Published online Aug 18, 2022. doi: 10.5312/wjo.v13.i8.760
Peer-review started: October 15, 2021
First decision: January 11, 2022
Revised: February 4, 2022
Accepted: August 5, 2022
Article in press: August 5, 2022
Published online: August 18, 2022
Processing time: 304 Days and 21.2 Hours
Alpha-defensin has been widely studied for the diagnosis of periprosthetic joint infection (PJI). However, there is a lack of detailed information regarding the proper laboratory technique of the enzyme-linked immunosorbent assay (ELISA) method, such as sample dilution.
To assess the influence of dilution in the synovial fluid during ELISA for the diagnosis of knee PJI; and determine which dilution presents a better performance.
Forty samples of synovial fluid from arthroplasty knees were included, 17 in the infected group and 23 in the aseptic group, according to Musculoskeletal Infection Society criteria. Initially, five synovial fluid samples from each group were assessed for quantitative analysis of alpha-defensin using ELISA. Different dilution ratios (1:10, 1:100, 1:500, 1:1000 and 1:5000) were tested based on the predetermined cutoff value of 5.2 mg/L. The dilutions that performed better were used to compare the results of all samples.
For infected cases, a gradual increase in the dilution of synovial fluid samples led to an equivalent increase in alpha-defensin level. The same was not observed in the aseptic cases. Both 1:1000 and 1:5000 dilutions presented satisfactory results to differentiate infected and aseptic cases. Further analyses were performed using 1:1000 and 1:5000 for all 40 samples. The 1:1000 dilution resulted in a sensitivity of 88.2% (95%CI, 66%-98%) and specificity of 95.7% (95%CI, 79%-99%), whereas the 1:5000 dilution presented a sensitivity of 94.1% (95%CI, 73%-99%) and a specificity of 100% (95%CI, 86%-100%).
The synovial fluid dilution had an important influence on the alpha-defensin ELISA results. Dilutions of 1:5000 showed the best performance for the diagnosis of knee PJI. The results of this study set the basis for a more reliable and reproducible alpha-defensin ELISA during the investigation of PJI, contributing to the expansion of this technique in different treatment centers worldwide.
Core Tip: Alpha-defensin is an antimicrobial peptide widely studied in patients with periprosthetic joint infection. Indeed, the analysis of alpha-defensin concentration in the synovial fluid by enzyme-linked immunosorbent assay (ELISA) has been gaining ground. However, there is a lack of information regarding the detailed technique for synovial fluid ELISA, particularly in regard to its dilution. Therefore, this study analyzed the influence of dilution in synovial fluid samples for the alpha-defensin ELISA method. We presume that this novel information may be helpful to make ELISA more reproducible and widely accessible for different treatment centers worldwide.
- Citation: Abdo RCT, Gobbi RG, Leite CBG, Pasoto SG, Leon EP, Lima ALLM, Bonfa E, Pécora JR, Demange MK. Quantitative alpha-defensin testing: Is synovial fluid dilution important? World J Orthop 2022; 13(8): 760-767
- URL: https://www.wjgnet.com/2218-5836/full/v13/i8/760.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i8.760
Periprosthetic joint infection (PJI) is an increasingly common and devastating complication that can lead to significant morbidity and mortality[1,2]. Since neither clinical examination nor laboratory tests are always trustworthy, the diagnosis of PJI remains a challenge[3]. Indeed, there is no gold-standard method for its diagnosis[4]. Therefore, several groups around the world have attempted to refine the diagnostic criteria of PJI[2,5,6]. The criteria score proposed (and later updated) by the Musculoskeletal Infection Society (MSIS) is the one most used by orthopedic surgeons[2].
Regarding the different strategies to improve the diagnosis of PJI, the use of biomarkers has been extensively studied in recent years[1,7,8]. Alpha-defensin has gained attention as one of the most reliable markers[9,10]. Defensins are antimicrobial peptides that act as part of the host's innate immune response against pathogen invasion and are produced in increased numbers during septic inflammation[11,12]. Thus, synovial-fluid alpha-defensin has shown excellent results in diagnosing PJI[13,14]. Two different modalities are available to measure synovial alpha-defensin: The qualitative alpha-defensin lateral flow test and the quantitative enzyme-linked immunosorbent assay (ELISA)[15]. Particularly, the ELISA method has presented the best results, so it was included as a criterion in the new International Consensus on Orthopedic Infections 2018 consensus[6].
Despite the importance and the many studies about alpha-defensin in synovial fluid[15,16], there is a lack of detailed information regarding the ELISA technique[13]. In fact, some crucial information about the reproducibility of the test and the standardization of specific cutoff values are unclear. Dilution of the synovial fluid sample is necessary for the test, but the ideal ratios are not known.
Therefore, this study aimed to evaluate the potential influence of dilution on the quantitative alpha-defensin ELISA test for the diagnosis of PJI in the synovial fluid of patients undergoing total knee arthroplasty (TKA). We hypothesized that changes in the dilution of the joint fluid would influence the test performance for the diagnosis of PJI.
The study was approved by the local ethics committee (No. 71039317.3.0000.0068). All the included patients signed the consent form.
Between January 2017 and December 2018, this study enrolled patients with primary TKA and any suspicious signs or symptoms of chronic infection, as follows: more than three months of persistent knee pain, effusion or local heat, presence of a draining sinus, early failure of the prosthesis (less than 5 years), fever and malaise. Patients who had used antibiotic therapy for at least four weeks before the evaluation or had an insufficient volume of synovial fluid during knee aspiration were excluded. Forty-five patients were assessed for recruitment. Of these, five did not have enough synovial fluid and were excluded from the study. The other 40 patients were included. They were divided into two groups: infected and noninfected (aseptic painful). Seventeen (42.5%) patients were considered to have infection, and 23 (57.5%) patients were classified as having aseptic painful TKA. Positive infection was confirmed if the MSIS criteria for PJI were fulfilled[17], as follows: presence of a draining sinus communicating with the joint or at least two positive cultures of the same microorganism, or if three out of the following five criteria were present: elevated serum C-reactive protein and erythrocyte sedimentation rate, elevated synovial white blood cell count, elevated percentage of synovial polymorphonuclear leukocytes (PMNs), positive histological analysis of periprosthetic tissue and a single positive culture of synovial fluid or periprosthetic tissue. For chronic PJI, synovial cell count and differential cutoff values were 3.000 cells/μL, 80% PMN.
Synovial fluid was collected from each patient. Using a sterile technique, the lateral suprapatellar approach was performed, and a 20-mL syringe with a 21-gauge needle was used to aspirate the joint fluid. A minimum of 0.5 mL was aspirated and stored in a tube with ethylenediaminetetraacetic acid (EDTA). The tubes were transported to the laboratory within one hour after aspiration at room temperature. Previous studies have estimated the stability of alpha-defensin levels in synovial fluid for a minimum of 48 h at room temperature[13]. The fluid was centrifuged for ten minutes at 2000 rpm according to our laboratory standardization, and the supernatant was frozen at -80°C until use[13].
Reagents from the commercially available ELISA test kit (Hycult Biotech®, Uden, The Netherlands) were used according to the manufacturer’s instructions. However, as anticipated, there was no manufacturer recommendation for synovial fluid dilution. In this study, ten samples (five from each group) were randomly selected and diluted in the buffer included in the kit at 1:10, 1:100, 1:500, 1:1000 and 1:5000. All dilutions were tested in duplicate. The reactions were read at 450 nanometers in an ELISA SpectraMax 190 reader (Molecular Devices, San Jose, United States) in accordance with the package insert instructions. The results of spectrophotometry were obtained in optical density (OD) units. The OD values were plotted on the vertical axis, and the corresponding concentrations were plotted on the horizontal axis (logarithmic scale). The concentration was multiplied by the dilution factor to obtain the actual alpha-defensin value in mg/L. A cutoff value of 5.2 mg/L was used since the same value was used as the cutoff in previous studies[13,18].
Initially, ten out of 40 cases were selected to define which dilutions would provide the best performance. After detecting which dilutions performed better, we expanded the analysis to all other cases. All samples were tested with the selected dilution ratio, following the same procedures as described above except for the serial dilution.
Statistical analysis was performed using GraphPad Prism version 8 (GraphPad Software, La Jolla, CA). The results of the alpha-defensin synovial fluid are expressed as mean and range. Differences between the two groups were compared using the Mann–Whitney test. To assess the performance of the test using 1:1000 and 1:5000 dilutions, the sensitivity, specificity, positive predictive value and negative predictive value were evaluated through the Wilson-Brown method. The 95% confidence interval (95%CI) was also calculated. P < 0.05 was considered to be statistically significant.
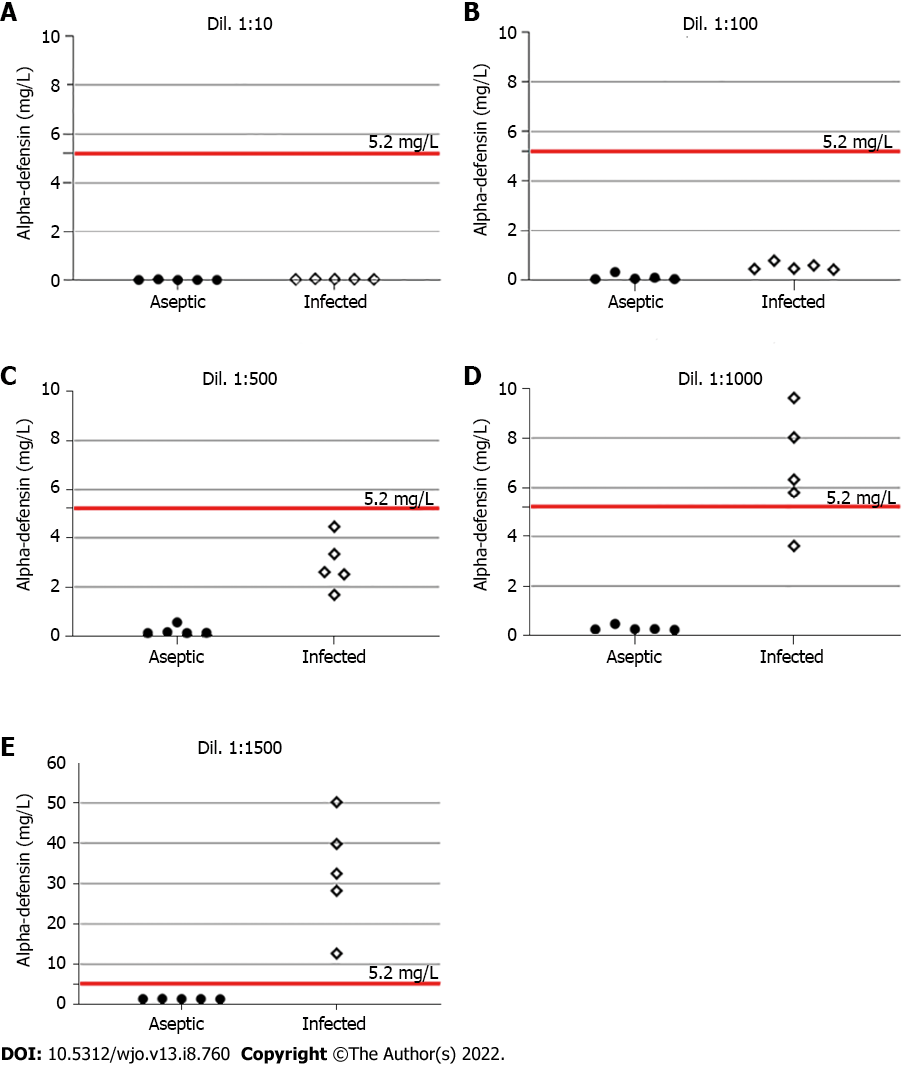
Table 1 shows the demographic and laboratory results of the included patients. Regarding the initial assessment, five samples from each group were tested using different dilutions. In the infected group, a gradual increase in synovial fluid dilution resulted in an equivalent elevation of alpha-defensin levels. The same response was not observed in the aseptic group (Figure 1). In addition, the best diagnostic performance of the test was obtained at 1:1000 and 1:5000 dilutions. Table 2 summarizes these findings.
| Total | Aseptic cases | Infected cases | |
| Sex | |||
| Male | 9 | 4 | 5 |
| Female | 31 | 19 | 12 |
| Age (range) | 67.57 (47-85) | 67.65 (47-85) | 67.47 (51-84) |
| Laterality | |||
| Right knee | 21 | 13 | 8 |
| Left knee | 19 | 10 | 9 |
| Inflammatory disease | |||
| RA | 8 | 5 | 3 |
| Gout | 2 | 0 | 2 |
| Major Criteria | |||
| Sinus Tract | 4 | 0 | 4 |
| 2 positive cultures | 2 | 0 | 2 |
| Minor criteria | |||
| CRP and ESR + | 9 | 0 | 9 |
| WBC count | 15 | 1 | 14 |
| PMN% | 14 | 0 | 14 |
| Hystological + | 5 | 0 | 5 |
| 1 culture | 12 | 1 | 11 |
| Dil. 1:10, mean (range) | Dil. 1:100, mean (range) | Dil. 1:500, mean (range) | Dil. 1:1000, mean (range) | Dil. 1:5000, mean (range) | |
| Infected cases | 0.04 (0.03-0.05) | 0.52 (0.40-0.76) | 2.92 (1.68-4.45) | 6.68 (3.62-9.62) | 32.67 (12.65-50.18) |
| Aseptic cases | 0.01 (0-0.03) | 0.09 (0.02-0.30) | 0.21 (0.12-0.55) | 0.3 (0.24-0.48) | 1.43 (1.4-1.49) |
| P | 0.016 | 0.008 | 0.008 | 0.008 | 0.008 |
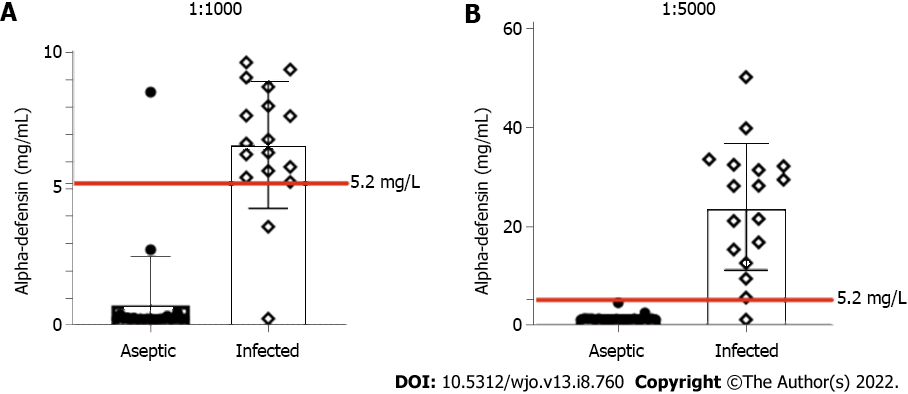
We next evaluated all of the remaining samples using these dilution ratios. The results of the alpha-defensin tests including all patients are displayed in Table 3 and Figure 2. At a dilution of 1:1000, statistical analysis indicated a sensitivity of 88.2% (95%CI, 66%-98%), a specificity of 95.7% (95%CI, 79%-99%), a positive predictive value of 93.8% (95%CI, 72%-99%) and a negative predictive value of 91.7% (95%CI, 74%-99%). In this situation, one false positive and two false negatives were found.
| Number of cases | Dilution of 1:1000 | Dilution of 1:5000 | |
| Infected cases | 17 (42.5%) | 6.60 (0.26-9.62) | 24.10 (5.72-50.18) |
| Aseptic cases | 23 (57.5%) | 0.74 (0.24-8.54) | 1.50 (1.22-4.66) |
| P | < 0.001 | < 0.001 |
At the 1:5000 dilution, a sensitivity of 94.1% (95%CI, 73%-99%), a specificity of 100% (95%CI, 86%-100%), a positive predictive value of 100% (95%CI, 81%-100%) and a negative predictive value of 95.8% (95%CI, 80%-99%) were observed. Only one false-negative result was identified within the infected group. This misdiagnosed case presented with a draining sinus and had positive intraoperative cultures.
To the best of our knowledge, there is a lack of studies detailing and testing the influence of different dilutions on the synovial fluid alpha-defensin ELISA test for the diagnosis of TKA infection. The novel results of this laboratory study support our hypothesis that the right synovial fluid dilution is crucial for a high diagnostic accuracy of the alpha-defensin ELISA test. Here, we found positive results using dilutions of 1:1000 and 1:5000, the best results occurring with a 1:5000 dilution ratio.
As mentioned above, in one of the first studies of synovial fluid alpha-defensin, a cutoff value of 5.2 mg/L of alpha-defensin was defined as the threshold for the diagnosis of PJI[19]. However, the dilutions applied to obtain this cutoff value were not described, making it hard to replicate the methods, even using the same alpha-defensin ELISA kit. In our study, using a 5.2 mg/L cutoff, the most effective dilution to distinguish between infection and noninfection was 1:5000. Using this dilution ratio, only one false-negative result was found. Similar to other patients[10,13], this patient presented with a drainage sinus, though our patient had positive intraoperative cultures, which was not seen in that other series. We also showed that although a dilution of 1:1000 had satisfactory results for detecting infected cases, it had lower sensitivity and specificity.
Another interesting consideration relates to the prozone phenomenon. The prozone phenomenon, also known as the hook effect, is a false-negative response that occurs in immunological tests as a consequence of an excess of either antigen or antibody[20]. In an alpha-defensin test, if the amount of antigen (i.e., alpha-defensin) is greater than the amount of antibody, the secondary antibody (i.e., peroxidase) will not bind properly, and the test will present a false result. This could be the reason for some false-negative values during the alpha-defensin assay. In this study, we did not directly assess the influence of dilution on the prozone phenomenon, but since lower dilutions are more likely to exhibit the prozone effect, the latest dilutions may be helpful to overcome this phenomenon[21], potentially allowing a more precise differentiation between TKA infection and asepsis.
Some limitations of our study must be considered. The small sample size may underpower the study. However, the consistent findings with different dilutions and the excellent results using a 1:5000 dilution reduced the need to increase the sample size. In addition, despite the exciting progressive improvement in the test performance with serial dilutions, we did not demonstrate the actual reason why it occurs. Further studies are necessary to answer this question. Even so, we describe in detail the analysis of synovial fluid alpha-defensin by ELISA, including the best dilutions for the most accurate diagnosis of knee PJI. Moreover, the information provided herein may ensure the reproducibility of the test across the laboratories, with more precise data.
Changes in synovial fluid dilution for ELISA had an important influence on the determination of alpha-defensin levels. Based on the cutoff value of 5.2 mg/L, dilutions of both 1:1000 and 1:5000 were adequate to support the diagnosis of TKA infection. The 1:5000 dilution showed the best performance in separating the infection and the aseptic cases, making it the most reliable dilution during knee PJI investigation. Therefore, the results of this study lay the foundation for a more reliable and reproducible alpha-defensin ELISA during the investigation of PJI, contributing to the expansion of this technique to different treatment centers worldwide.
Periprosthetic joint infection (PJI) is a serious complication post-surgery that is associated with substantial morbidity and financial burden to the healthcare system. The alpha-defensin quantitative enzyme-linked immunosorbent assay (ELISA) is one of the most useful methods to investigate PJI.
Although the alpha-defensin ELISA is currently the most reliable test to diagnose PJI, there is a lack of detailed information regarding its proper laboratory technique, including the best sample dilution.
This study aimed to assess the influence of dilution in the synovial fluid during ELISA for the diagnosis of knee PJI and determine which dilution presents a better performance.
In this prospective study, 40 cases of total knee arthroplasty were evaluated: 17 classified as PJI and 23 classified as aseptic knees. Initially, 5 synovial fluid samples from each group were assessed by ELISA using different dilution ratios (1:10, 1:100, 1:500, 1:1000 and 1:5000). The dilutions had better performance were used to compare the results of all samples.
For infected cases, a gradual increase in the synovial fluid dilution led to an equivalent increase in alpha-defensin level, which was not seem in the aseptic cases. Both 1:1000 and 1:5000 dilutions presented satisfactory results to differentiate infected and aseptic cases. Further analyses were performed using 1:1000 and 1:5000 for all 40 samples. The 1:1000 dilution resulted in a sensitivity of 88.2% (95%CI, 66%-98%) and specificity of 95.7% (95%CI, 79%-99%), whereas the 1:5000 dilution presented a sensitivity of 94.1% (95%CI, 73%-99%) and a specificity of 100% (95%CI, 86%-100%).
Synovial fluid dilution appears to influence the alpha-defensin ELISA results. Dilutions of 1:5000 showed the best performance for the diagnosis of knee PJI.
The results of this current study may set the basis for more reliable and reproducible alpha-defensin ELISA during PJI investigation, contributing to the expansion of this technique in different treatment centers worldwide.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hu H, China; Kuiper JW, Netherlands S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Chen Y, Kang X, Tao J, Zhang Y, Ying C, Lin W. Reliability of synovial fluid alpha-defensin and leukocyte esterase in diagnosing periprosthetic joint infection (PJI): a systematic review and meta-analysis. J Orthop Surg Res. 2019;14:453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, Shohat N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J Arthroplasty. 2018;33:1309-1314.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 885] [Cited by in RCA: 1408] [Article Influence: 201.1] [Reference Citation Analysis (0)] |
| 3. | Tan TL, Kheir MM, Shohat N, Tan DD, Kheir M, Chen C, Parvizi J. Culture-Negative Periprosthetic Joint Infection: An Update on What to Expect. JB JS Open Access. 2018;3:e0060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Goswami K, Parvizi J, Maxwell Courtney P. Current Recommendations for the Diagnosis of Acute and Chronic PJI for Hip and Knee-Cell Counts, Alpha-Defensin, Leukocyte Esterase, Next-generation Sequencing. Curr Rev Musculoskelet Med. 2018;11:428-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 5. | Elkins JM, Kates S, Lange J, Lichstein P, Otero J, Soriano A, Wagner C, Wouthuyzen-Bakker M. General Assembly, Diagnosis, Definitions: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34:S181-S185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Shohat N, Bauer T, Buttaro M, Budhiparama N, Cashman J, Della Valle CJ, Drago L, Gehrke T, Marcelino Gomes LS, Goswami K, Hailer NP, Han SB, Higuera CA, Inaba Y, Jenny JY, Kjaersgaard-Andersen P, Lee M, Llinás A, Malizos K, Mont MA, Jones RM, Parvizi J, Peel T, Rivero-Boschert S, Segreti J, Soriano A, Sousa R, Spangehl M, Tan TL, Tikhilov R, Tuncay I, Winkler H, Witso E, Wouthuyzen-Bakker M, Young S, Zhang X, Zhou Y, Zimmerli W. Hip and Knee Section, What is the Definition of a Periprosthetic Joint Infection (PJI) of the Knee and the Hip? J Arthroplasty. 2019;34:S325-S327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 7. | Balato G, de Matteo V, Ascione T, Di Donato SL, De Franco C, Smeraglia F, Baldini A, Mariconda M. Laboratory-based versus qualitative assessment of α-defensin in periprosthetic hip and knee infections: a systematic review and meta-analysis. Arch Orthop Trauma Surg. 2020;140:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Bonanzinga T, Ferrari MC, Tanzi G, Vandenbulcke F, Zahar A, Marcacci M. The role of alpha defensin in prosthetic joint infection (PJI) diagnosis: a literature review. EFORT Open Rev. 2019;4:10-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472:4006-4009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | Bonanzinga T, Zahar A, Dütsch M, Lausmann C, Kendoff D, Gehrke T. How Reliable Is the Alpha-defensin Immunoassay Test for Diagnosing Periprosthetic Joint Infection? Clin Orthop Relat Res. 2017;475:408-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 11. | Chalifour A, Jeannin P, Gauchat JF, Blaecke A, Malissard M, N'Guyen T, Thieblemont N, Delneste Y. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104:1778-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 1030] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 13. | Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-Defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96:1439-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 14. | Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin Accuracy to Diagnose Periprosthetic Joint Infection-Best Available Test? J Arthroplasty. 2016;31:456-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Eriksson HK, Nordström J, Gabrysch K, Hailer NP, Lazarinis S. Does the Alpha-defensin Immunoassay or the Lateral Flow Test Have Better Diagnostic Value for Periprosthetic Joint Infection? Clin Orthop Relat Res. 2018;476:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Marson BA, Deshmukh SR, Grindlay DJC, Scammell BE. Alpha-defensin and the Synovasure lateral flow device for the diagnosis of prosthetic joint infection: a systematic review and meta-analysis. Bone Joint J. 2018;100-B:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Parvizi J, Gehrke T; International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29:1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 691] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 18. | Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Booth RE Jr, Parvizi J. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473:198-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 19. | Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472:3254-3262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 20. | Hoofnagle AN, Wener MH. The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J Immunol Methods. 2009;347:3-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 416] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 21. | Kim HS, Choi AR, Yang M, Oh EJ. EDTA Treatment for Overcoming the Prozone Effect and for Predicting C1q Binding in HLA Antibody Testing. Ann Lab Med. 2019;39:572-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |