Published online May 18, 2022. doi: 10.5312/wjo.v13.i5.538
Peer-review started: December 12, 2021
First decision: January 22, 2022
Revised: February 4, 2022
Accepted: April 24, 2022
Article in press: April 24, 2022
Published online: May 18, 2022
Processing time: 151 Days and 7.3 Hours
Gastrointestinal stromal tumors (GISTs) are rare primary neoplasms of the gastrointestinal tract, accounting for 1% to 2% of all gastrointestinal neoplasms worldwide. GISTs are frequently discovered incidentally during workup for other diagnosis or intestinal obstruction, as they can present with few or no symptoms. Simultaneously, GISTs confer a high degree of malignant transformation, with a progression in about 10% to 30% of cases.
A 63-year-old healthy female presented to our institution with complaints of right knee pain and limited passive and active motion in the setting of a previous right total knee arthroplasty (TKA). One year after TKA, the patient was incidentally diagnosed with a GIST, which was successfully removed. After removal, the patient continued to have limited range of motion of the right knee and subsequently underwent revision TKA. Intraoperatively significant fibrotic adhesions were found encapsulating the femoral and tibial components. The patient’s pain improved postoperatively, however, she continued to have decreased range of motion with difficulty ambulating.
We propose that this case may demonstrate a proinflammatory milieu arising from a GIST, which had a direct influence on the outcome of recent total knee arthroplasty. This proposed mechanism between neoplastic cytokinetic activity and adhesion formation could have implications on preoperative and postoperative orthopedic management of total knee arthroplasty.
Core Tip: This case demonstrated a patient who had recurrent adhesion formation resulting in reduced clinical outcomes, hypothesized to be secondary to a gastrointestinal stromal tumor (GIST) inflammatory response. Currently, there is a paucity of literature documenting GIST tumors and potential adhesion formation and decreased clinical outcomes in patients with prosthetic joints.
- Citation: Mitchell S, Lee A, Stenquist R, Yatsonsky II D, Mooney ML, Shendge VB. Extensive adhesion formation in a total knee replacement in the setting of a gastrointestinal stromal tumor: A case report. World J Orthop 2022; 13(5): 538-543
- URL: https://www.wjgnet.com/2218-5836/full/v13/i5/538.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i5.538
Adhesion formation is a serious cause of adverse outcomes in the medical field. Several triggers of adhesion formation include inflammation, foreign body sensation and iatrogenic causes[1-3]. Currently, the underlying mechanisms of adhesion remain unclear but the regulation of fibrinolytic activity, leading to fibrosis, is thought to play an important role in adhesion formation[1]. Gastrointestinal stromal tumors (GISTs) are especially of interest because circulating levels of cell adhesion marker L1 Cellular Adhesion Molecule (L1CAM) which has been proposed as a prognostic marker[1,4]. Cell adhesion molecule L1CAM (CD171), a 200–220 kDa type I glycoprotein of the immunoglobulin superfamily, plays a role in development of the nervous system by regulating cell interactions, including neuronal migration[5]. L1CAM has also been shown to have other interactions with cell regulators such as integrins which play an important role in inflammation and fibrosis[6]. This case report demonstrates a patient with GIST that presented with extensive adhesion formation status-post total knee replacement, resulting in limited range of motion and need for revision surgery.
Our patient is a 63-year-old female with complaints of right knee osteoarthritis causing her significant discomfort.
The patient has had complaints of right knee osteoarthritis since 2015. The patient’s other conditions included long-standing hypertension, controlled type II diabetes mellitus, and moderate obesity. Radiographs of the right knee demonstrated moderate tricompartmental osteoarthritis of the knee with notable valgus deformity. Initially, she was managed conservatively with non-steroidal anti-inflammatory drugs (NSAIDs), bracing, intra-articular cortisone injections, and physical therapy. After failing two years of conservative treatment, she elected to undergo right total knee arthroplasty in January 2017. The patient underwent right total knee arthroplasty with a Stryker Triathlon X3 implant system with posterior stabilization. The postoperative course was unremarkable; she was instructed to continue knee range of motion exercises and was referred to physical therapy.
In June 2018, the patient returned to the orthopedics clinic with pain and stiffness of the right knee. Physical exam revealed right knee range of motion at 95˚ flexion to full extension. Radiographs were unremarkable with no change of the components noted from the previous. The patient had been compliant with her physical therapy and was ambulating without assistive devices. The patient was instructed to continue strengthening exercises and was referred to physical therapy.
In February 2018, the patient presented to an outside facility for complaints of severe fatigue and melena. Subsequent computed tomography imaging and esophagogastroduodenoscopy revealed diffuse gastric wall thickening with a fungating mass and focal calcifications within the greater curvature of the stomach suspicious for neoplastic disease. Subsequent biopsy of this mass revealed a CD117-positive tumor, while being negative for pancytokeratin, CD20 (B-cell marker), and Helicobacter pylori, which was consistent with an ulcerated gastrointestinal stromal tumor. This tumor was successfully excised in April 2018 via open gastrectomy without complications; postoperative pathology confirmed the diagnosis of spindle-type GIST with adequate negative surgical margins. The patient was subsequently started on long-term imatinib mesylate therapy and placed on an annual surveillance program.
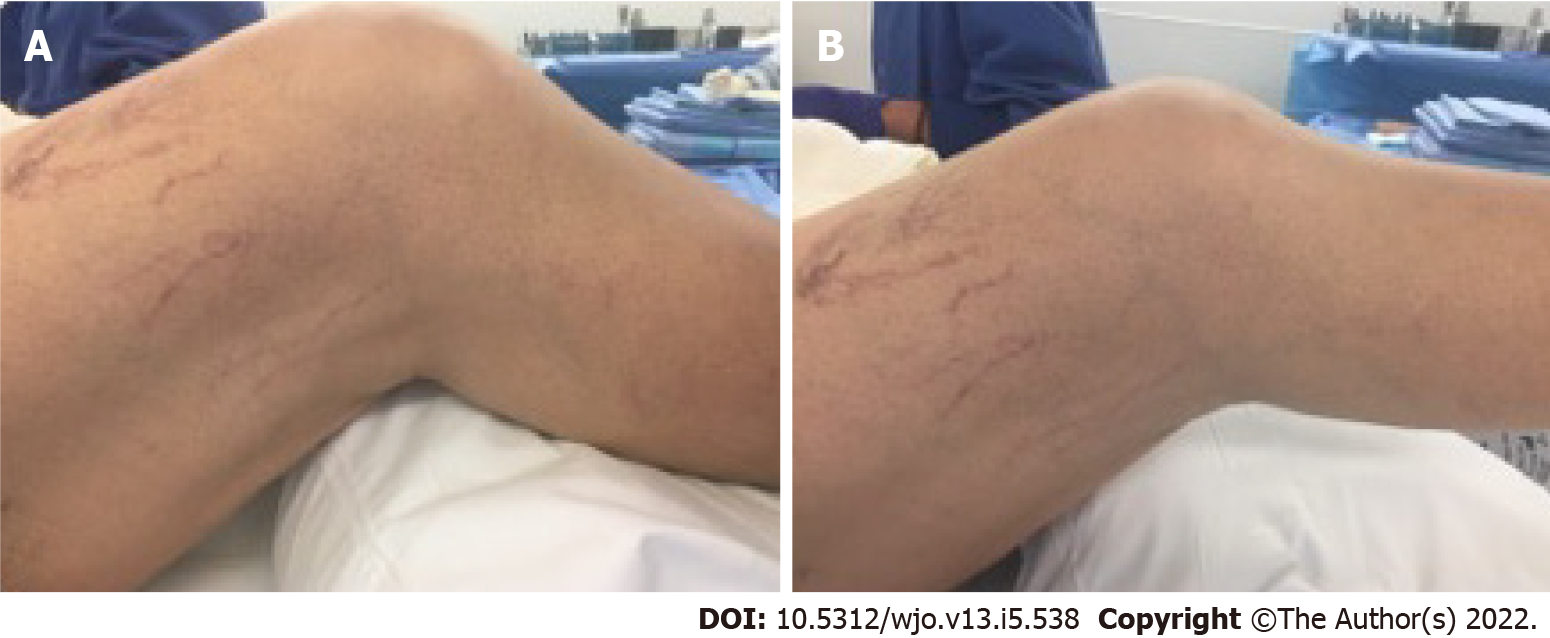
In November 2018, the patient elected to undergo joint manipulation under general anesthesia due to continuing knee pain. Prior to manipulation, the patient's right knee range of motion was lacking 10-15˚ of full extension and flexion to 50˚. Post-procedure range-of-motion (ROM) demonstrated significant improvement in the ROM, with full extension to 105˚ of flexion. However, the patient quickly re-developed right knee stiffness. Two months after knee manipulation under anesthesia, clinical examination revealed a fixed flexion deformity of 25° and active flexion to 65° (Figure 1A and B). Radiographs demonstrated evidence of early polyethylene wear.
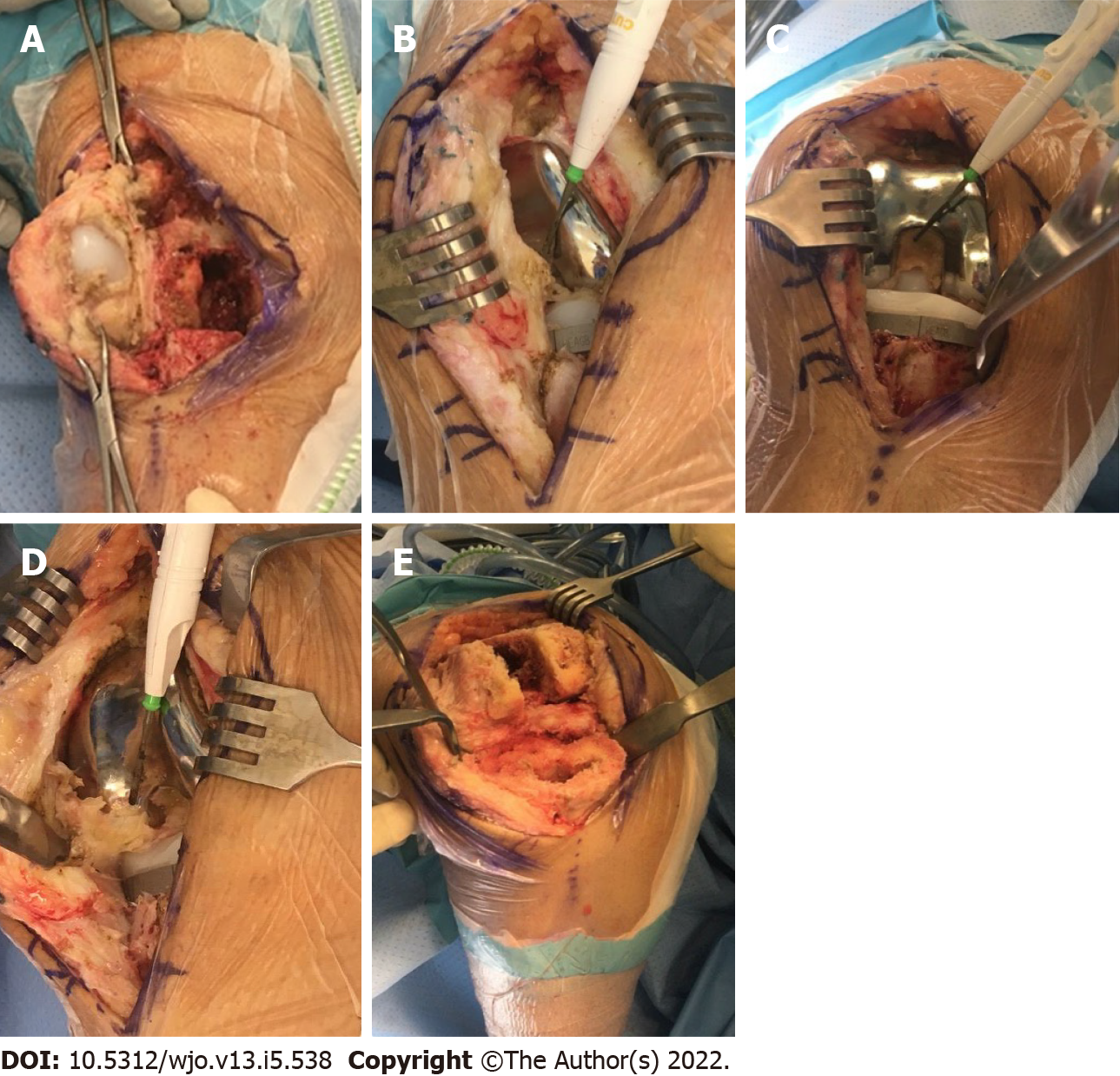
In July 2020, three-and-a-half years after her initial prosthesis implantation and after multiple attempts at physical therapy, pain management, and an unsuccessful joint manipulation, the patient elected for a single-stage revision of her right knee prosthesis. Intraoperative findings demonstrated significant adhesion formation in the suprapatellar fossa extending from the anterior aspect of the patella into the intercondylar notch and posterior capsule (Figure 2A and B). Severe calcification was noted within the posterior compartment of the knee, and significant adhesions were appreciated between the polyethylene liner and the femoral component, as well as within the lateral and medial gutters (Figure 2C and D). Multiple intraoperative specimens were sent for culture demonstrating aseptic, dense fibrous tissue with abundant fibrin and chronic inflammation. After extensive debridement, the patient underwent successful revision with a Stryker Triathlon implant system including posterior stabilization (Figure 2E).
The final diagnosis of the presented case is tricompartmental osteoarthritis of the knee with notable valgus deformity.
The patient was initially managed conservatively with NSAIDs, bracing, intra-articular cortisone injections, and physical therapy. After failing two years of conservative treatment, she elected to undergo right total knee arthroplasty in January 2017. The patient underwent right total knee arthroplasty with a Stryker Triathlon X3 implant system with posterior stabilization. One year post-operatively, the patient returned to the clinic with pain and stiffness of the right knee and elected to undergo joint manipulation under general anesthesia 18 mo after TKA. Three-and-a-half years after her initial prosthesis implantation and after multiple attempts at physical therapy, pain management, and an unsuccessful joint manipulation, the patient elected for a single-stage revision of her right knee prosthesis. After extensive debridement, the patient underwent successful revision with a Stryker Triathlon implant system including posterior stabilization.
Four months after TKA revision, the patient presented with moderate stiffness and was now utilizing a cane. On exam, the patient demonstrated 5˚ of total motion in her right knee with minimal pain. Radiographs obtained at this visit suggested a progressive scarring process in the posterior aspect of the knee. Due to this recurring scarring and limited outcome overall, this patient will be followed in a long-term setting with strengthening exercises and other conservative management constituting her primary treatment, as we discussed with this patient that she may have reached the maximal medical improvement following this revision surgery.
GISTs are primary neoplasms of the gastrointestinal tract that are rare, accounting for 1% to 2% of all gastrointestinal neoplasms, and are frequently discovered incidentally during workup for other diagnosis or intestinal obstruction. GISTs are often clinically silent and confer a high degree of malignant transformation, with a progression in about 10% to 30% of cases[1,7]. Søreide et al[6] found that the median age for GISTs was 65 with a 1:1 male to female ratio. Tumors are often 2-30 cm at time of diagnosis and cause symptoms either by mass-effect or ulcerative anemia. These tumors were not well-characterized until the 1990s, and literature suggests that GISTs have been underdiagnosed for decades[8].
Most GISTs are of sporadic etiology (97%), thought to arise from an activating mutation in either KIT or platelet-derived growth factor A[7]. Typically, these tumors are composed of a nearly uniform population of spindle cells (70%), epithelioid cells (20%) or a mix of these two morphologies[1]. Over 95% of GIST tumors are associated with KIT Tyrosine activity (CD117), while over 80% of GISTs are associated with BCL2, and 70% associated with CD34[4,5,9]. Since there are a variety of presentations, this tumor is often mistaken with fibromatosis and leiomyosarcoma[10].
Key components in metastatic neoplastic lineages are mutations that either downregulate adhesion genes or upregulate motility-promoting genes. One such motility-promoting molecule is called L1CAM. L1CAM has been shown to promote angiogenesis and pro-fibrotic environments, and is associated with mediating overall cell survival[9,11,12]. Concurrently, L1CAM has been found to be expressed in at least 74% of GISTs, with higher L1CAM concentrations being associated with worse outcomes[10]. L1CAM’s extracellular domains are associated with activation of integrin-dependent processes, as well as interactions with fibroblast growth factor receptor (FGFR). Prior research utilized peptides to block the L1CAM-FGFR signaling pathway, which resulted in reduced glioma cell migration and motility[13]. Double knockdown of L1CAM expression and FGFR activity in glioblastoma cells significantly reduced cellular motility (96% reduction) while knockdown of either L1CAM or FGFR displayed partially reduced cell migration[13]. These studies suggested an important relationship between L1CAM-FGFR interactions and the development of metastatic disease. Due to the role that the L1CAM-FGFR interaction plays in metastatic spread and negative outcomes in gliomas and other tumors, this patient’s recurrent adhesion formation could be attributed to their underlying GIST diagnosis. Furthermore, FGFR has been shown to regulate a vast network of profibrotic mediators, including transforming growth factor beta, endothelin-1, and connective tissue growth factor amongst many other profibrotic factors[14-17]. Subsequent activation of FGFRs through the L1CAM molecule that is commonly expressed in GISTs may have led to the generation of fibrotic adhesions within this patient’s prosthetic knee joint.
However, it is also possible that the multiple surgeries this patient underwent activated an inflammatory response within the knee joint, creating a fibrotic environment that resulted in the adhesions observed. While the exact underlying mechanism of intra-articular adhesions is unclear, researchers believe that many cytokines and growth factors can contribute to fibrous tissue hyperplasia which may be responsible for adhesion formation. These cytokines and growth factors are part of the inflammatory response and it is generally recognized that surgical trauma can activate this response. Additional research should clarify the exact mechanisms in which GISTs can promote adhesions in patients who undergo surgery to reduce post-operative complications as well as appropriate management and treatment of these complications. This patient demonstrates a unique case in which a GIST diagnosis may have impacted her ability to regain full motion and use of her knee after total knee arthroplasty.
Our case demonstrated a patient with extensive fibrotic scarring in a relatively new knee prosthesis in the setting of a gastrointestinal stromal tumor. This rare neoplasm has been hypothesized to induce a pro-fibrotic milieu, which has the potential to disrupt artificial devices. Further analysis of patients diagnosed with GISTs and concomitant joint arthroplasty is warranted to elucidate the exact mechanism behind the formation of these adhesions, as well as what potential therapies exist to mitigate joint complications.
The authors would like to thank the orthopedic surgery department of University of Toledo Medical Center for facilitating the resources necessary for publication of this case. We would also like to thank Dr. Vithal Shendge for his significant contributions toward this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen D, China; Dhali A, India S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 560] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Daluga D, Lombardi AV Jr, Mallory TH, Vaughn BK. Knee manipulation following total knee arthroplasty. Analysis of prognostic variables. J Arthroplasty. 1991;6:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 131] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Yatsonsky Ii D, Pan K, Shendge VB, Liu J, Ebraheim NA. Linkage of microbiota and osteoporosis: A mini literature review. World J Orthop. 2019;10:123-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Zander H, Rawnaq T, von Wedemeyer M, Tachezy M, Kunkel M, Wolters G, Bockhorn M, Schachner M, Izbicki JR, Kaifi J. Circulating levels of cell adhesion molecule L1 as a prognostic marker in gastrointestinal stromal tumor patients. BMC Cancer. 2011;11 189:1-189:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Inaguma S, Wang Z, Lasota JP, Miettinen MM. Expression of neural cell adhesion molecule L1 (CD171) in neuroectodermal and other tumors: An immunohistochemical study of 5155 tumors and critical evaluation of CD171 prognostic value in gastrointestinal stromal tumors. Oncotarget. 2016;7:55276-55289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813-3825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 838] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 7. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 8. | Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (1)] |
| 9. | Maten MV, Reijnen C, Pijnenborg JMA, Zegers MM. L1 Cell Adhesion Molecule in Cancer, a Systematic Review on Domain-Specific Functions. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Kaifi J, Strelow A, Schurr P. L1 (CD171) is highly expressed in gastrointestinal stromal tumors. Mod Pathol. 2006;19:399-406. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Geismann C, Arlt A, Bauer I, Pfeifer M, Schirmer U, Altevogt P, Müerköster SS, Schäfer H. Binding of the transcription factor Slug to the L1CAM promoter is essential for transforming growth factor-β1 (TGF-β)-induced L1CAM expression in human pancreatic ductal adenocarcinoma cells. Int J Oncol. 2011;38:257-266. [PubMed] |
| 12. | Lappegård KT, Bergseth G, Riesenfeld J, Pharo A, Magotti P, Lambris JD, Mollnes TE. The artificial surface-induced whole blood inflammatory reaction revealed by increases in a series of chemokines and growth factors is largely complement dependent. J Biomed Mater Res A. 2008;87:129-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Mohanan V, Temburni MK, Kappes JC, Galileo DS. L1CAM stimulates glioma cell motility and proliferation through the fibroblast growth factor receptor. Clin Exp Metastasis. 2013;30:507-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Sahni A, Sahni SK, Simpson-Haidaris PJ, Francis CW. Fibrinogen binding potentiates FGF-2 but not VEGF induced expression of u-PA, u-PAR, and PAI-1 in endothelial cells. J Thromb Haemost. 2004;2:1629-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17:4545-4553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 281] [Cited by in RCA: 309] [Article Influence: 22.1] [Reference Citation Analysis (4)] |
| 16. | Zhao S, Sun Y, Li X, Wang J, Yan L, Chen H, Wang D, Dai J, He J. Reduction of intraarticular adhesion of knee by local application of rapamycin in rabbits via inhibition of fibroblast proliferation and collagen synthesis. J Orthop Surg Res. 2016;11:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Dees C, Chakraborty D, Distler JHW. Cellular and molecular mechanisms in fibrosis. Exp Dermatol. 2021;30:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |