Published online Jun 18, 2022. doi: 10.5312/wjo.v13.i6.587
Peer-review started: January 18, 2022
First decision: March 24, 2022
Revised: April 4, 2022
Accepted: May 7, 2022
Article in press: May 7, 2022
Published online: June 18, 2022
Processing time: 149 Days and 7.4 Hours
The evaluation of the efficacy of platelet-rich plasma (PRP) in clinical practice yields conflicting results and raises numerous controversies. This may be due to different concentrations of biologically active components in PRP obtained with the use of different methods of gravity separation.
To compare the content, repeatability and correlations between biologically active components in PRP obtained with four different commercial systems.
From a whole blood sample of each of 12 healthy male volunteers, 4 PRP samples were prepared using 4 different commercial kits [Arthrex Autologous Condi
Significantly higher concentrations of platelets (PLT), white blood cells (WBC) and red blood cells (RBC) were found in PRP obtained with the use of Mini GPS III than in PRP obtained using other systems. Significant differences in the content of growth factors and cytokines in PRP were found. A positive correlation of the amount of PLT, RBC and WBC with the concentration of most of the growth factors was found but in only three inflammatory cytokines. The obtained correlations between blood cell components and cytokines differed between the systems in terms of statistical significance, which may be due to insufficient sample size. The repeatability of the obtained PLT concentration also varied between protocols with the lowest in Xerthra and the highest in Arthrex ACP.
Significant differences in the content of biologically active components and their repeatability were found in PRP obtained by various methods, providing new data for further research.
Core Tip: The presented study showed important differences between blood cell components and levels of selected growth factors and inflammatory cytokines in platelet-rich plasma obtained with four different commercial preparation systems in a single-donor model. The range of cytokines analyzed far exceeded the ranges investigated in earlier publications. This was also the first study to pay attention to the repeatability of the quality of the obtained platelet-rich plasma (PRP). New positive correlations were found between platelet content in PRP and several cytokines (Hepatocyte growth factor, Interleukin-1β, Monocyte chemoattractant protein-1, Interleukin-8, Interleukin-18). The demonstrated positive correlation between red blood cell content in PRP and cytokines has never been described before.
- Citation: Dejnek M, Witkowski J, Moreira H, Płaczkowska S, Morasiewicz P, Reichert P, Królikowska A. Content of blood cell components, inflammatory cytokines and growth factors in autologous platelet-rich plasma obtained by various methods. World J Orthop 2022; 13(6): 587-602
- URL: https://www.wjgnet.com/2218-5836/full/v13/i6/587.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i6.587
Autologous platelet-rich plasma (PRP) therapy is a method known for many years used in the treatment of various diseases. Nowadays, despite numerous controversies, it is widely used, especially in aesthetic medicine, orthopedics and sports medicine[1-6]. The method involves preparation from the patient's blood sample of plasma with a concentration of platelets higher than physiological and then its injection into pathological tissues[2,6-8].
In the human body, platelets are involved in the repairing processes, inter alia, by releasing different cytokines including growth factors from their α granules[2,6,9-11]. Inflammatory cytokines have also an important role in the initiation of the healing process, chemotaxis and keratinocyte proliferation but their excess can impair regeneration[12].
The essence of treatment with PRP is to accelerate the repairing processes by delivering a high concentration of platelet-derived growth factors and other cytokines directly to the affected area[6,13]. Attempts have been made to use PRP for treating diseases in which the repairing processes are impaired or when PRP was expected to accelerate healing as much as possible. This applies especially to chronic overuse injuries such as enthesopathies or tendinopathies, to the acceleration of the healing process of many different sports injuries of ligaments, tendons and muscles but also to bone union disorders and hard-healing wounds[6,13-16]. Intraarticular PRP injections used in osteoarthritis may be beneficial in the alleviation of chronic pain helping to increase the physical activity of patients[4,17-19].
The production of autologous platelet-rich plasma using commercial kits is a fast, convenient, affordable and safe way to obtain high concentrations of the desired growth factors[13,20]. By now it is known that too low platelet concentration is insufficient to induce a tissue response but too high a concentration can even have some negative impact on tissue healing[21,22].
There is an increasing number of manufacturers providing commercial kits that enable the quick, easy and safe preparation of PRP in an outpatient setting. Such kits differ from one another by some parameters such as the amount of material collected from the patient, the type of anticoagulant, the structure of the separator, the time and speed of centrifugation, the method of extraction and activation of the obtained plasma. They also differ in the assumed concentration that they allow to obtain and the presence of leukocytes in the final product[2,20,23-25]. Such a multitude of variables makes it impossible to reliably assess the effectiveness of therapies with different autologous platelet-rich plasma preparations without providing detailed information[21]. Furthermore, only a small number of studies on relatively small populations have shown significant differences in the desired cytokine content by testing only a few from many commercially available PRP systems. Despite many years of clinical use of PRP, there is still a remarkable scarcity of relevant and reliable information about its biologically active compounds. This lack of information was the main reason for the previous preliminary study conducted by the present authors[26]. Its results encouraged the authors to expand the study with a larger population and a greater amount of analyzed cytokines.
The goal of this study was to compare the content of blood cell components, selected inflammatory cytokines and selected growth factors in PRP samples obtained with the use of various commercial kits, to find any correlations between those biologically active components and to establish their repeatability within each method.
The study was designed as a single-center prospective non-randomized case-series descriptive laboratory study and was conducted in a medical university laboratory in the autumn of 2020 by clinicians from the Department of Trauma and Hand Surgery and the Department of Sports Medicine in cooperation with laboratory researchers from the Faculty of Pharmacy.
It was conducted in accordance with the standards of Good Clinical Practice. The study was approved by the institutional bioethics committee. All participants signed informed consent to participate in the study. The study followed the Minimum Information for Studies Evaluating Biologics in Orthopedics (MIBO) guidelines[27].
The study involved 12 healthy male volunteers, aged 24-35 years (mean ± SD, 28.92 ± 3.15 y.o.). The mean height, body weight and body mass index were 182.42 ± 5.14 cm, 86.92 ± 8.73 kg and 26.16 ± 2.83 kg/m², respectively. The inclusion criteria were: the age of 20-35 years, the absence of serious diseases and conditions that could affect the amount and function of blood cell components (in particular diabetes, blood dyscrasia, inflammatory conditions, cancer, pre-existing joint pathologies). Volunteers who, in the two weeks preceding the study, were taking drugs that may interfere with the function of platelets or may affect the quantity or quality of blood cell components (in particular antiplatelet or anti-inflammatory drugs) were excluded from the study.
Each volunteer, after signing the informed consent, was asked to complete a questionnaire with personal information about age, height, weight, diseases, medications, sports activities and nicotine addiction. A vast majority of the respondents (91.67%) declared practicing sports at least once a week, and none of them regularly smoked cigarettes.
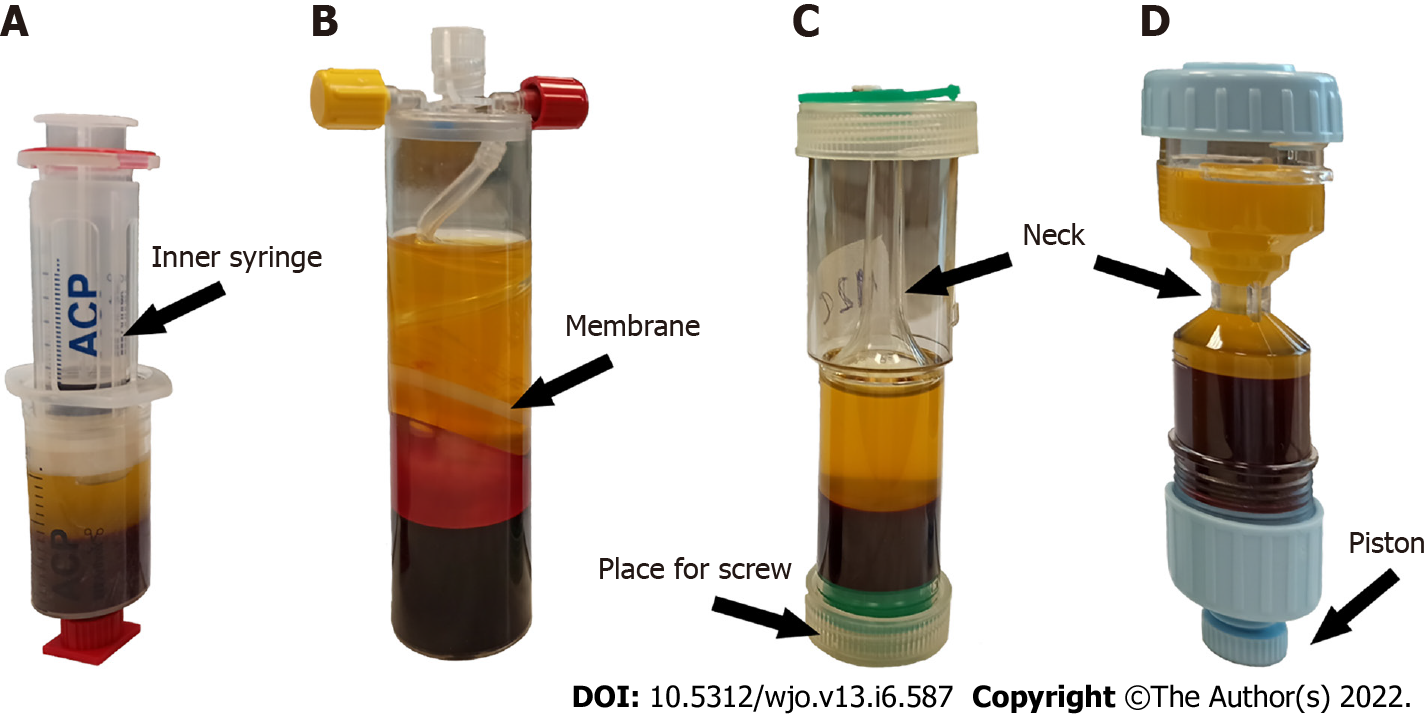
Four commercially available systems for the preparation of PRP intended for use in orthopedics and sports medicine were selected: the Arthrex Autologous Conditioned Plasma (ACP) Double Syringe System (Arthrex Inc., United States), the Mini GPS III Platelet Concentration System (Biomet Inc., United States), the Xerthra PRP kit (Biovico Sp. z o.o., Poland), Dr. PRP (Rmedica, Republic of Korea).
Following the principles of asepsis, 74 mL of whole blood was collected and divided immediately into:
- 2 mL to a probe with ethylenediaminetetraacetic acid (EDTA).
- 13.5 mL to a double-syringe with 1.5 mL of anticoagulant citrate dextrose solution A (ACD-A) from Arthrex ACP.
- 27 mL to a syringe with 3 mL of ACD-A for the Mini GPS III kit.
- 13.5 mL to a syringe with 1.5 mL of 3.13% sodium citrate for the Xerthra kit.
- 18 mL to a syringe with 2 mL of 3.13% sodium citrate for the Dr. PRP kit.
The four samples of liquid-form PRP were prepared simultaneously from blood obtained from each volunteer using 4 different commercial kits according to the manuals provided by the manufacturers. The main characteristics of the PRP protocols are listed in Table 1 and illustrated in Figure 1.
| Arthrex ACP | Mini GPS III | Xerthra | Dr. PRP | |
| Amount of blood collected | 13.5 mL | 27 mL | 13.5 mL | 18 mL |
| Final amount of PRP | 4 mL | 3 mL | 1.5 mL | 3 mL |
| Drawn blood/obtained PRP | 3.38:1 | 9:1 | 9:1 | 6:1 |
| Anticoagulant type | ACD-A | ACD-A | 3.13% SOD CITR | 3.13% SOD CITR |
| Anticoagulant amount | 1.5 mL | 3 mL | 1.5 mL | 2 mL |
| Number of spins | 1 | 1 | 1 | 1 |
| RPM | 1500 | 3200 | 3500 | 3100 |
| Centrifugation time | 5’ | 15’ | 5’ | 4’ |
| Removal of PPP | no | yes | yes | yes |
| Expected WBC content | low | high, 5x | low | low |
| Expected PLT concentration | 2-3 x | 9.3 x | 2-13 x | 4-5 x |
| Dedicated centrifuge | Yes | Yes | Yes | Yes |
| Activation method | In vivo | In vivo | In vivo | In vivo |
| Type of PRP | Conditioned Plasma | LR-PRP | LP-PRP | LP-PRP |
First, the whole blood samples collected for EDTA were analyzed. The time between blood draw, PRP processing, extraction and activation did not exceed 1 h. All preparations were conducted in daylight and at room temperature. The whole blood count and blood cell composition of PRP samples were analyzed using an automatic laboratory analyzer Mindray BC-5150 (Shenzhen Mindray Bio-Medical Electronics Co., PRC) which needs 20 µL for each analysis. Immediately after PRP preparation, each sample was transferred into Eppendorf polypropylene tubes and then shaken gently for 30 s directly before analysis. Platelet capture efficiency (PCE) was calculated with the formula below, described previously by J. Magalon[23].
PCE (%) = [Volume of PRP obtained (mL) × platelet concentration in PRP (G/L)]/[Net volume of whole blood collected (mL) × platelet concentration in whole blood (G/L)].
The remaining PRP (1 ml) was dispensed into Eppendorf polypropylene tubes and then activated through a double freeze-thaw process (30 min for each step) according to the procedure described by R. Zimmermann[25]. The activated samples were frozen to the temperature of -80 °C and stored for further analysis.
The samples were thawed to room temperature and centrifuged for 5 min at 2500 revolutions per minute in a Micro Star 17 microcentrifuge (VWR International Company, Thermo Electron LED, Germany) immediately before performing the composition analysis of selected cytokines using flow cytometry.
A LEGENDplexTM Custom Human 7-plex Panel (BioLegend, United States) was used to quantify the following platelet growth factors:
- Transforming Growth Factor-β1 (TGF-β1, free active).
- Epidermal growth factor (EGF).
- Fibroblast Growth Factor- basic (FGF-basic).
- Vascular endothelial growth factor (VEGF).
- Hepatocyte growth factor (HGF).
- Platelet-Derived Growth Factor-AA (PDGF-AA).
- Platelet-Derived Growth Factor-BB (PDGF-BB).
LEGENDplexTM Human Inflammation Panel 1 (BioLegend, United States) was used to quantitatively measure 13 human inflammatory cytokines:
- Interleukin-1β (IL-1β).
- Interferon-α2 (IFN-α2).
- Interferon-γ (IFN-γ).
- Tumor Necrosis Factor α (TNF-α).
- Monocyte Chemoattractant Protein-1 (MCP-1; CCL2).
- Interleukin-6 (IL-6).
- Interleukin-8 (CXCL8).
- Interleukin-10 (IL-10).
- Interleukin-12p70 (IL-12p70).
- Interleukin-17A (IL-17A).
- Interleukin-18 (IL-18).
- Interleukin-23 (IL-23).
- Interleukin-33 (IL-33).
BioLegend’s LEGENDplexTM assays are bead-based multiplex immunoassays that use fluorescence-encoded beads and flow cytometer measurements. The concentrations of particular cytokines were determined by means of a standard curve generated during the performance of the test. The analyses were conducted according to the manufacturer’s procedure. The samples were acquired on CyFlow SPACE and CyFlow CUBE flow cytometer (Sysmex-Partec, Germany) using a 488 nm laser with a 536/40 (BP) filter for the PE fluorochrome, and a 638 nm laser with 675/20 (BP) for the APC fluorochrome. The results were analyzed with LEGENDplexTM Data Analysis Software V.8.0 (Vigene Tech Inc., United States).
The results were statistically analyzed using Statistica 13.3 software (TIBCO Software Inc, United States). Arithmetic means ± SD were calculated. The Shapiro-Wilk test was used to analyze the compliance of the distribution of the analyzed variables with normal distribution. The Kruskal-Wallis test by ranks (one-way analysis of variance on ranks) with Dunn’s post-hoc test was used to compare the concentrations of platelets (PLT), white blood cells (WBC), red blood cells (RBC) and cytokines between each protocol. Spearman's rank correlation coefficient was used to find any relationship between blood cell components and cytokines. The strength of the correlation was determined for ρ-value: negligible for ρ ≤ 0.2, week for ρ ≤ 0.4, moderate for ρ ≤ 0.6, strong for ρ ≤ 0.8 and very strong for ρ > 0.8. The repeatability of the results obtained for each of the methods was estimated as the coefficient of variation (CV) which is the ratio of the standard deviation to the mean, multiplied by 100%. High repeatability was established as a CV < 25%, moderate as a CV = 25%-44%, weak as a CV = 45%-99% and very weak as a CV > 100%. Statistical significance was established at the level of P ≤ 0.05.
Power analysis for PRP comparison was performed using automatic software calculation for several means one-way ANOVA with the use of Root Mean Square Standardized Effect. The statistical power of the tests was set at 0.8. Based on the magnitude of the effect calculated according to the preliminary study, it was estimated that in order to show significant blood cell component differences between the PRP groups with a test power of 0.8, each group should have a minimum of 6 subjects. Similarly, the population needed to show differences in GF levels differs depending on the factor tested (from 8 to 61 subjects). Group size could not be estimated for differences in inflammatory cytokine levels as there were no previous studies to provide the necessary data. The authors calculated that 48 PRP samples divided into four groups should be sufficient to achieve the assumed goal of the study.
The blood counts of all participants are shown in the Table 2.
| Differential leukocyte | Blood count |
| RBC (1012/L) | 4.97 ± 0.43 |
| PLT (109/L) | 240.67 ± 49.85 |
| WBC (109/L) | 6.49 ± 1.49 |
| Neutrophils (109/L) | 3.79 ± 1.29 |
| Lymphocytes (109/L) | 2.08 ± 0.45 |
| Monocytes (109/L) | 0.45 ± 0.13 |
| Eosinophils (109/L) | 0.14 ± 0.08 |
| Basophils (109/L) | 0.04 ± 0.01 |
The highest concentrations of PLT, WBC and RBC in PRP were obtained with Mini GPS III. Platelet concentration in PRP obtained with Mini GPS III was significantly higher than that obtained with Artherx ACP (P < 0.001), Xerthra (P < 0.001) and Dr. PRP (P < 0.008). The differences between the remaining systems were not significant (P > 0.05). The situation was similar with the ability to concentrate PLT above the baseline with 5.05 × for Mini GPS III which was significantly higher than for other systems, for which it ranged from 1.47 to 2.14 (P < 0.05). Four PRP samples prepared on Xerthra did not exceed the whole blood baseline level of PLT concentration and the other four exceeded it by more than 2 times. Only one sample prepared with Dr. PRP and none of the samples prepared with Mini GPS III and Arthrex failed to exceed the baseline level.
WBC concentration and neutrophil count also significantly differ only when comparing Mini GPS III with other systems (P < 0.005) but they do not differ significantly between those other systems (P > 0.05). Lymphocytes, monocytes, eosinophils and basophils were on a detectable level only in Mini GPS III PRPs.
The highest RBC contamination in the samples was observed for Mini GPS III and it was significantly higher compared to other systems (P < 0.001). RBC concentrations in Arthrex ACP, Xerthra and Dr. PRP were all barely detectable and amounted to 0.05, 0.02 and 0.01 × 1012/L, respectively.
Several means one-way ANOVA power analysis of these multiple comparisons reached levels above 0.99. All blood cell components are shown in Table 3.
| Arthrex ACP | Mini GPS III | Xerthra | Dr. PRP | |
| PLT (109/L) | 357.33 ± 99.01 | 1212.67 ± 268.63 | 455.27 ± 362.92 | 499.75 ± 153.46 |
| WBC (109/L) | 0.87 ± 1.01 | 34.19 ± 11.18 | 1.80 ± 2.55 | 0.60 ± 0.87 |
| Neutrophils (109/L) | 0.87 ± 1.01 | 16.71 ± 9.89 | 1.80 ± 2.55 | 0.60 ± 0.87 |
| RBC (1012/L) | 0.05 ± 0.08 | 1.49 ± 0.86 | 0.02 ± 0.02 | 0.01 ± 0.01 |
| xPLT | 1.47 ± 0.18 | 5.05 ± 0.67 | 1.96 ± 1.71 | 2.14 ± 0.73 |
| xWBC | 0.14 ± 0.17 | 5.27 ± 1.41 | 0.29 ± 0.4 | 0.10 ± 0.16 |
| xRBC | 0.01 ± 0.02 | 0.30 ± 0.17 | 0.00 | 0.00 |
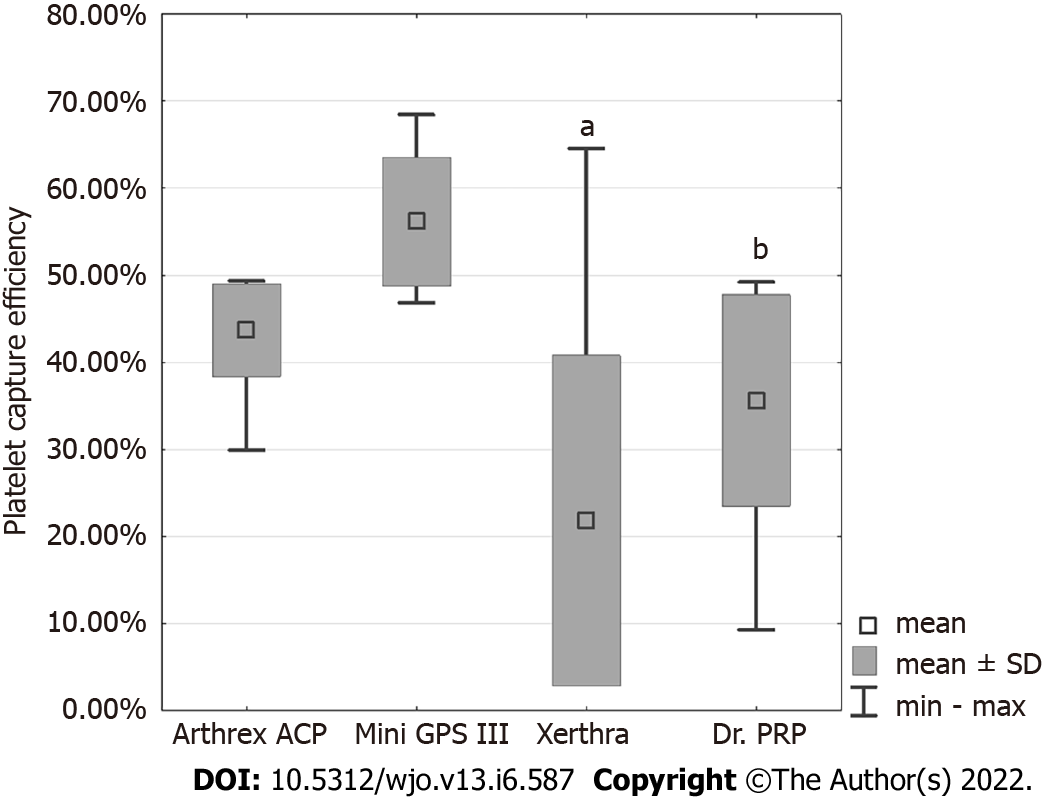
PCE values, in descending order, were obtained for Mini GPS III at 56.15% ± 7.44%, Arthrex ACP at 43.68% ± 5.32%, Dr. PRP at 35.61% ± 12.13% and for Xerthra at 21.79% ± 18.98% (Figure 2). Statistical analysis showed significant differences only between Mini GPS III and Xerthra (P < 0.001) and Dr. PRP (P = 0.001).
The coefficient of variation (CV) showed the highest repeatability of PLT concentrations for Arthrex ACP (12.18%) and the Biomet GPS III system (13.25%). The least predictable PLT concentrations were provided by the Xerthra PRP kit (95.95%). The results of CV for WBC and RBC concentrations seem noteworthy only for LR-PRP obtained for Mini GPS III. The repeatability was moderate for WBC (CV = 26.79%) and weak for RBC (CV = 56%). All CV results are shown in Table 4.
| WBC | RBC | PLT | |
| Arthrex ACP [%] | 114.80 | 175.69 | 12.18 |
| Mini GPS III [%] | 26.79 | 56.83 | 13.25 |
| Xerthra [%] | 149.38 | 133.98 | 95.95 |
| Dr. PRP [%] | 151.45 | 95.10 | 34.05 |
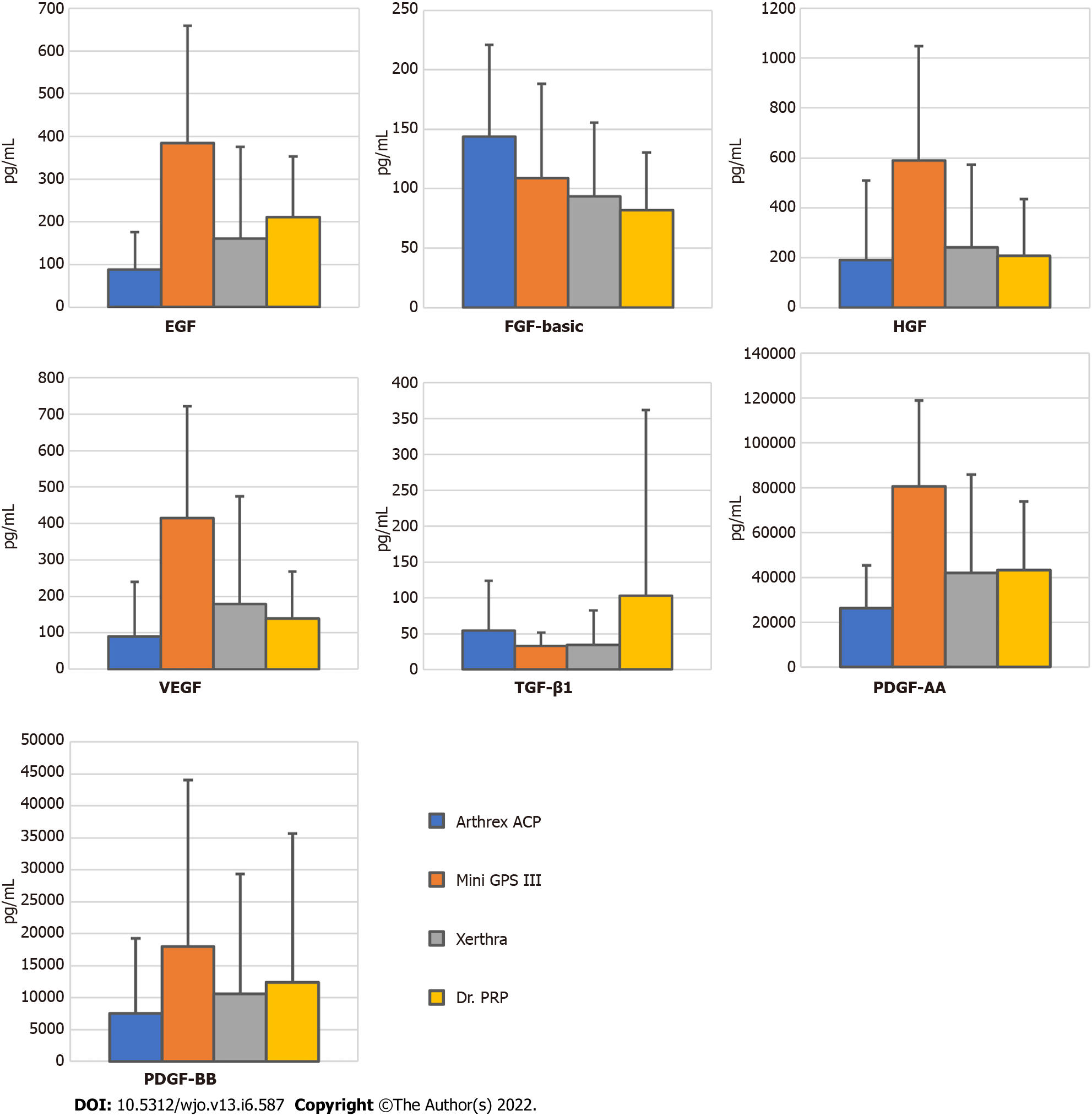
The highest concentrations of EGF, VEGF, HGF, PDGF-AA and PDGF-BB were found in PRP samples obtained with Mini GPS III and the lowest in samples obtained with Arthrex ACP, and the differences for the first four were statistically significant with P values = 0.005, 0.02, 0.01 and 0.006, respectively. A statistically significant difference was also found between Mini GPS III and Xerthra in the concentration of EGF (P = 0.04) and PDGF-AA (P = 0.04). Several means one-way ANOVA power analysis of these multiple comparisons showed insufficient levels (< 0.8) for TGF-β1, FGF-basic, VEGF, HGF and PDGF-BB suggesting the insufficient sample size in this regard. Mean results with standard deviations of all tested growth factors are included in Supplementary Table 1 and highlighted in Figure 3.
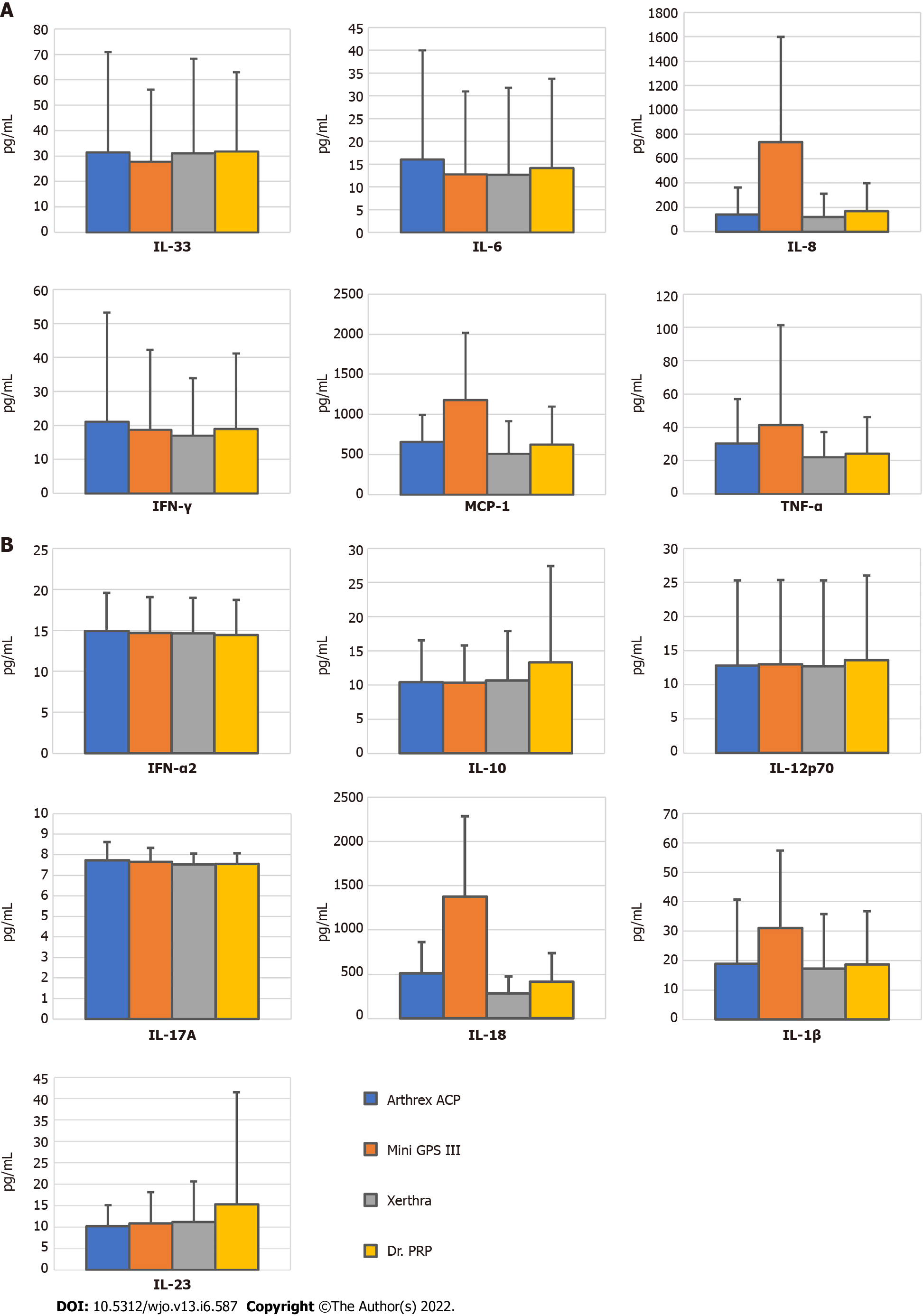
Among all tested inflammatory cytokines, statistically significant differences between systems were found only in the levels of IL-8 and IL-18. IL-8 concentration in PRP obtained with Mini GPS III (734.85 pg/mL) was higher than in that obtained with Arthrex ACP (139.53 pg/mL, P = 0.02) and the Xerthra PRP kit (122.98 pg/mL, P = 0.004). IL18 concentration was the highest in PRP from MiniGPS III (1377 pg/mL) with a significant difference compared to Arthrex ACP (509.41 pg/mL, P = 0.04), the Xerthra PRP kit (283.01 pg/mL, P < 0.001) and Dr. PRP (414.02 pg/mL, P = 0.007). Unfortunately, several means one-way ANOVA power analysis of these multiple comparisons showed levels above 0.8 only for IL-18, which makes correct interpretation of the obtained results much more difficult. Mean results with standard deviations of all the tested cytokines are included in Supplementary Table 1 and highlighted in Figure 4.
Significant positive correlations of PLT, WBC and RBC concentrations with the following growth factors: EGF, VEGF, HGF, PDGF-AA, PDGF-BB were found. Most of them were weak or moderate. A strong Spearman correlation was found between PLT and EGF (ρ = 0.602, P < 0.001), PLT and PDGF-AA (ρ = 0.637, P < 0.001). All correlations are presented in Supplementary Figure 1.
Positive significant correlations of PLT, WBC and RBC concentrations with the following inflammatory cytokines: IL-1β, MCP-1, IL-8, IL-18 were found. A strong Spearman correlation was found only between PLT and IL-18 (ρ = 0.627, P < 0.001). All correlations are presented in Supplementary Figure 1.
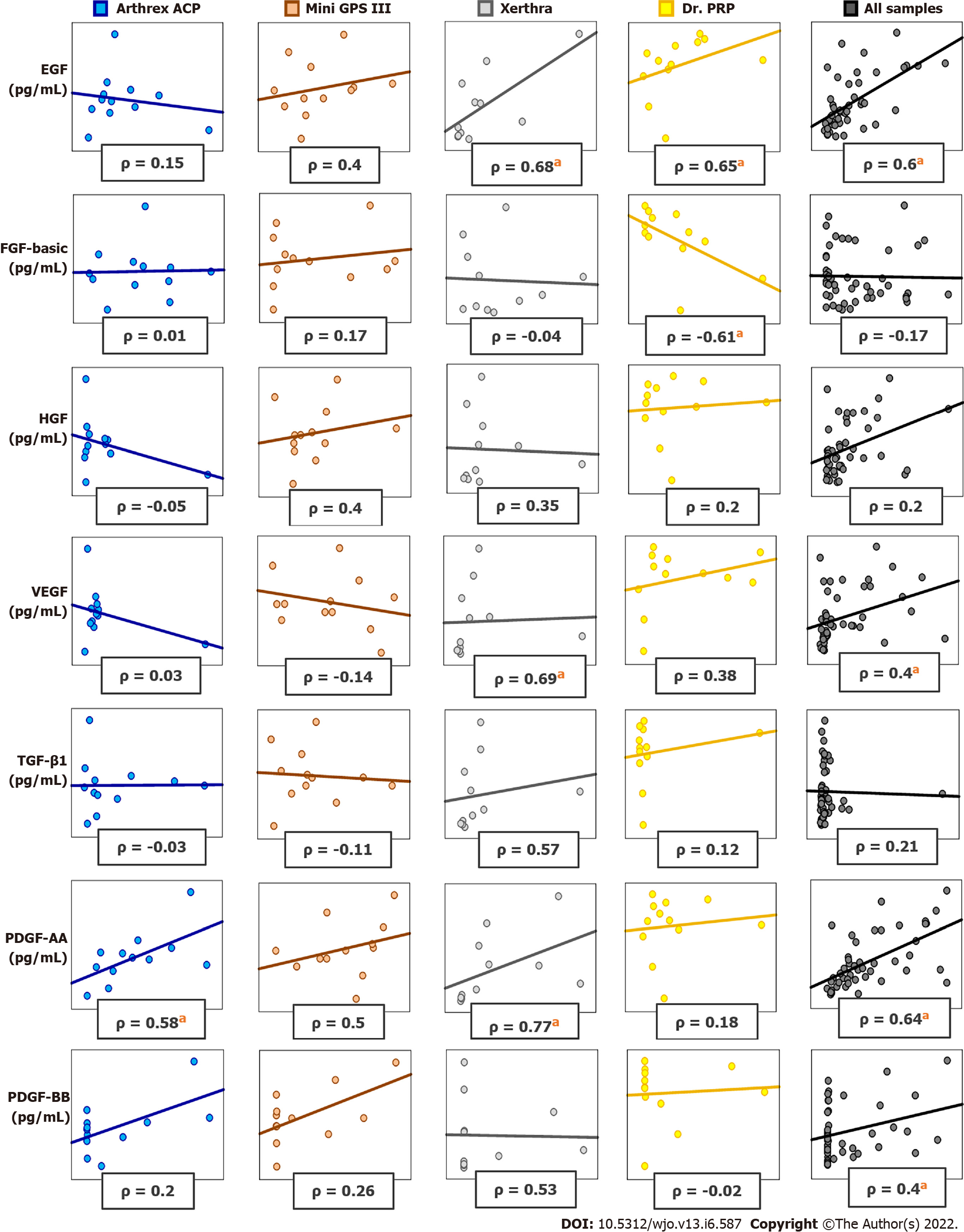
The correlations between blood cell components and growth factors or cytokines in PRP samples vary widely between different systems. In PRP samples obtained with Arthrex ACP, a significant Spearman correlation was found between PLT and PDGF-AA, INF- γ, IL-8. In PRP samples prepared with Mini GPS III System, a significant positive correlation was found between RBC and IL-1β, IL-18. In PRP prepared with Xerthra, a significant positive correlation was found between PLT and EGF, VEGF, PDGF-AA, IL-18; between WBC and TGF-β1, EGF, VEGF, PDGF-AA; between RBC and EGF, VEGF, PDGF-AA. In PRP samples prepared with Dr. PRP Kit, a significant correlation was found between PLT and EGF, FGF-basic; between WBC and IL-18, IL-23. All significant Spearman correlation values are presented in Supplementary Table 2. Selected differences in correlations obtained with different systems are shown in Figure 5.
The results of the study revealed significant differences in PRP obtained with various commercial kits. Other studies had already confirmed the existence of such differences, therefore it was not the essence of the study[20]. The main goal was to highlight these differences, especially in the case of kits that have not been tested in this way before, such as Xerthra and Dr. PRP. Another novelty was the range of the investigated cytokines, significantly exceeding the ranges investigated in previous publications[10,20,23]. This study was also the first to pay attention to the repeatability of the quality of the obtained PRP, which is particularly important when planning a therapy or a clinical trial. This study also showed interesting differences in the results of PRP analysis and the correlations found as compared to the available literature.
It is believed that for therapeutic effect, PRP should have a platelet concentration above the baseline level. All PRP preparation systems were able to produce PRP with the mean platelet concentrations above this level but 4 samples prepared using Xerthra and 1 sample prepared using Dr. PRP did not meet this criterion. It seems to be due to the construction of separators in these systems. Immediately after centrifugation, the PRP is not physically separated from PPP and RBC which is why during the separation and extraction process it is not difficult to partially mix the content. The Arthrex ACP system uses a different approach because after centrifugation it has only two layers - RBC and easily removable plasma. That is probably why the manufacturer called its product Autologous Conditioned Plasma instead of Platelet-Rich Plasma. In his paper, L. Mazzucco considered PLT > 200 × 103/mL as sufficient for therapeutic effect, and all investigated systems reached this value[28]. The US Food and Drug Administration (FDA) requires a platelet concentration of at least 250 × 103/mL for PRP products which was also achieved by all systems. Other authors recommend a PLT concentration of about 1000 × 103[29]. Among systems included in this study, only Mini GPS III meets this criterion.
The content of PLT in PRP differed among systems but this difference was significant only when comparing Mini GPS III to other systems. The results of this study concerning PLT concentration for Mini GPS III and Arthrex ACP were similar to those obtained by other researchers[10,23,30,31]. The present authors found a positive correlation of PLT content with EGF, VEGF, HGF, PDGF-AA, PDGF-BB, IL-1β, MCP-1, IL-8 and IL-18 but not with FGF-basic, TGF-1β and the rest of the investigated inflammatory cytokines. Other authors support the correlation of PLT in PRP with PDGF[20], PDGF, VEGF[10], PDGF-AB, VEGF, EGF[23], PDGF-AB[31]. Contrary to the results presented in this study, J. Magalon and E.A Sundman found a positive correlation of PLT with TGF1β[23,31]. According to the present authors’ knowledge, the positive correlations of PLT with MCP-1, IL-8 and IL-18 in PRP samples are presented in a scientific paper for the first time.
There are some controversies about leukocyte-rich platelet-rich plasma which is expected to have WBC concentration above the baseline. Its negative effect on tissue healing was demonstrated in in vitro studies, which, however, was not confirmed by studies on living organisms. This effect may be due to the high content of proteinases and hydrolases in neutrophils and should be taken into consideration when planning the therapy[8,13,31,32]. WBC content could also have an antibacterial effect[33]. On the other hand, in the presented study, high leukocyte content positively correlates with the levels of important growth factors and cytokines such as EGF, VEGF, HGF, PDGF-AA, PDGF-BB, IL-1β, MCP-1, IL-8 and IL-18. Authors of similar papers do not frequently report results of correlations between WBC and GF. J. Magalon has shown a significant positive correlation of WBC content with EGF and VEGF but not with PDGF-AB and TGF-β1[23]. T. N. Castillo also found a positive correlation of WBC with VEGF and PDGF-ββ but not with PDGF-AB or TGF-β1[24]. The presented results support and greatly extend the above. The highest WBC content was found in PRP prepared using the Mini GPS III System as it is defined as leukocyte-rich platelet-rich plasma (L-PRP)[21,34].
There was also significantly higher content of RBC in Mini GPS III than in other systems. This is undesirable evidence of imperfect separation of PRP and may explain why other authors did not provide detailed information about RBC and its correlation with growth factors and cytokines. Surprisingly, a positive correlation was again demonstrated with EGF, VEGF, HGF, PDGF-AA, PDGF-BB, IL-1β, MCP-1, IL-8 and IL-18. This has never been reported by other authors in the context of PRP evaluation and requires further research.
Significant differences in the levels of EGF, VEGF, HGF, PDGF-AA, PDGF-BB, IL-8 and IL-18 were found between Mini GPS III and Arthrex, for EGF, IL-8 and IL-18 between Mini GPS III and Xerthra and for IL-18 between Mini GPS III and Dr. PRP. The presented results support and extend the current state of knowledge[10,23,24,30,31,35]. Further studies are needed to explain the origin of these differences in the cases in which the correlation between blood cell components and cytokines/growth factors cannot simply explain it.
For the first time in the literature, the repeatability of obtained concentrations of PLT, WBC and RBC in different PRP preparation systems was clearly evaluated. The presented study demonstrated considerable differences between systems. In the authors’ opinion, especially Xerthra PRP kit requires some improvements in the provided protocol for better repeatability. Due to the fact that two PRP samples prepared with Xerthra had a PLT increase > 4x above the baseline level, the authors still believe that there is great potential in this system.
For clinical practice and further studies, the most important issue is what should be expected from differences in PRP cytokine levels. Multiple studies have demonstrated a beneficial effect of PDGF and FGF on the healing process, both in animal models and in patients with wound healing disorders. However, in vivo functions of many growth factors remain largely unconfirmed[12]. In this study, PDGF-AA and PDGF-BB were selected as representatives of the PDGF family which stimulates cell (neutrophil, monocyte, fibroblast) migration to the wound site, enhances the proliferation of fibroblasts and production of extracellular matrix[6,12]. FGF-basic, as an FGF family member has well described mitogenic activity, regulates migration and cell differentiation, and has a cytoprotective effect on cells under stress conditions[12]. VEGF is involved in the regulation of angiogenesis during wound healing, HGF was discovered as a stimulator of dissociation of epithelial cells, migration, proliferation and new blood vessel formation, EGF induces cell differentiation of both ectodermal and mesodermal origin, and TGF-β1 has an important role in controlling cell proliferation and differentiation during the repairing process[6,12]. Other important cytokines that play a positive role in tissue healing are MCP-1 as a major macrophage chemoattractant, IL-8 as a neutrophil chemoattractant and stimulant of reepithelialization, IL-10 as an inhibitor of inflammation and scar formation[12]. Proinflammatory cytokines such as IL-1α and β, IL-6 and TNF-α are also involved in the repairing process by stimulation of keratinocyte and fibroblast proliferation, synthesis and breakdown of extracellular matrix proteins, fibroblast chemotaxis and regulation of the immune response[12].
It is expected that high levels of the above cytokines in PRP should result in better wound healing. In the presented study, a positive but not always significant Spearman correlation was found between all blood cell components and growth factors such as PDGF-AA, PDGF-BB, EGF, VEGF, HGF, MCP-1, IL-8 and IL-1 β, however, their correlation with TGF-β1, FGF-basic, TNF-α, IL-6 and IL-10 was found to be negligible. These results suggest that the best clinical outcome should be expected from PRP with the highest concentration of blood cell components.
Proinflammatory cytokines such as IFN-α2, IFN-γ, IL-12p70, IL-17A, IL-18, IL-23 and IL-33 may play an additional role in wound healing by regulating the secretion of other cytokines, regulating the immune response and antimicrobial activity. However, their function in tissue repair is poorly understood. In the presented study, a significant correlation was found only between all blood cell components and IL-18. Since IL-18 is able to induce severe inflammatory reactions, the implications of its correlation with highly concentrated PRP require further investigation.
Significant differences in the content of blood cell components and growth factors between different methods of platelet-rich plasma separation have already been demonstrated in previous publications[10,20,23,24,30,35]. However, none of the studies analyzed the Xerthra and Dr. PRP systems in such great detail. The medical market offers much more commercially available PRP preparation systems than those included in the study. The present authors evaluated only four of them but still, most of similar studies analyzed a smaller or comparable number of different systems[10,23,24,30,35].
Another limitation of the study is the size of the population. However, the number of participants is larger compared to the majority of similar studies[20,23,24,30,35]. The group was kept as homogeneous as possible to eliminate additional factors that could distort the results, therefore the study included a group of healthy men of similar age. The post-hoc test power analysis showed a sufficient sample size for the PRP group comparison of PCE, blood cell components, EGF, PDGF-AA, IL-18 and slightly insufficient for VEGF, HGF, MCP-1 and IL-8. To achieve the test power level of 0.8 in order to compare most other cytokines, the sample size should be considerably increased from 3 (FGF-basic) to more than 100 times (IFN-α2, IL-12p70 or IL-33). This data may help other researchers to design their studies appropriately.
In the study presented in this paper, the degree of platelet activation was not tested. This was based on the available literature which showed no significant differences in platelet activation between different separation methods[23,36]. Moreover, according to the literature, the storage time of PRP in which there is no significant activation of the platelets, should not exceed 6 h at a temperature of 20°C[37]. All PRP samples obtained in this study were tested for the content of blood cell components immediately after production and then they were frozen at -80 °C waiting for cytokine analysis.
Not all of the known cytokines and growth factors that may affect the healing and regeneration processes occurring in platelet-rich plasma were analyzed. However, the focus was on the most important ones. This study analyzes a wider range of cytokines than most publications on this topic[10,20,23,24,30,35].
Significant differences were found in the content of biologically active components in PRP obtained with the use of different methods and considerable differences were demonstrated in their repeatability within each method. Significant correlations between blood cell components and growth factors/ inflammatory cytokines were presented, contributing new data to the current state of knowledge.
Due to the considerable heterogeneity of PRPs, the presented results support the recommendations for studies reporting the use of autologous blood-based therapies to provide detailed information about the characteristics of all PRP samples (MIBO)[27]. The study also provides detailed information about the desired content of growth factors and their correlation with blood cell components in four different commercially available systems for PRP preparation, which could help clinicians to choose one depending on their expectations. The results of this study also demonstrate the need for producers to improve the existing solutions in order to improve the provided protocols to increase the repeatability of the parameters of PRP samples.
In clinical practice, it seems reasonable to suggest the use of commercial PRP kits that allow obtaining the highest blood cell concentration with the highest predictable reproducibility. It is worth noting that not only the level of PLT but also WBC and RBC levels significantly correlated with cytokines involved in the healing process, which may encourage researchers to look for new “blood cell concentrate” treatment methods.
Autologous platelet-rich plasma (PRP) therapy is a method used to treat a variety of diseases related to soft tissue degeneration. The main idea behind this is to improve local healing and stimulate regeneration by administering large amounts of platelet-derived growth factors and cytokines. There are many commercial kits available to assist in obtaining PRP in an outpatient setting.
Due to the wide variety of PRP preparation systems, there are justified doubts about the quality of the obtained samples. Differences in the content of biologically active compounds between some PRP systems have already been demonstrated. However, only a small number of available systems and a limited number of cytokines and growth factors have been investigated.
To compare PRP obtained using four different commercial preparation systems in terms of the content of biologically active components, correlations between those components and their repeatability in each method.
After obtaining informed consent from participants, whole blood was collected from 12 young healthy male volunteers, and 4 different PRP samples were prepared from each of them in a single-donor model. PRP samples were prepared using different commercial kits: Arthrex Autologous Conditioned Plasma (ACP) Double Syringe System (Arthrex Inc., United States), the Mini GPS III Platelet Concentration System (Biomet Inc., United States), the Xerthra PRP kit (Biovico Sp. z o.o., Poland) and Dr. PRP (Rmedica, Republic of Korea). The content of cellular components in each sample was assessed using an automatic laboratory analyzer Mindray BC-5150 (Shenzhen Mindray Bio-Medical Electronics Co., PRC). To quantify the content of seven selected growth factors (Epidermal growth factor (EGF), Fibroblast Growth Factor- basic, Hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), Transforming Growth Factor-β1, Platelet-Derived Growth Factor-AA, Platelet-Derived Growth Factor-BB and thirteen inflammatory cytokines [(Interferon-α2, Interleukin-10, Interleukin-12p70, Interleukin-17A, Interleukin-18 (IL-18), Interleukin-23 (IL-23), Interleukin-33, Interleukin-6, Interleukin-8, Interferon-γ, Monocyte Chemoattractant Protein-1 (MCP-1), Tumor Necrosis Factor α (TNF-α)], bead-based multiplex immunoassays LEGENDplexTM (BioLegend, United States) that use fluorescence-encoded beads and flow cytometer measurements were performed.
Differences between PRPs obtained with various preparation systems were found in terms of cellular composition, repeatability, platelet capture efficiency, concentrations of growth factors and inflammatory cytokines. The highest ability to concentrate platelets (PLT) above the baseline was obtained with Mini GPS III (5.05 x) and the lowest with Arthrex ACP (1.47 x). Those two systems had the best repeatability of platelet concentrations assessed as the coefficient of variation of 13.25% and 12.18%, respectively. The highest concentrations of Epidermal growth factor, hepatocyte growth factor, vascular endothelial growth factor, platelet-derived growth factor-AA, platelet-derived growth factor-BB, IL-18, Interleukin-1β, Interleukin-8, MCP-1 and TNF-α were found in PRP with the highest PLT, white blood cells and red blood cells concentrations (obtained with Mini GPS III), and positive significant (P < 0.05) correlations between cell components and these paracrine factors (except TNF-α) were revealed.
The study provided new data on the differences between PRP obtained with the various commercial systems. The range of analyzed cytokines far exceeded the ranges investigated in earlier publications. The presented findings should help researchers and clinicians choose the system that best meets their expectations.
Further research should be focused on the comparison of PRPs obtained using different techniques in the context of their biological effect on soft tissues in vitro and their clinical efficacy in various diseases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haque N, Bangladesh; Yunus MHM, Malaysia S-Editor: Wang LL L-Editor: A P-Editor: Li X
| 1. | Creaney L, Hamilton B. Growth factor delivery methods in the management of sports injuries: the state of play. Br J Sports Med. 2008;42:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Middleton KK, Barro V, Muller B, Terada S, Fu FH. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop J. 2012;32:150-163. [PubMed] |
| 3. | Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107:229-37; discussion 238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 277] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Tietze DC, Geissler K, Borchers J. The effects of platelet-rich plasma in the treatment of large-joint osteoarthritis: a systematic review. Phys Sportsmed. 2014;42:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Dhillon RS, Schwarz EM, Maloney MD. Platelet-rich plasma therapy - future or trend? Arthritis Res Ther. 2012;14:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 6. | Sánchez-González DJ, Méndez-Bolaina E, Trejo-Bahena NI. Platelet-rich plasma peptides: key for regeneration. Int J Pept. 2012;2012:532519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Roh YH, Kim W, Park KU, Oh JH. Cytokine-release kinetics of platelet-rich plasma according to various activation protocols. Bone Joint Res. 2016;5:37-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Kobayashi Y, Saita Y, Nishio H, Ikeda H, Takazawa Y, Nagao M, Takaku T, Komatsu N, Kaneko K. Leukocyte concentration and composition in platelet-rich plasma (PRP) influences the growth factor and protease concentrations. J Orthop Sci. 2016;21:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Degen RM, Conti MS, Camp CL, Altchek DW, Dines JS, Werner BC. Epidemiology and Disease Burden of Lateral Epicondylitis in the United States: Analysis of 85,318 Patients. HSS J. 2018;14:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Oh JH, Kim W, Park KU, Roh YH. Comparison of the Cellular Composition and Cytokine-Release Kinetics of Various Platelet-Rich Plasma Preparations. Am J Sports Med. 2015;43:3062-3070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 411] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 12. | Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2305] [Cited by in RCA: 2411] [Article Influence: 109.6] [Reference Citation Analysis (0)] |
| 13. | Wasterlain AS, Braun HJ, Dragoo JL. Contents and Formulations of Platelet-Rich Plasma. Oper Tech Orthop. 2012;22:33-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Monto RR. Platelet rich plasma treatment for chronic Achilles tendinosis. Foot Ankle Int. 2012;33:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Suthar M, Gupta S, Bukhari S, Ponemone V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci. 2017;24:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 16. | Mi B, Liu G, Zhou W, Lv H, Liu Y, Wu Q, Liu J. Platelet rich plasma vs steroid on lateral epicondylitis: meta-analysis of randomized clinical trials. Phys Sportsmed. 2017;45:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Shen L, Yuan T, Chen S, Xie X, Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 18. | Lisi C, Perotti C, Scudeller L, Sammarchi L, Dametti F, Musella V, Di Natali G. Treatment of knee osteoarthritis: platelet-derived growth factors vs. hyaluronic acid. A randomized controlled trial. Clin Rehabil. 2018;32:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of Intra-articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Systematic Review. Arthroscopy. 2016;32:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 261] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 20. | Kushida S, Kakudo N, Morimoto N, Hara T, Ogawa T, Mitsui T, Kusumoto K. Platelet and growth factor concentrations in activated platelet-rich plasma: a comparison of seven commercial separation systems. J Artif Organs. 2014;17:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 452] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 22. | Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 358] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 23. | Magalon J, Bausset O, Serratrice N, Giraudo L, Aboudou H, Veran J, Magalon G, Dignat-Georges F, Sabatier F. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthroscopy. 2014;30:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 24. | Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 330] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 25. | Zimmermann R, Arnold D, Strasser E, Ringwald J, Schlegel A, Wiltfang J, Eckstein R. Sample preparation technique and white cell content influence the detectable levels of growth factors in platelet concentrates. Vox Sang. 2003;85:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Dejnek M, Moreira H, Płaczkowska S, Morasiewicz P, Barg E, Witkowski J, Reichert P. Analysis and comparison of autologous platelet-rich plasma preparation systems used in the treatment of enthesopathies: A preliminary study. Adv Clin Exp Med. 2021;30:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Murray IR, Geeslin AG, Goudie EB, Petrigliano FA, LaPrade RF. Minimum Information for Studies Evaluating Biologics in Orthopaedics (MIBO): Platelet-Rich Plasma and Mesenchymal Stem Cells. J Bone Joint Surg Am. 2017;99:809-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 28. | Mazzucco L, Balbo V, Cattana E, Guaschino R, Borzini P. Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang. 2009;97:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 242] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 29. | Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 1036] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 30. | Mazzocca AD, McCarthy MB, Chowaniec DM, Cote MP, Romeo AA, Bradley JP, Arciero RA, Beitzel K. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 316] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 31. | Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39:2135-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 447] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 32. | McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94:e143(1-e1438). [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 245] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 33. | Cieślik-Bielecka A, Bold T, Ziółkowski G, Pierchała M, Królikowska A, Reichert P. Antibacterial Activity of Leukocyte- and Platelet-Rich Plasma: An In Vitro Study. Biomed Res Int. 2018;2018:9471723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1190] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 35. | Everts PA, Brown Mahoney C, Hoffmann JJ, Schönberger JP, Box HA, van Zundert A, Knape JT. Platelet-rich plasma preparation using three devices: implications for platelet activation and platelet growth factor release. Growth Factors. 2006;24:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 36. | Pignatelli P, Pulcinelli FM, Ciatti F, Pesciotti M, Sebastiani S, Ferroni P, Gazzaniga PP. Acid citrate dextrose (ACD) formula A as a new anticoagulant in the measurement of in vitro platelet aggregation. J Clin Lab Anal. 1995;9:138-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Bausset O, Giraudo L, Veran J, Magalon J, Coudreuse JM, Magalon G, Dubois C, Serratrice N, Dignat-George F, Sabatier F. Formulation and storage of platelet-rich plasma homemade product. Biores Open Access. 2012;1:115-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |