Published online Jun 18, 2022. doi: 10.5312/wjo.v13.i6.564
Peer-review started: February 28, 2022
First decision: April 13, 2022
Revised: April 26, 2022
Accepted: June 14, 2022
Article in press: June 14, 2022
Published online: June 18, 2022
Processing time: 108 Days and 7 Hours
Osteoporotic vertebral compression fractures (OVCFs) are the most common fragility fracture and significantly influence the quality of life in the elderly. Currently, the literature lacks a comprehensive narrative review of the management of OVCFs. The purpose of this study is to review background information, diagnosis, and surgical and non-surgical management of the OVCFs. A comprehensive search of PubMed and Google Scholar for articles in the English language between 1980 and 2021 was performed. Combinations of the following terms were used: compression fractures, vertebral compression fractures, osteoporosis, osteoporotic compression fractures, vertebroplasty, kyphoplasty, bisphosphonates, calcitonin, and osteoporosis treatments. Additional articles were also included by examining the reference list of articles found in the search. OVCFs, especially those that occur over long periods, can be asymptomatic. Symptoms of acute OVCFs include pain localized to the mid-line spine, a loss in height, and decreased mobility. The primary treatment regimens are pain control, medication management, vertebral augmentation, and anterior or posterior decompression and reconstructions. Pain control can be achieved with aceta
Core Tip: Management of osteoporotic vertebral compression fractures must be combined with multiple approaches. Appropriate exercises and activity modification are important in fracture prevention. Medication with different mechanisms of action is a critical long-term causal treatment strategy. The minimally invasive surgical interventions such as vertebroplasty and kyphoplasty are reserved for patients not responsive to conservative therapy and are recognized as efficient stopgap treatment methods. Posterior decompression and fixation or Anterior decompression and reconstruction may be required if neurological deficits are present.
- Citation: Patel D, Liu J, Ebraheim NA. Managements of osteoporotic vertebral compression fractures: A narrative review. World J Orthop 2022; 13(6): 564-573
- URL: https://www.wjgnet.com/2218-5836/full/v13/i6/564.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i6.564
Osteoporotic vertebral compression fractures (OVCFs) are the most common fragility fracture and are a marker of osteoporosis[1]. In the United States, approximately 700,000 OVCFs occur annually[2]. OVCFs and hip fractures are estimated to cost between $10 and $15 billion annually, indicating the immense burden of these conditions[3]. Along with the financial burden, osteoporotic fractures are associated with a decrease in health-related quality of life and quality-adjusted life years[4-6]. Therefore, it is imperative to better understand this prevalent condition and how to prevent and treat it.
Despite how common VCFs are, there has not been a definitive treatment course for patients suffering from these conditions. Overall, managing an acute OVCF includes pain control, activity modification, education, and treatment of the underlying osteoporosis. Patients can be treated conservatively with rest and/or medications or surgical management, such as vertebroplasty and kyphoplasty. However, there are no treatment algorithms to decide the best course of action. The American Academy of Orthopedic Surgeons (AAOS) developed a clinical practice guideline with 11 recommendations for treating osteoporotic VCFs, but only 1 has a strong recommendation and 1 has a moderate recommendation; the other 9 are weak or inconclusive[7]. This lack of robust evidence for specific treatment methods over others provides clinicians with leniency to make decisions based primarily on personal experience.
This review aims to provide a comprehensive overview of the presentation and treatment of osteoporotic vertebral compression fractures.
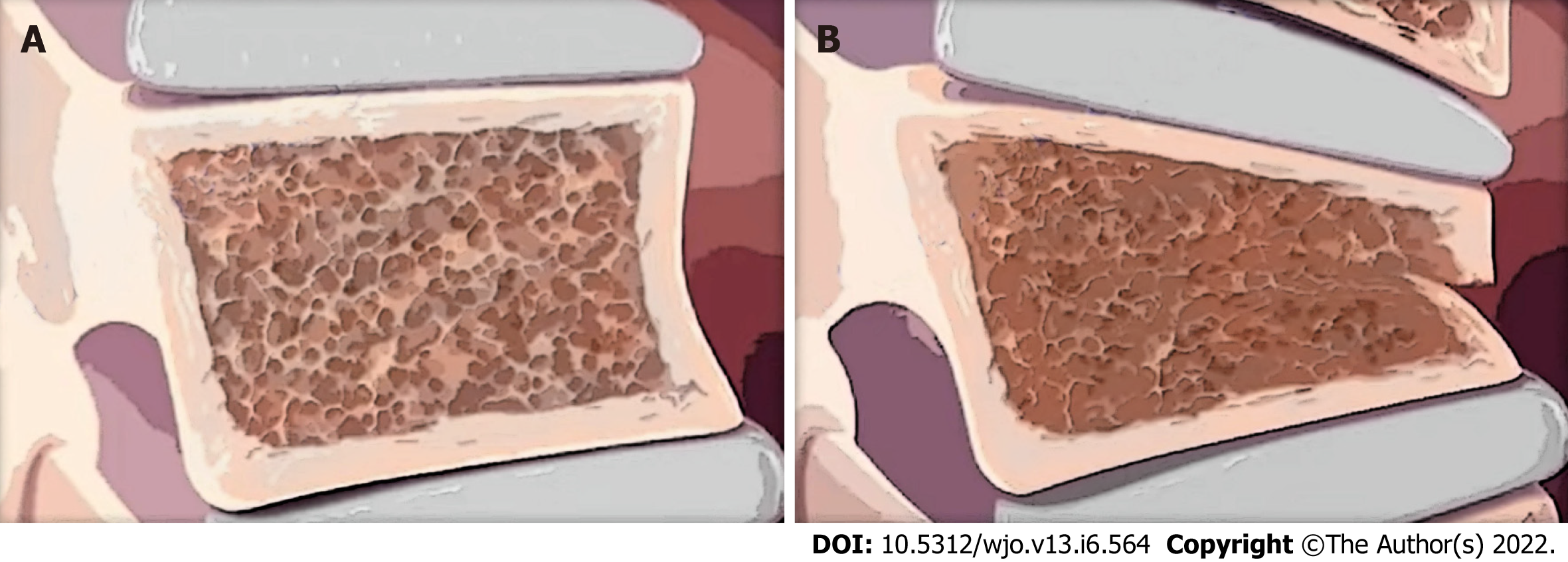
Even though OVCFs are very common, they are often undiagnosed because patients can be asymptomatic, especially those that occur slowly over time[1]. Further contributing to the lack of diagnosis is the fact that neurological complications are rare and acute symptoms can be overlooked[8,9]. Some signs that can be indicative of OVCFs are a decrease in height, change in kyphosis (when adjusted for age, Figure 1), acute-onset back pain that increases when changing posture and decreases when lying supine, and limited mobility[1,9]. These acute fractures typically occur in the lower thoracic vertebra and can be due to a fall in people with moderate osteoporosis or minor activities such as coughing in people with severe osteoporosis[1]. The pain is usually well localized to the midline spine but often refers to a unilateral or bilateral pattern into the flank, anterior abdomen, or the posterior superior iliac spine[10]. Diagnosis can be determined based on history and physical exam (e.g., tenderness over vertebrae; neurological assessment) and imaging. Magnetic resonance imaging (MRIs) and computed tomography (CT) scans are used to determine the timing and extent of the fracture along with soft-tissue or spinal cord involvement[11].
Evaluation for osteoporosis should be done as soon as possible after the VCF if not already diagnosed. Dual-energy X-ray absorptiometry (DEXA) is used to measure bone mineral density (BMD) and confirm the diagnosis of osteoporosis[12]. Osteoporosis is the most prevalent bone disorder worldwide and is defined as a T-score of -2.5 SD below the BMD of a healthy younger person of the same sex[13-15]. Low bone density, or osteopenia, is defined as a T-score between -1 and -2.5. The decrease in BMD is primarily due to loss of gonadal function and aging, but by two different mechanisms[16]. The loss of gonadal function, specifically a decrease in estrogens especially seen in postmenopausal women, results in excessive osteoclast activity, whereas in aging, the demand for osteoblasts outweighs the supplies[16,17]. These two different causes of osteoporosis also differ in the location they affect: postmenopausal bone loss is mainly in trabecular bone whereas aging-associated bone loss occurs mainly in cortical bone[16]. The excessive increase in reabsorption or decreased formation leads to compromised bone volume and integrity, thus increasing the risk of fracture. Other risk factors for osteoporosis include inadequate calcium and vitamin D levels. Vitamin D improves the absorption of calcium from the gut and helps ensure there are adequate calcium levels for normal bone mineralization[18]. The evaluation of an OVCF also includes assessment for neurologic findings, which may necessitate further imaging and consultation with a spine surgeon depending on the results; laboratory evaluation to assess for malignancy, such as multiple myeloma, and secondary causes of osteoporosis, including adverse effects of drug therapy, immobilization, and endocrine disorders, may be indicated[19].
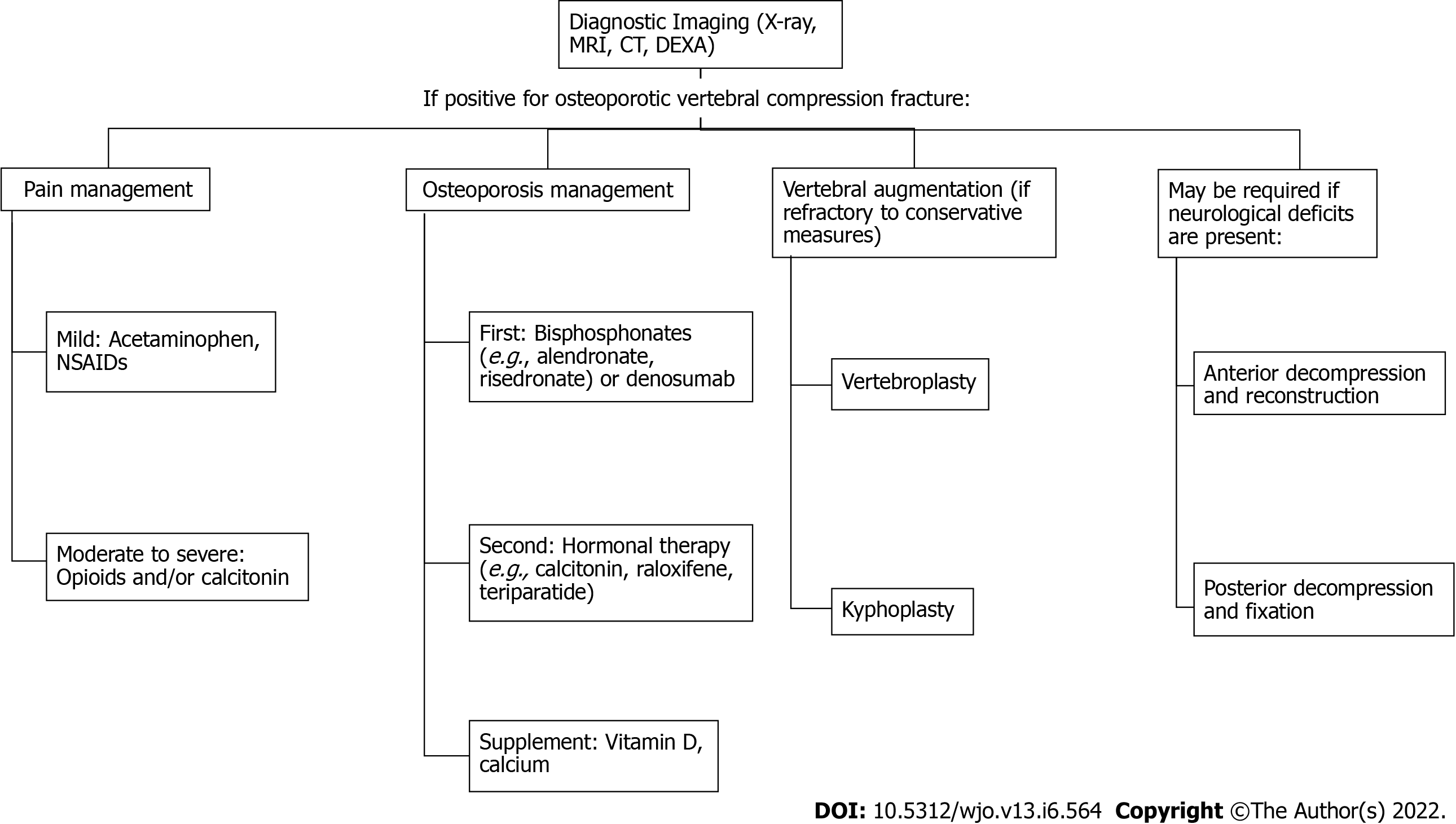
Treatments of OVCF remain challenging. They usually include pain management, Anti-osteoporosis medications, vertebral augmentation, and surgical decompression and reconstruction (Figure 2).
Nonsteroidal anti-inflammatory drugs (NSAIDs) and opioid analgesics are the most commonly used medications for pain control[20]. The choice of the initial agent depends upon the severity of pain[21]. Acetaminophen or NSAIDs, such as ibuprofen and naproxen, are the first-line options for patients with mild pain. These medications are easily accessible over the counter and used due to their perceived safety and relatively low cost[22]. Even though NSAIDs are used first is partially due to the incidence of more serious risks associated with other medications such as opioids and tricyclic depressants, they are not without their own adverse effects[22]. Some significant complications from the use of NSAIDs include gastrointestinal toxicity, cardiovascular thrombotic events, stroke, and renal toxicity; there is an increased risk of these complications in older patients and those with preexisting conditions (e.g., cardiovascular disease, heart failure, impaired renal function), which are more prevalent in older populations[22-24]. Clinicians should be aware of potential contraindications for NSAID treatment in their patients and consider gastrointestinal protective therapy for those prescribed NSAIDs.
If the pain is not managed with initial medications or is moderate to severe, opioids and calcitonin are options. Calcitonin is unique because it has a positive impact on bone health, as discussed later in this review, and can provide pain relief possibly through modulating nociception in the central nervous system[25]. Opioids are typically required at the outset for patients with severe pain with an acute (0 to 4 wk) VCF. It has been suggested that initial treatment with an opioid is combined with low-dose acetaminophen. There is sufficient evidence of the short-term efficacy of opioids, but the long-term effectiveness is diminished because of serious adverse reactions[26]. These adverse events include constipation, nausea and vomiting, dizziness, drowsiness, dependence, and abuse[27]. Because the risks of opioid use for chronic back pain outweighs the benefits, the American Academy of Neurology recommends against using opioid pain medications in this patient demographic[26]. Similarly, the AAOS are unable to recommend for or against opioids/analgesics for neurologically intact and symptomatic VCF patients[7]. Muscle relaxants can be used initially, but their long-term use is not recommended for similar reasons as long-term opioid use[28].
A brace can also be used for pain relief because it stabilizes the spine. Bracing can be used initially in the acute and subacute phases but should not be used long-term because atrophy of the core musculature can occur with prolonged use[28]. Additionally, the efficacy of bracing in preventing future vertebral body collapse has not been established and it is often poorly tolerated in older patients[29]. The AAOS is also inconclusive in its stance on bracing and other forms of therapy such as exercise[7]. If patients choose to exercise, programs should be implemented to improve flexibility, muscle strength, and core and gait stability[30]. It is important to note that exercise regimens should be tapered as pain improves[11]. Another potential treatment method for fracture repair is low-level laser therapy (LLLT). Although the exact mechanisms of LLLT are unknown, it is hypothesized to increase angiogenesis and bone formation while decreasing inflammation, as tested in animal studies[31,32]. LLLT could accelerate fracture healing and thus improve patient outcomes by enhancing callus formation, but there is limited evidence for its use in patients with bone fractures[33]. Further research into the mechanisms of LLLT and the appropriate parameters for therapeutic use and minimal side effects need to be studied before clinical application.
Patient selection is a critical factor for both the decision to perform vertebral augmentation and the selection of the type of procedure. Surgical interventions are typically reserved for those who have not experienced relief from medical management. For patients with incapacitating pain from acute and subacute vertebral compression fractures who are unable to taper parenteral or transition to oral opioids within seven d of admission or have intolerable sedation, constipation, or delirium from this therapy, it has been suggested that vertebral augmentation be performed rather than continue medical management. This is typically performed during the initial hospitalization. A vertebral augmentation is also an option for those without improvement in pain despite four to six weeks of conservative management with oral opioids and calcitonin, or for those who are intolerant of oral opioids.
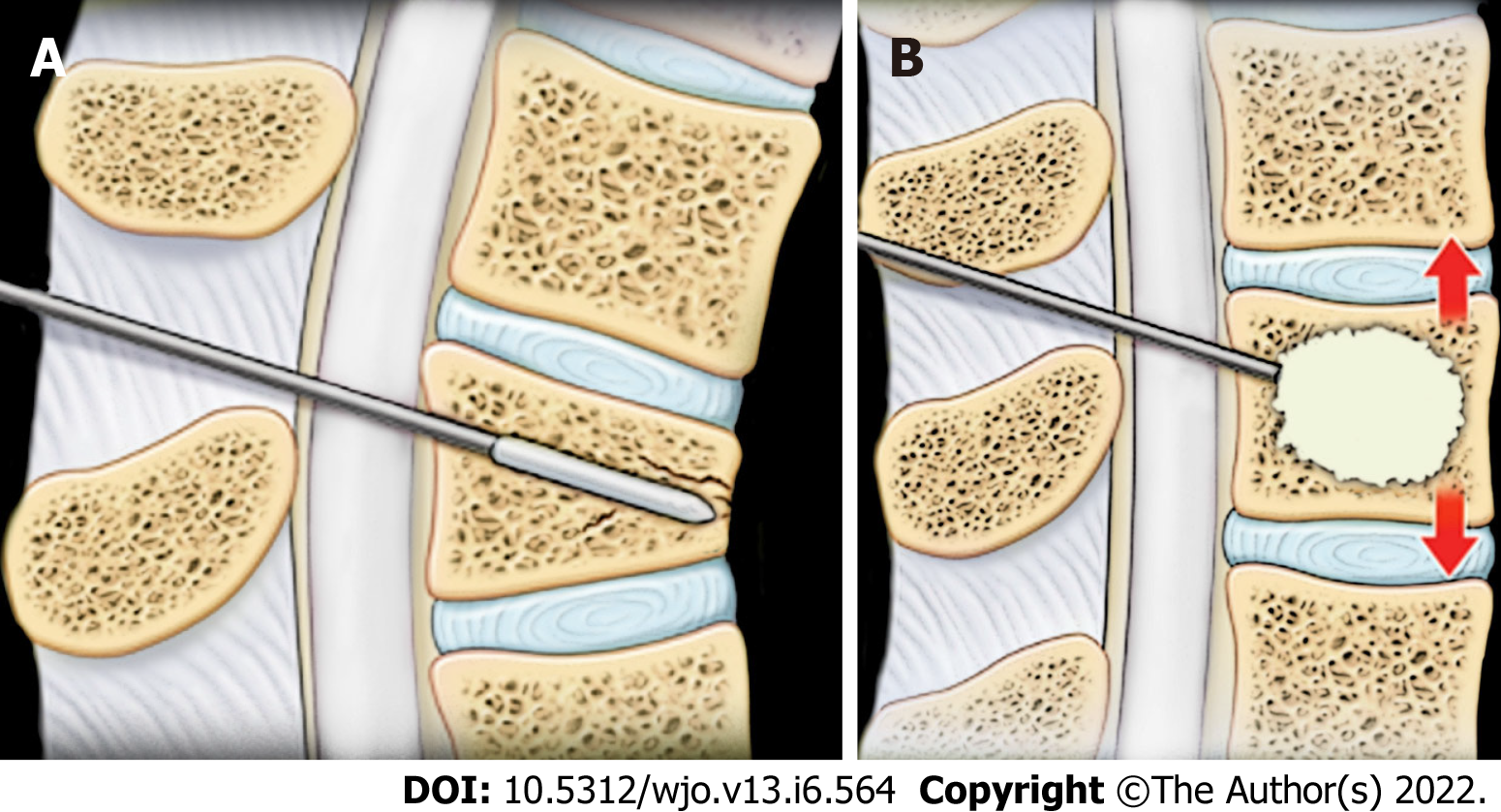
The two main surgical treatment options are vertebroplasty and kyphoplasty. Vertebroplasty involves injecting cement, such as polymethylmethacrylate, directly into the fractured vertebrae for pain relief and fracture stabilization[34]. Success rates for pain relief and functional improvement after vertebroplasty range between 78% and 90%[35-39]. Some complications include cement extravasation (the most common), rib fracture, radiculopathy, spinal cord compression, and cement pulmonary embolism[40-43]. However, studies have demonstrated no significant difference in pain improvement or pain-related disability when comparing patients who underwent vertebroplasty and a control group[44-46].
Kyphoplasty involves inflating a balloon in the bone and then injecting cement into the cavity[34]. This method has similar success to vertebroplasty in pain relief and functional improvement but is more successful at restoring the kyphotic angle[47]. However, there have been fewer complications associated with kyphoplasty compared to vertebroplasty, partly due to decreasing the risk of cement leakage (Figure 3)[34,48-51].
The characteristics of the VCF can influence which surgical procedure is performed. The AAOS strongly recommends against vertebroplasty in patients with an osteoporotic VCF on imaging with correlating signs and symptoms and are neurologically intact[7]. Kyphoplasty is weakly recommended in this patient demographic[7]. The AAOS cannot recommend or be against any treatment for patients who are not neurologically intact[7]. Based on the recommendations of the AAOS and relatively fewer complications, kyphoplasty is most likely to be the preferred surgical method in treating symptomatic VCFs. However, kyphoplasty is more technically challenging than vertebroplasty as it involves bilateral placement in nearly all cases and relies on the use of a balloon tamponade system that can have its own technical difficulties. Additionally, vertebroplasty does not commit to a bipedicular approach, does not rely on the performance of a balloon system, and is less expensive by approximately $5000[52]. The difference between these two procedures should be discussed with the patient, so they can be involved in the decision-making process.
Other surgical interventions include decompression then screw implantation and vertebral fixation. Decompression is indicated if the patient has neurological deficits due to neural compression. Additionally, screw augmentation can be used to increase the pull-out strength and may be combined with cement injection if fenestrated screws are used[53]. Vertebral fixation, specifically posterior vertebral fixation, has limited indications, including burst fractures, multiple VCFs causing kyphotic deformity, and malunion[53]. Both of these interventions can be combined with cement injection, so they can result in similar complications as vertebroplasty and kyphoplasty, such as cement extravasation.
Pharmaceutical therapy can also be initiated for treatment or prevention and is the recommended therapy for long-term treatment of OVCFs. The first-line drug treatment for osteoporosis is bisphosphonates. The FDA has approved this drug class for the prevention and treatment of osteoporosis. Bisphosphonates work by inhibiting osteoclast activation and decreasing bone resorption, leading to a decrease in bone loss[54-56]. Alendronate, an aminobisphosphonate, is often the specific first-line treatment because its efficacy in reducing fracture risk and favorable safety profile[57]. Alendronate significantly decreases the risk of fractures, specifically by 44% for radiographic vertebral fractures and 57% for osteoporotic women or those with an existing vertebral fracture[59,59]. Another aminobisphosphonate considered first-line, risedronate, has been shown to have a relative risk reduction in vertebral fractures of 39%[60]. Over three years, risedronate decreased the vertebral fracture risk by 41%[61]. The main adverse effects of bisphosphonates are gastrointestinal complications, such as reflux, esophagitis, and gastric ulcers[20]. Bisphosphonates, especially oral bisphosphonates, are contraindicated for patients who cannot tolerate the gastrointestinal side effects or have esophageal dysmotility.
Denosumab is another popular medication used for treating osteoporosis that can increase BMD[62,63]. It is a human monoclonal antibody that inhibits RANKL and thus prevents the formation and activation of osteoclasts. Unlike other osteoporosis medications, denosumab is administered subcutaneously every six mo, which can be more convenient for the patient and increase compliance, given that compliance with anti-osteoporotic therapy is lacking[64]. When administered for three years, denosumab has been shown to decrease the risk of vertebral, nonvertebral, and hip fractures in postmenopausal women[65]. Importantly, there were no differences in the incidence of adverse events (e.g., infection, hypocalcemia, cancer, cardiovascular events) between patients who received denosumab and those who received a control[66]. Because of the infrequent administration of denosumab, its effectiveness, and its lack of adverse effects, denosumab is a promising treatment option.
Calcitonin is a thyroid hormone (TH) that can also be used for the prevention and treatment of postmenopausal osteoporosis[66]. Calcitonin decreases the resorption of bone by osteoclasts and can increase bone density in postmenopausal women while potentially reducing the risk of VCFs[67,68]. The AAOS moderately recommends a four-week regiment of calcitonin for patients with an acute (0-5 d after identifiable event or onset of symptoms) symptomatic osteoporotic VCF (confirmed with imaging) and who are neurologically intact[7]. There are other hormone therapies available, such as selective estrogen receptor modulators like raloxifene or parathyroid hormone analogs like teriparatide that have a positive impact on reducing fracture risk, but the use of hormone therapy for osteoporosis has declined[69,70]. The increase in the risk of cardiovascular complications (e.g., stroke, coronary heart disease) and breast cancer have led to a decrease in the use of hormone therapy[71,74].
Supplementation can also have a positive impact on bone health. Postmenopausal osteoporotic women should receive 1500 mg of calcium and 400 IU of vitamin D daily[29]. Calcium plus vitamin D supplementation has been shown to significantly reduce the risk of fractures by 15%[18]. Exercise and physical therapy can also strengthen bones to prevent fractures. Strengthening the spinal extensor muscles can increase bone density and decrease the risk of VCFs[75]. Additional lifestyle modifications can also be implemented to reduce the risk of vertebral fractures. For example, patients should be advised to quit smoking and consume less alcohol (< 3 drinks/d) to decrease their risk for osteoporotic vertebral fractures[28]. Table 1 summarizes the different pharmaceutical managements of osteoporosis and their efficacy; different medications have different routes of administration (PO = orally, IV = intravenous, SQ = subcutaneous, IN = intranasal) and different effects on the relative risk of fracture (RR). It is important to note that certain side effects, such as renal toxicity and atypical fracture are extremely rare, as depicted by the asterisk.
| Drug | Dosing | Efficacy | Adverse effects |
| Bisphosphonates | |||
| Alendronate | 10 mg daily, PO; 70 mg weekly, PO | RR of 0.55 in postmenopausal women compared to placebo | Esophageal irritation; Dyspepsia; Acute phase reaction; Atrial fibrillation; Musculoskeletal pain; Renal toxicity; Atypical fracture; Osteonecrosis of the jaw |
| Ibandronate | 2.5 mg daily, PO; 150 mg monthly, PO; 3 mg every 3 mo, IV | RR between 0.50 and 0.62 in postmenopausal women compared to placebo | |
| Risedronate | 5 mg daily, PO; 35 mg weekly, PO; 150 mg monthly, PO | RR of 0.64 in postmenopausal women compared to placebo | |
| Zoledronate | 5 mg annually, IV | 7% increase in spine BMD; RR of 0.23 in postmenopausal women compared to placebo | |
| Rankl inhibitors | |||
| Denosumab | 60 mg every 6 mo, SQ | 68% decrease in incidence of radiographic vertebral fractures; 9.2% increase in lumbar spine BMD | Fatigue; Weakness; Diarrhea; Hypophosphatemia |
| Hormones | |||
| Raloxifene (SERM) | 60 mg daily, PO; 120 mg daily, PO | RR of 0.65 in postmenopausal women compared to placebo (60 mg); RR of 0.54 in postmenopausal women compared to placebo (120 mg); 2.6% increase in lumbar spine BMD (60 mg); 2.5% in lumbar spine BMD (120 mg) | Flu syndrome; Vasodilation; Endometrial cavity fluid; Peripheral edema |
| Teriparatide (PTH) | 20 microg daily, SQ; 40 microg daily, SQ | 65% decrease in fracture risk (20 microg); 69% decrease in fracture risk (40 microg); 9% increase in lumbar spine BMD (20 microg); 13% increase in lumbar spine BMD (40 microg) | Nausea; Headache; Dizziness; Leg cramps |
| Calcitonin (TH) | 200 mg daily, IN | 33% decrease in fracture risk; 1%-1.5% increase in lumbar spine BMD | Epistaxis; Rhinitis; Ulceration of nasal mucosa; Hypocalcemia |
| Supplementations | |||
| Vitamin D | 400 IU daily, PO | 15% decrease in fracture risk | - |
| Calcium | 1500 mg daily, PO | ||
Osteoporosis is a chronic disease with a great clinical impact on the increasing geriatric population and OVCFs are seen most frequently. Therefore, it is essential to understand the manifestation and treatment of these conditions. Steps for diagnosis and treatment of OVCFs can follow these recommendations: (1) Obtain diagnostic imaging (X-ray, MRI, or CT) and DEXA scan; (2) Pain management: (a) Mild: Acetaminophen or NSAIDs; and (b) Moderate to severe: Opioids and/or calcitonin; (3) Osteoporosis management: (a) First-line: bisphosphonates (e.g., alendronate, risedronate, etc.) or denosumab; (b) Second-line: hormonal therapy (e.g., calcitonin, raloxifene, teriparatide, etc.); and (c) Supplements: Vitamin D and calcium; (4) Vertebral augmentation (if refractory to above): Vertebroplasty, or Kyphoplasty; and (5) Posterior decompression and fixation or Anterior decompression and reconstruction may be required if neurological deficits are present.
This narrative review summarized the current literature surrounding OVCFs and serves as a guideline for managing these fractures. The detailed pathogenesis and related targeted treatment options still need to be developed for better clinical outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ma X, China; Mostafavinia A, Iran A-Editor: Lin FY, United States S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Kutsal FY, Ergin Ergani GO. Vertebral compression fractures: Still an unpredictable aspect of osteoporosis. Turk J Med Sci. 2021;51:393-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 2. | Riggs BL, Melton LJ 3rd. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995;17:505S-511S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 866] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 4. | Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, Kanis J. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res. 2000;15:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 344] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Oleksik AM, Ewing S, Shen W, van Schoor NM, Lips P. Impact of incident vertebral fractures on health related quality of life (HRQOL) in postmenopausal women with prevalent vertebral fractures. Osteoporos Int. 2005;16:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Tosteson AN, Gabriel SE, Grove MR, Moncur MM, Kneeland TS, Melton LJ 3rd. Impact of hip and vertebral fractures on quality-adjusted life years. Osteoporos Int. 2001;12:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 166] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Esses SI, McGuire R, Jenkins J, Finkelstein J, Woodard E, Watters WCI. The Treatment of Symptomatic Osteoporotic Spinal Compression Fractures. J Am Acad Orthop Surg. 2011;19:176-182. [RCA] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Picazo DR, Villaescusa JR, Martínez EP, Pérez FD. Late collapse osteoporotic vertebral fracture in an elderly patient with neurological compromise. Eur Spine J. 2014;23:2696-2702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Varacallo MA, Fox EJ. Osteoporosis and its complications. Med Clin North Am. 2014;98:817-831, xii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Rosen HN, Walega DR. Osteoporotic thoracolumbar vertebral compression fractures: Clinical manifestations and treatment. In: Rosen CJ, Mulder JE, editors. UpToDate, 2020. [cited 20 January 2022]. Available from: https://www.uptodate.com/contents/osteoporotic-thoracolumbar-vertebral-compression-fractures-clinical-manifestations-and-treatment#!. |
| 11. | McCarthy J, Davis A. Diagnosis and Management of Vertebral Compression Fractures. Am Fam Physician. 2016;94:44-50. [PubMed] |
| 12. | Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R; National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25:2359-2381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1953] [Cited by in RCA: 2134] [Article Influence: 194.0] [Reference Citation Analysis (0)] |
| 13. | Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129. [PubMed] |
| 14. | Coughlan T, Dockery F. Osteoporosis and fracture risk in older people. Clin Med (Lond). 2014;14:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 15. | Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. The Lancet. 2002;359:1929-1936. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1433] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 16. | Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1138] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 17. | Parfitt A. Bone-forming cells in clinical condictions. The osteoblast and osteocyte. 1990;. |
| 18. | Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, Looker AC, Wallace TC, Wang DD. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 374] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 19. | Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc. 2002;77:453-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Genev IK, Tobin MK, Zaidi SP, Khan SR, Amirouche FML, Mehta AI. Spinal Compression Fracture Management: A Review of Current Treatment Strategies and Possible Future Avenues. Global Spine J. 2017;7:71-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Toyoda H, Takahashi S, Hoshino M, Takayama K, Iseki K, Sasaoka R, Tsujio T, Yasuda H, Sasaki T, Kanematsu F, Kono H, Nakamura H. Characterizing the course of back pain after osteoporotic vertebral fracture: a hierarchical cluster analysis of a prospective cohort study. Arch Osteoporos. 2017;12:82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | McCarberg BH. NSAIDs in the older patient: balancing benefits and harms. Pain Med. 2013;14 Suppl 1:S43-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Singh G. Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. Am J Med. 1998;105:31S-38S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 285] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43:1905-1915. [PubMed] [DOI] [Full Text] |
| 25. | Gennari C. Analgesic effect of calcitonin in osteoporosis. Bone. 2002;30:67-70. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 26. | Franklin GM; American Academy of Neurology. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology. 2014;83:1277-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 27. | Busse JW, Wang L, Kamaleldin M, Craigie S, Riva JJ, Montoya L, Mulla SM, Lopes LC, Vogel N, Chen E, Kirmayr K, De Oliveira K, Olivieri L, Kaushal A, Chaparro LE, Oyberman I, Agarwal A, Couban R, Tsoi L, Lam T, Vandvik PO, Hsu S, Bala MM, Schandelmaier S, Scheidecker A, Ebrahim S, Ashoorion V, Rehman Y, Hong PJ, Ross S, Johnston BC, Kunz R, Sun X, Buckley N, Sessler DI, Guyatt GH. Opioids for Chronic Noncancer Pain: A Systematic Review and Meta-analysis. JAMA. 2018;320:2448-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 491] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 28. | Garg B, Dixit V, Batra S, Malhotra R, Sharan A. Non-surgical management of acute osteoporotic vertebral compression fracture: A review. J Clin Orthop Trauma. 2017;8:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Kim DH, Vaccaro AR. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J. 2006;6:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 30. | Sinaki M. Exercise for Patients With Osteoporosis: Management of Vertebral Compression Fractures and Trunk Strengthening for Fall Prevention. PM R. 2012;4:882-888. [RCA] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Kazem Shakouri S, Soleimanpour J, Salekzamani Y, Oskuie MR. Effect of low-level laser therapy on the fracture healing process. Lasers Med Sci. 2010;25:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Medalha CC, Santos AL, Veronez Sde O, Fernandes KR, Magri AM, Renno AC. Low level laser therapy accelerates bone healing in spinal cord injured rats. J Photochem Photobiol B. 2016;159:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Arjmand B, Khodadost M, Jahani Sherafat S, Rezaei Tavirani M, Ahmadi N, Hamzeloo Moghadam M, Okhovatian F, Rezaei Tavirani S, Rostami-Nejad M. Low-Level Laser Therapy: Potential and Complications. J Lasers Med Sci. 2021;12:e42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 34. | Teyssédou S, Saget M, Pries P. Kyphopasty and vertebroplasty. Orthop Traumatol Surg Res. 2014;100:S169-S179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Deramond H, Depriester C, Galibert P, Le Gars D. Percutaneous vertebroplasty with polymethylmethacrylate. Technique, indications, and results. Radiol Clin North Am. 1998;36:533-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 567] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 36. | Gangi A, Guth S, Imbert JP, Marin H, Dietemann J-L. Percutaneous Vertebroplasty: Indications, Technique, and Results. RadioGraphics. 2003;23:e10-e. [RCA] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Hulme PA, Krebs J, Ferguson SJ, Berlemann U. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine (Phila Pa 1976). 2006;31:1983-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 493] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 38. | Peters KR, Guiot BH, Martin PA, Fessler RG. Vertebroplasty for osteoporotic compression fractures: current practice and evolving techniques. Neurosurgery. 2002;51:S96-103. [PubMed] |
| 39. | Predey TA, Sewall LE, Smith SJ. Percutaneous vertebroplasty: new treatment for vertebral compression fractures. Am Fam Physician. 2002;66:611-615. [PubMed] |
| 40. | Mathis JM, Barr JD, Belkoff SM, Barr MS, Jensen ME, Deramond H. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol. 2001;22:373-381. [PubMed] |
| 41. | Cotten A, Dewatre F, Cortet B, Assaker R, Leblond D, Duquesnoy B. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200:525-530. [RCA] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 567] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 42. | Deramond H, Depriester C, Toussaint P, Galibert P. Percutaneous Vertebroplasty. Semin Musculoskelet Radiol. 1997;1:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Padovani B, Kasriel O, Brunner P, Peretti-Viton P. Pulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 1999;20:375-377. [PubMed] |
| 44. | Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, Graves S, Staples MP, Murphy B. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 906] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 45. | Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569-579. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1145] [Cited by in RCA: 933] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 46. | McCullough BJ, Comstock BA, Deyo RA, Kreuter W, Jarvik JG. Major medical outcomes with spinal augmentation vs conservative therapy. JAMA Intern Med. 2013;173:1514-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Kurra S, Metkar U, Lieberman IH, Lavelle WF. The Effect of Kyphoplasty on Mortality in Symptomatic Vertebral Compression Fractures: A Review. Int J Spine Surg. 2018;12:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Lee MJ, Dumonski M, Cahill P, Stanley T, Park D, Singh K. Percutaneous treatment of vertebral compression fractures: a meta-analysis of complications. Spine (Phila Pa 1976). 2009;34:1228-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Zhang JD, Poffyn B, Sys G, Uyttendaele D. Comparison of vertebroplasty and kyphoplasty for complications. Orthop Surg. 2011;3:158-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Garnier L, Tonetti J, Bodin A, Vouaillat H, Merloz P, Assaker R, Court C; French Society for Spine Surgery. Kyphoplasty vs vertebroplasty in osteoporotic thoracolumbar spine fractures. Short-term retrospective review of a multicentre cohort of 127 consecutive patients. Orthop Traumatol Surg Res. 2012;98:S112-S119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Li X, Yang H, Tang T, Qian Z, Chen L, Zhang Z. Comparison of kyphoplasty and vertebroplasty for treatment of painful osteoporotic vertebral compression fractures: twelve-month follow-up in a prospective nonrandomized comparative study. J Spinal Disord Tech. 2012;25:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Mehio AK, Lerner JH, Engelhart LM, Kozma CM, Slaton TL, Edwards NC, Lawler GJ. Comparative hospital economics and patient presentation: vertebroplasty and kyphoplasty for the treatment of vertebral compression fracture. AJNR Am J Neuroradiol. 2011;32:1290-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Prost S, Pesenti S, Fuentes S, Tropiano P, Blondel B. Treatment of osteoporotic vertebral fractures. Orthop Traumatol Surg Res. 2021;107:102779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 54. | Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1179] [Cited by in RCA: 1063] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 55. | Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest. 1996;97:2692-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 561] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 56. | Russell LA. Osteoporosis and Orthopedic Surgery: Effect of Bone Health on Total Joint Arthroplasty Outcome. Current Rheumatology Reports. 2013;15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 57. | Ensrud KE, Schousboe JT. Clinical practice. Vertebral fractures. N Engl J Med. 2011;364:1634-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 58. | Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1643] [Cited by in RCA: 1476] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 59. | Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR; Fracture Intervention Trial. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85:4118-4124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 554] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 60. | Wells G, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;CD004523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 61. | Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1762] [Cited by in RCA: 1582] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 62. | Gu HF, Gu LJ, Wu Y, Zhao XH, Zhang Q, Xu ZR, Yang YM. Efficacy and Safety of Denosumab in Postmenopausal Women With Osteoporosis: A Meta-Analysis. Medicine (Baltimore). 2015;94:e1674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 63. | Hildebrand GK, Kasi A. Denosumab. 2021 Dec 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022. [PubMed] |
| 64. | Rabenda V, Vanoverloop J, Fabri V, Mertens R, Sumkay F, Vannecke C, Deswaef A, Verpooten GA, Reginster JY. Low incidence of anti-osteoporosis treatment after hip fracture. J Bone Joint Surg Am. 2008;90:2142-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 65. | Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2264] [Cited by in RCA: 2278] [Article Influence: 142.4] [Reference Citation Analysis (0)] |
| 66. | Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 343] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 67. | Kallio DM, Garant PR, Minkin C. Ultrastructural effects of calcitonin on osteoclasts in tissue culture. J Ultrastruct Res. 1972;39:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 105] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Cranney A, Tugwell P, Zytaruk N, Robinson V, Weaver B, Shea B, Wells G, Adachi J, Waldegger L, Guyatt G; Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis. VI. Meta-analysis of calcitonin for the treatment of postmenopausal osteoporosis. Endocr Rev. 2002;23:540-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Delmas PD, Ensrud KE, Adachi JD, Harper KD, Sarkar S, Gennari C, Reginster JY, Pols HA, Recker RR, Harris ST, Wu W, Genant HK, Black DM, Eastell R; Mulitple Outcomes of Raloxifene Evaluation Investigators. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609-3617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 285] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 70. | Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3315] [Cited by in RCA: 3049] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 71. | Ralston S. Bisphosphonates for Osteoporosis. In: Harrison-Woolrych M, editor. Medicines For Women. Cham: Springer International Publishing, 2015: 345-371. [DOI] [Full Text] |
| 72. | Jeremiah MP, Unwin BK, Greenawald MH, Casiano VE. Diagnosis and Management of Osteoporosis. Am Fam Physician. 2015;92:261-268. [PubMed] |
| 73. | Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB; Women's Health Initiative Investigators. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. JAMA. 2003;290:1729-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 781] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 74. | Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11239] [Cited by in RCA: 10436] [Article Influence: 453.7] [Reference Citation Analysis (0)] |
| 75. | Sinaki M, Itoi E, Wahner HW, Wollan P, Gelzcer R, Mullan BP, Collins DA, Hodgson SF. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone. 2002;30:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 211] [Article Influence: 9.2] [Reference Citation Analysis (0)] |