the lack of an alternative treatment mode in the control group to compare the efficacy of Thermobalancing therapy with available first line treatment modalities for low back pain. In line with previous clinical trials on this treatment modality for other chronic diseases, such as chronic prostatitis, BPH and KSD, blinding was not performed due to the impracticality of a placebo group using such a wearable medical device for a long duration.
This research confirms that the use of non-invasive Dr Allen’s Device gradually relieves chronic low back pain and improves the level of activity in patients with LDH or NSLBP. The study highlights the importance of this novel out-of-hospital treatment option as all participants in the treatment group experienced a significant improvement in their health-related quality of life as a result of using Dr Allen’s Device at home during a 3-mo period and reported no side effects or complications. Overall, the study demonstrates high efficacy and safety of thermobalancing therapy and Dr Allen’s Device for low back treatment in male and female patients with chronic low back pain. Thus, this wearable medical device can be used as the first-line therapy for chronic pain in the lower spine, and for effective at-home management of low back pain due to LDH and other non-specific causes.
Chronic low back pain (CLBP) is a highly prevalent cause of disability worldwide. Of the numerous causes of low back pain, lumbar disc herniation (LDH) is a major contributor accounting for around 9% of CLBP in the population. The main symptom of LDH is chronic pain in the lower spine area that can impact the quality of life and heavily burden individuals, their families, and society. Standard treatments include pain management with acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), opioids and anticonvulsants, steroid injections, and spinal surgeries.
Multiple adverse effects of the standard treatment options for low back pain limit their use and highlight the pressing need for a new conservative, safe and effective mode of treatment.
This is the first clinical study that attempts to demonstrate the efficacy and safety of Dr Allen’s Device for Low Back Pain Treatment in patients with chronic low back pain due to lumbar disc herniation (LDH) or non-specific low back pain (NSLBP), as well as the ability of this therapy to improve health-related quality of life.
A total of 55 patients with chronic low back pain were recruited and randomised for the clinical trial. Thermobalancing therapy with Dr Allen’s Device for Low Back Pain Treatment was used as a monotherapy for a 3-month period. The Numerical Pain Rating Scale (NPRS) and the Japanese Orthopedic Association Back Pain Evaluation Questionnaire (JOABPEQ) were used to assess the effectiveness of treatment. The effect on chronic low back pain was assessed as the primary health outcome.
The study showed that most patients in the treatment group experienced a significant pain reduction in the low back area, an improvement in the lumbar function, walking ability, social life, and mental health, with no patients showing worsening of these parameters. It was confirmed that the treatment with Dr Allen’s Device was effective and safe in patients with non-specific low back pain and lumbar disc herniation.
Thermobalancing therapy with Dr Allen’s Device can be recommended for an effective at-home management of chronic low back pain.
The results suggest that this treatment may also be effective and safe for other types of chronic back pain and further research in this direction is needed.
We are grateful to Fine Treatment for advancing population health through novel medical research, to Government College University Faisalabad (GCUF) for independently conducting this clinical trial, and to the Oxfordshire Local Enterprise Partnership (OxLEP) for their support in developing this medical innovation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohey NM, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY
| 1. | Gianola S, Bargeri S, Del Castillo G, Corbetta D, Turolla A, Andreano A, Moja L, Castellini G. Effectiveness of treatments for acute and subacute mechanical non-specific low back pain: a systematic review with network meta-analysis. Br J Sports Med. 2022;56:41-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 2. | Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, Williams G, Smith E, Vos T, Barendregt J, Murray C, Burstein R, Buchbinder R. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:968-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1799] [Article Influence: 163.5] [Reference Citation Analysis (0)] |
| 3. | Grabovac I, Dorner TE. Association between low back pain and various everyday performances: Activities of daily living, ability to work and sexual function. Wien Klin Wochenschr. 2019;131:541-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Wehling M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: management and mitigation of risks and adverse effects. Eur J Clin Pharmacol. 2014;70:1159-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 5. | Enthoven WT, Roelofs PD, Deyo RA, van Tulder MW, Koes BW. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350:g6380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 382] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 7. | Abdel Shaheed C, Maher CG, Williams KA, Day R, McLachlan AJ. Efficacy, Tolerability, and Dose-Dependent Effects of Opioid Analgesics for Low Back Pain: A Systematic Review and Meta-analysis. JAMA Intern Med. 2016;176:958-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 241] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 8. | Suri P, Pashova H, Heagerty PJ, Jarvik JG, Turner JA, Comstock BA, Bauer Z, Annaswamy TM, Nedeljkovic SS, Wasan AD, Friedly JL. Short-term improvements in disability mediate patient satisfaction after epidural corticosteroid injections for symptomatic lumbar spinal stenosis. Spine (Phila Pa 1976). 2015;40:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Deyo RA, Mirza SK. CLINICAL PRACTICE. Herniated Lumbar Intervertebral Disk. N Engl J Med. 2016;374:1763-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 221] [Article Influence: 24.6] [Reference Citation Analysis (1)] |
| 10. | Allen S, Adjani A. Therapeutic Device and Method, United States Patent and Trademark Office. U.S. Patent No: US9408744B2, 9 August 2016. (accessed June 11, 2023). Available from: https://www.google.com/patents/US9408744. |
| 11. | Allen S, Aghajanyan IG. Benign Prostatic Hyperplasia Treatment with New Physiotherapeutic Device. Urol J. 2015;12:2371-2376. [PubMed] |
| 12. | Allen S, Aghajanyan IG. Thermobalancing conservative treatment for moderate-to-low-degree lower urinary tract symptoms (LUTS) secondary to prostate enlargement. Cogent Medicine. 2016;3:1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Allen S, Aghajanyan IG. Effect of thermobalancing therapy on chronic prostatitis and chronic pelvic pain syndrome. J Clin Urol. 2017;10:347-354. |
| 14. | Allen S, Adjani A. Benign Prostatic Hyperplasia and Kidney Stone Disease Thermobalancing Therapy with Dr Allen’s Device: Key to Successful Ageing Without Medications, Surgery, and Risky Exposure to Coronavirus Infection. Nephro-Urol Mon. 2021;13:e110771. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Suzuki H, Aono S, Inoue S, Imajo Y, Nishida N, Funaba M, Harada H, Mori A, Matsumoto M, Higuchi F, Nakagawa S, Tahara S, Ikeda S, Izumi H, Taguchi T, Ushida T, Sakai T. Clinically significant changes in pain along the Pain Intensity Numerical Rating Scale in patients with chronic low back pain. PLoS One. 2020;15:e0229228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 16. | Hashizume H, Konno S, Takeshita K, Fukui M, Takahashi K, Chiba K, Miyamoto M, Matsumoto M, Kasai Y, Kanamori M, Matsunaga S, Hosono N, Kanchiku T, Taneichi H, Tanaka N, Kanayama M, Shimizu T, Kawakami M. Japanese orthopaedic association back pain evaluation questionnaire (JOABPEQ) as an outcome measure for patients with low back pain: reference values in healthy volunteers. J Orthop Sci. 2015;20:264-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 971] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 18. | Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine (Phila Pa 1976). 2005;30:1331-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 885] [Cited by in RCA: 1039] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 19. | Fukui M, Chiba K, Kawakami M, Kikuchi S, Konno S, Miyamoto M, Seichi A, Shimamura T, Shirado O, Taguchi T, Takahashi K, Takeshita K, Tani T, Toyama Y, Yonenobu K, Wada E, Tanaka T, Hirota Y; Subcommittee of the Clinical Outcome Committee of the Japanese Orthopaedic Association on Low Back Pain and Cervical Myelopathy Evaluation. JOA Back Pain Evaluation Questionnaire (JOABPEQ)/JOA Cervical Myelopathy Evaluation Questionnaire (JOACMEQ). The report on the development of revised versions. April 16, 2007. The Subcommittee of the Clinical Outcome Committee of the Japanese Orthopaedic Association on Low Back Pain and Cervical Myelopathy Evaluation. J Orthop Sci. 2009;14:348-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Knezevic NN, Candido KD, Vlaeyen JWS, Van Zundert J, Cohen SP. Low back pain. Lancet. 2021;398:78-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 665] [Article Influence: 166.3] [Reference Citation Analysis (0)] |
| 21. | Husky MM, Ferdous Farin F, Compagnone P, Fermanian C, Kovess-Masfety V. Chronic back pain and its association with quality of life in a large French population survey. Health Qual Life Outcomes. 2018;16:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 22. | Yao M, Xu BP, Li ZJ, Zhu S, Tian ZR, Li DH, Cen J, Cheng SD, Wang YJ, Guo YM, Cui XJ. A comparison between the low back pain scales for patients with lumbar disc herniation: validity, reliability, and responsiveness. Health Qual Life Outcomes. 2020;18:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Ouchi K, Watanabe M, Tomiyama C, Nikaido T, Oh Z, Hirano T, Akazawa K, Mandai N. Emotional Effects on Factors Associated with Chronic Low Back Pain. J Pain Res. 2019;12:3343-3353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Chan CW, Mok NW, Yeung EW. Aerobic exercise training in addition to conventional physiotherapy for chronic low back pain: a randomized controlled trial. Arch Phys Med Rehabil. 2011;92:1681-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Alturkistani LH, Hendi OM, Bajaber AS, Alhamoud MA, Althobaiti SS, Alharthi TA, Atallah AA. Prevalence of Lower Back Pain and its Relation to Stress Among Medical Students in Taif University, Saudi Arabia. Int J Prev Med. 2020;11:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Traeger AC, Buchbinder R, Elshaug AG, Croft PR, Maher CG. Care for low back pain: can health systems deliver? Bull World Health Organ. 2019;97:423-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 27. | Adjani A, Allen S. The at-home delivery of treatment for benign prostate enlargement and chronic prostatitis enabled by Dr Allen’s Device is a valuable healthcare innovation during a pandemic. Medico Research Chronicles. 2021;8:334-344. [DOI] [Full Text] |
| 28. | Allen S. Innovative Thermobalancing therapy and Dr Allen’s Device for the first time employ body energy to treat chronic prostatic diseases effectively. Int J Qual Innovation. 2020;6. [DOI] [Full Text] |
| 29. | Allen S. The Vascular Factor Plays the Main Role in the Cause of Pain in Men with Chronic Prostatitis and Chronic Pelvic Pain Syndrome: The Results of Clinical Trial on Thermobalancing Therapy. Diseases. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Allen S, Aghajanyan I. Use of thermobalancing therapy in ageing male with benign prostatic hyperplasia with a focus on etiology and pathophysiology. Aging Male. 2017;20:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |