Published online Apr 18, 2022. doi: 10.5312/wjo.v13.i4.388
Peer-review started: June 26, 2021
First decision: October 18, 2021
Revised: October 31, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: April 18, 2022
Processing time: 289 Days and 22.3 Hours
The direct anterior approach for total hip arthroplasty (DAA-THA) is increasing in popularity due to some advantages such as less surgical trauma, minimal dissection of soft tissues, shorter rehabilitation times, faster return to daily activities, lower incidence of dislocation. On the other hand, the literature reports a high rate of intraoperative complications, with many different rates and complication types in the published papers.
To analyze our complications comparing results with the literature; to report measures that we have taken to reduce complications rate.
All DAA-THA patients with one year minimum follow up who were operated at a single high-volume centre, between January 2010 and December 2019 were included in this retrospective study. All surgeries were performed using cementless short anatomical or straight stems and press fit cups. Patients’ follow-up was performed, at 6 wk, 3 mo, then annually post-surgery with clinical and radiological evaluation. Primary outcomes were stem revision for aseptic loosening and all-cause stem revision. Second outcome was intra-operative and post-operative complications identification.
A total of 394 patients underwent DDA-THA from January 2010 and December 2019, for a total of 412 hips; twelve patients lost to follow-up and one patient who died from causes not related to surgery were excluded from the study. The average age at the time of surgery was 61 years (range from 28 to 78 years). Mean follow-up time was 64.8 mo (range 12-120 mo). Seven stems were revised. One cortical perforation, one trochanteric and lateral cortical wall intraoperative fracture, one diaphyseal fracture, three clinically symptomatic early subsidence and one late aseptic loosening. We also observed 3 periprosthetic fractures B1 according to the Vancouver Classification. Other minor complications not requiring stem revision were 5 un-displaced fractures of the calcar region treated with preventive cerclage, one early infection, one case of late posterior dislocation, 18 case of asymptomatic stem subsidence, 6 cases of lateral cutaneous femoral nerve dysesthesia.
DAA is associated to good outcomes and lower incidence of dislocation. Complication rate can be reduced by mindful patient selection, thorough preoperative planning, sufficient learning curve and use of intraoperative imaging.
Core Tip: Direct anterior approach for total hip arthroplasty is increasing in popularity due to some advantages such as less surgical trauma, shorter rehabilitation times, faster return to daily activities, lower incidence of dislocation. Moreover, the literature reports a high rate of intraoperative complications, with many different rates and complication types in the published papers. The aim of this paper is to analyze our complications comparing the results obtained in a total of 412 hips at a mean follow-up of 64.8 mo with those reported in the literature and to describe measures that we have taken to reduce complications rate.
- Citation: Rivera F, Comba LC, Bardelli A. Direct anterior approach hip arthroplasty: How to reduce complications - A 10-years single center experience and literature review. World J Orthop 2022; 13(4): 388-399
- URL: https://www.wjgnet.com/2218-5836/full/v13/i4/388.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i4.388
The direct anterior approach (DAA) was first described by Heuter in 1881 but popularized by Judet starting in 1947[1]. However, it has only recently become popular. In particular, this happened in 2005 following publications by Matta et al[2,3]. The increase in popularity of DDA is due to the advantages such as less surgical trauma, minimal dissection of soft tissues, shorter rehabilitation times, faster return to daily activities, lower incidence of dislocation. However, the literature reports a higher rate of perioperative DAA complications than other approaches[4-8] like periprosthetic fracture, prosthesis loosening and nerve injury, but the reported complication rates vary widely in the published literature both in the incidence rate and in the type of complication[9-13]. There is not yet consensus concerning the best approach for total hip arthroplasty (THA) and debates are ongoing[14]. The interest on DAA complications is growing in the recent literature, also compared with those of other hip approaches[15-17]. The aim of our study is to analyze the complication incidence rate in a study group with a maximum follow up of 10 years and compare the results with the literature. Furthermore, from the analysis of our learning curve, we can derive the technical and organizational measures that we have taken to reduce the incidence of complications with the use of DAA for THA.
All DAA-THA patients with one year minimum follow up who were operated at a single high-volume centre (> 450 arthroplasties/year), between January 2010 and December 2019 were included in this study. The exclusion criteria for the use of DAA in our practice were: age more than 80 years, arthroplasties in hip fractures or in osteolytic lesions, patients with body mass index > 35. Clinical data (gender, age, weight and height) and comorbidities (cardiovascular, respiratory, gastrointestinal, nutritional, endocrine, genitourinary) were collected retrospectively from medical records and outpatient control cards. Radiographic data was taken from the hospital database (Picture archiving and communication system).
All surgeries were performed using cementless short stems and press fit cup by three surgeons experienced in DAA. Two cementless design of the stem were used. Minimax stem and Versafit press-fit cup (Medacta International, Castel San Pietro, Switzerland) and Fitmore stem and Continuum press-fit cup (Zimmer Biomet, Warsaw, IN, USA). Minimax is anatomically designed, collarless, and made of titanium-niobium alloy (Ti-6Al-7Nb) stem. The neck has a 127 degrees neck-shaft angle with an anteversion of 9 degrees. Minimax stem can be classified as type 6 according to Khanuja et al[18] because it is curved, anatomic stems that match the proximal femoral endosteal geometry. Fitmore is a straight designed, tapered, collarless and made of titanium stem which is coated proximally with Ti-VPS (Titanium Vacuum Plasma Spray) and rough-blasted distally. The neck has a neck-shaft angle of 127°, 129°, 137° or 140° without anteversion. Fitmore stem can be classified as type 2 according to Khanuja et al[18] because it is a tapered, wedged and proximal porous coated stem that achieves fixation proximally. According to short stem classification[19], Fitmore stem can be classified as type 4, shortened conventional wedged design.
Final decision whether to use DAA was made by the orthopaedic surgeon, in compliance with the exclusion criteria, operating based on age, fragility, bone morphology, and level of activity of the patient. In a same way, decision whether implant straight or anatomical stem was made by orthopaedic surgeon in compliance with proximal femoral anatomy after pre-operative planning[20,21]. Ceramic on ceramic coupling was chosen and 32 mm or 36 mm head diameter were used in all cases.
All surgeries were done in all patients with the support of the standard operating room table or the Amis Mobile Leg positioner (Medacta International, Castel San Pietro, Switzerland).
Primary outcomes were stem revision for aseptic loosening and all-cause stem revision. Second outcome was intra-operative and post-operative complications identification. Patient follow-up was performed at 6 wk, 3 mo and then annually post-surgery.
During preoperative and postoperative radiographic controls, anteroposterior and axial hip radiographs were taken with the foot in a neutral rotational position. Femoral geometries were categorized according to the Door classification system[22] using preoperative anteroposterior radiographs of the hip. The calcar-to-canal ratio was calculated by dividing the canal width, measured at 10 cm below the lesser trochanter, by the calcar width, measured at the middle level of the lesser trochanter. Femurs with a ratio of 0-0.5 were considered type A, 0.5-0.75 as type B, and 0.75-1 as type C[23,24].
Alignment of the stem was considered neutral when the vertical axis of the stem was between 0 and 2° with respect to the femoral shaft axis. A varus-valgus alignment was classified as mild in case of misalignment between 2° and 5° and severe when the misalignment of the stem was greater than 5°. The most recent radiographs were compared to the first postoperative clinic radiographs to evaluate bony remodeling and changes in implant positioning. Stem subsidence was diagnosed in the presence of a stem sinking greater than 3 mm, measured on a perpendicular line drawn from the greater trochanter to the lateral edge of the implant. Implant loosening was diagnosed in the presence of subsidence and/or axial deviation in varus/valgus. We judged early subsidence or axis deviation within 6 mo of surgery, late when observed after 6 mo post-operative.
We retrospectively reviewed a group of 394 consecutive patients who underwent DDA-THA from January 2010 to December 2019 of whom 18 were operated bilaterally in a single procedure, for a total of 412 hips; twelve patients lost to three-month follow-up and one patient who died from causes not related to surgery were excluded from the study. The remaining 381 patients (399 hips) were 263 (65.9%) female and 136 (34.1%) male. The average age at the time of surgery was 61 years (range from 28 to 78 years). The preoperative diagnosis was primary osteoarthritis in 328 cases (14 bilateral), avascular necrosis of the femoral head in 38 cases (3 bilateral), rheumatoid arthritis in 11 cases (1 bilateral), traumatic osteoarthritis in 18 cases, and other causes in 4 cases. Demographic data of patients are sumarized in Table 1. Mean follow-up time was 64.8 mo (range 12-120 mo). In 238 cases an anatomical stem was used, in 161 cases a straight stem was used. Three hundred forty-four surgeries were performed via the DAA using a standard operating room table (86.2%) and 44 (13.8%) surgeries were performed using the AMIS mobile leg.
| Parameters | Values |
| No. of patients | 381 |
| No. of hips | 399 |
| Gender (male/female), n (%) | 136 (34.1)/263 (65.9) |
| mean age (yr) | 61 (28-78) |
| mean follow-up (mo) | 64,8 (12 –120) |
| Diagnosis (No. hips) | |
| Osteoarthritis | 328 (14 bilateral) |
| Avascular necrosis | 38 (3 bilateral) |
| Rheumatoid arthritis | 11 (1 bilateral) |
| Traumatic osteoarthritis | 18 |
| Other causes | 4 |
| Surgical technique, n (%) | |
| Standard operating room table | 445 (86.4) |
| AMIS mobile leg positioner | 54 (19.4) |
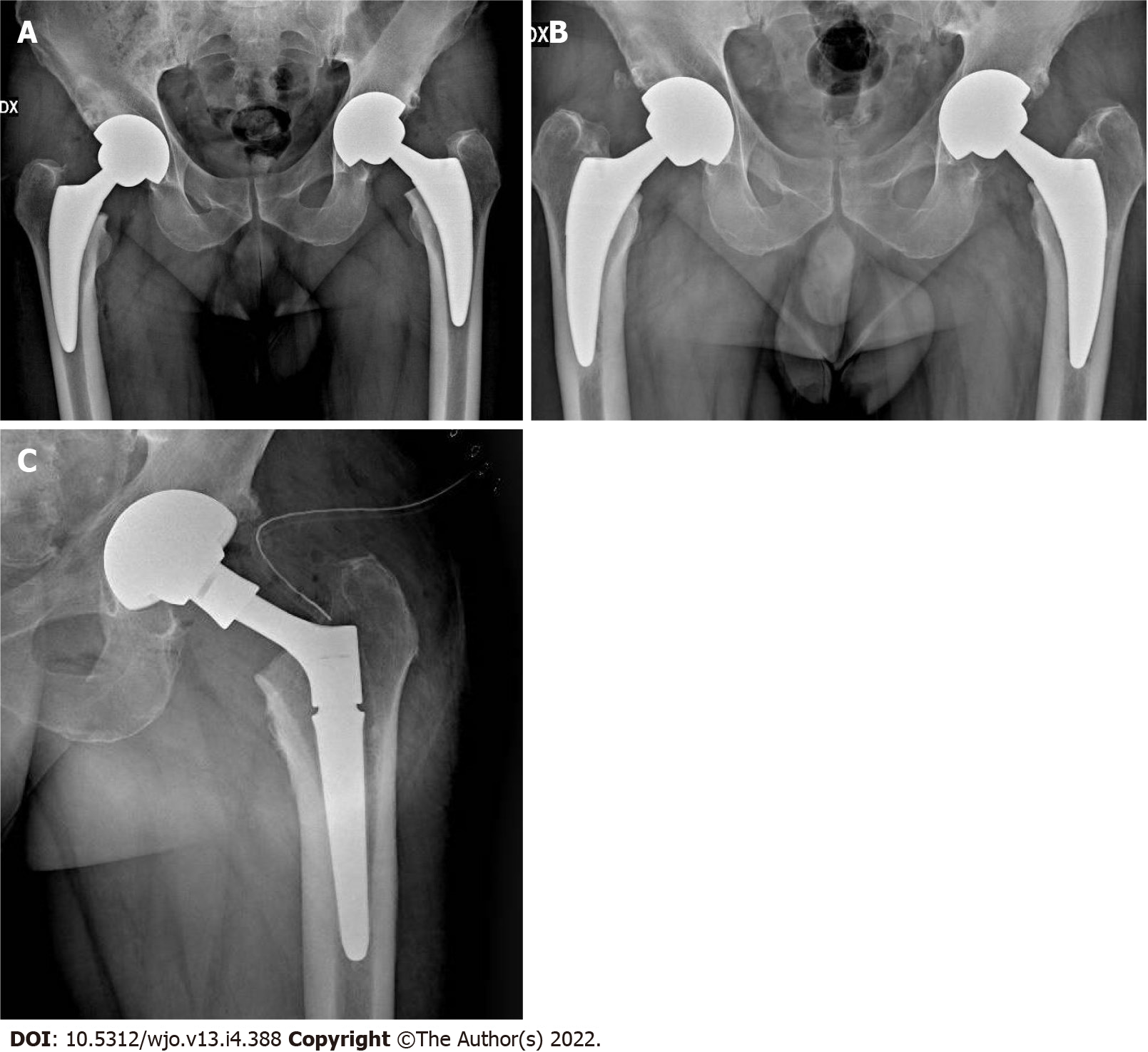
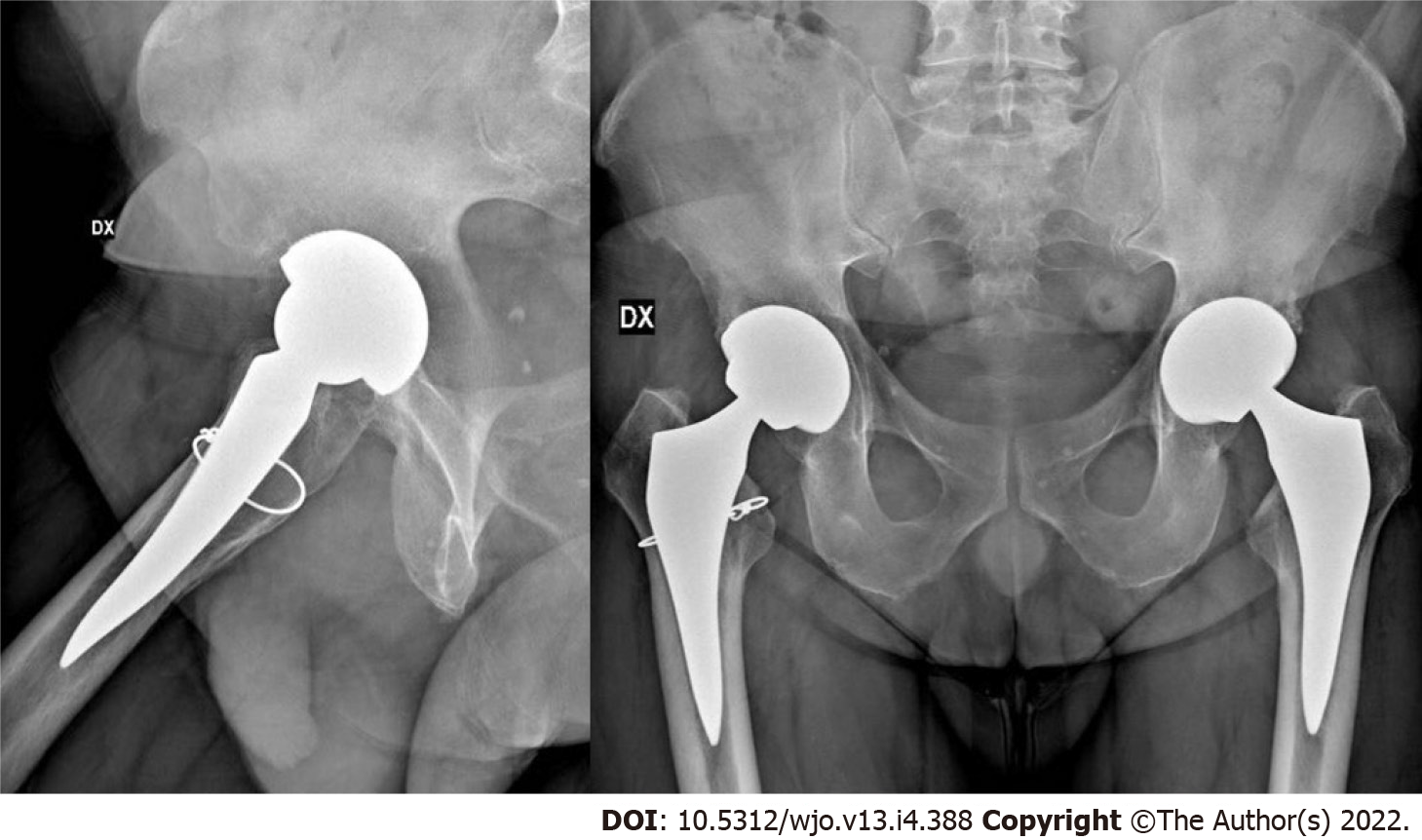
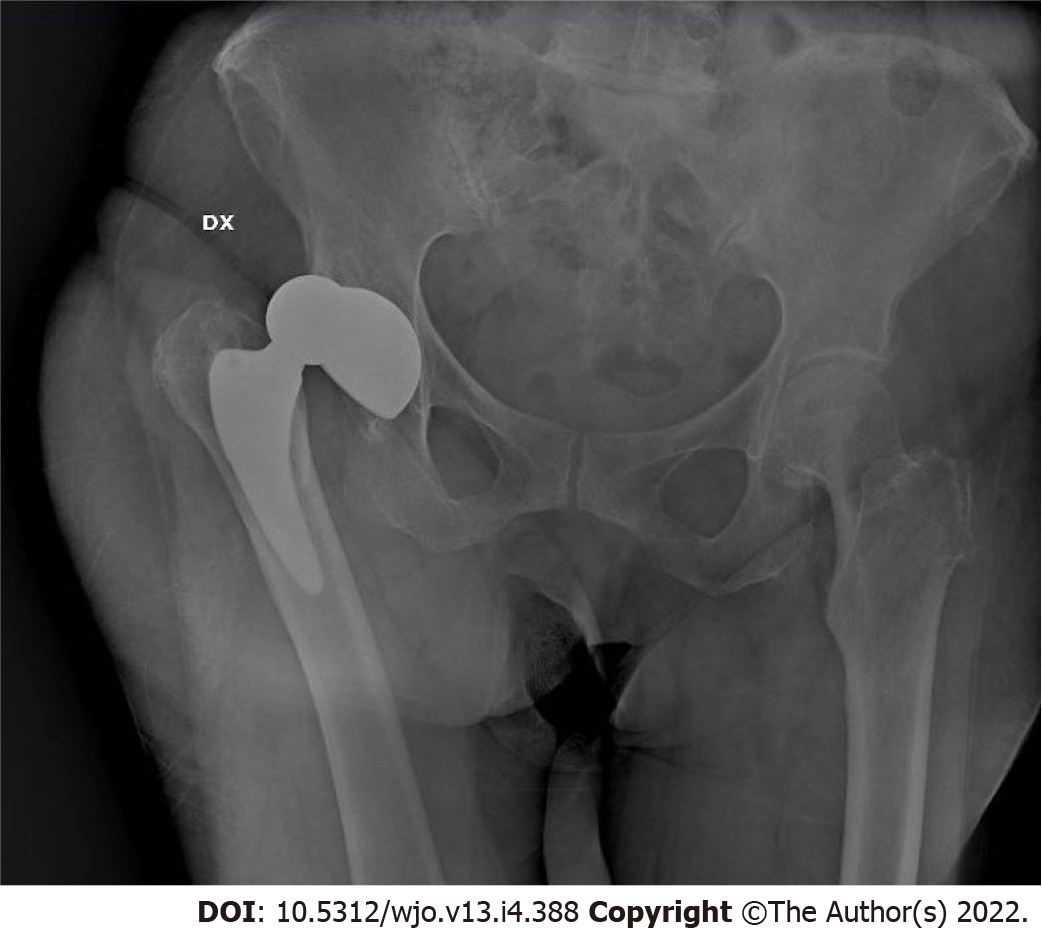
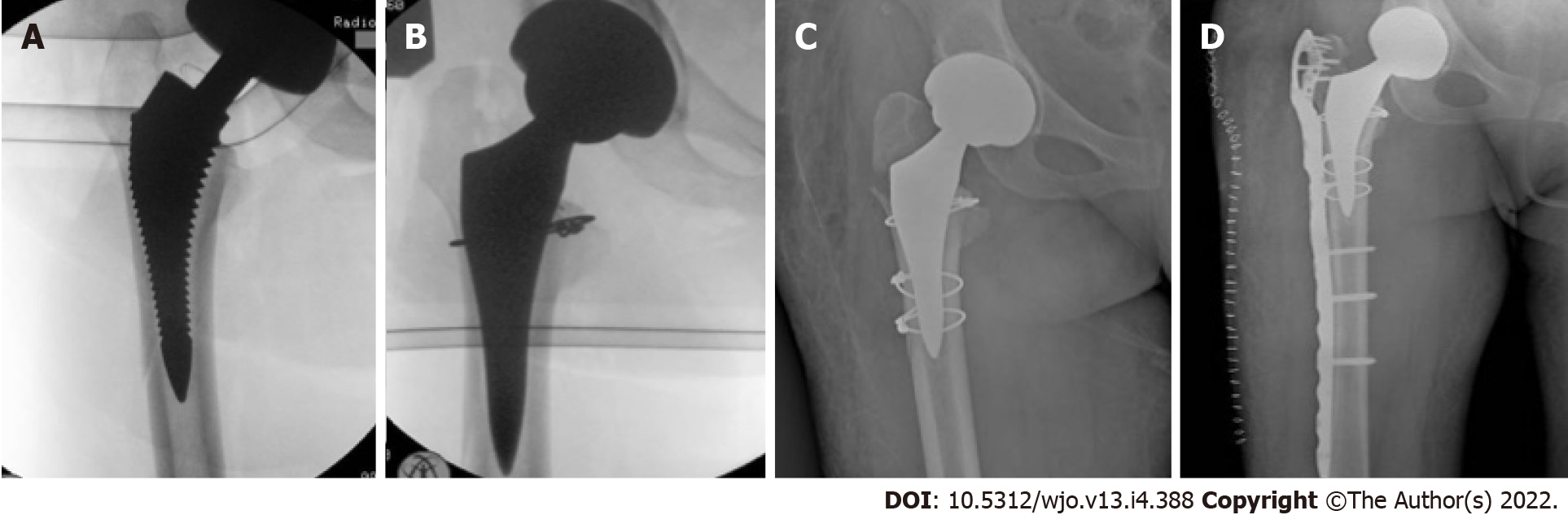
Seven stems were revised. One cortical perforation was observed on postoperative radiographic control and then revised. One trochanteric and lateral cortical wall fracture was intraoperative observed and fixed with cerclage and stem revision. One diaphyseal fracture was treated by plate fixation. Three clinically symptomatic early subsidence revised respectively 5, 6 and 7 mo after surgery. One aseptic loosening 4 years after surgery (Figure 1). We also observed 3 periprosthetic fractures B1 according to the Vancouver Classification. The intra operative complications observed, in addition to the cortical perforation, the diaphyseal fracture and the trochanteric fracture, were 5 un-displaced fractures of the calcar region treated with preventive cerclage (Figure 2). One early infection was treated with surgical washing and head and liner revision followed by antibiotic therapy. One posterior dislocation was observed one year after surgery (Figure 3). Patient referred dislocation during a squat on the ground with the loss of balance. Dislocation was reduced without anesthesia, no further dislocations were observed one year after reduction. Six patients referred numbness or paresthesias within the cutaneous distribution of the anterolateral thigh at follow-up.
According to Dorr classification[17], 168 hips (42%) were graded as Dorr A, 192 hips (48%) as Door B and 39 hips (10%) as Dorr C. In our experience pre-operative planning showed more suitable Fitmore stem for a femoral geometry type A (84/168), according to the Door classification system. This is due to the tapering of the tip adaptable to a narrow femoral canal. Minimax stem is instead more suitable for a femoral geometry type C due to more filling metaphyseal portion (37/39). For this reason, the two groups of patients treated with Fitmore stem and Minimax stem appear not homogeneous and their incidence of complications is not comparable.
In 307 (77%) cases the alignment of the stem was considered neutral, in 76 (19%) cases it was considered mild varus-valgus and in 15 (4%) cases severe varus-valgus. Trabeculae hypertrophy was observed in 84 cases (21%) at zone 3, in 45 cases (11%) at zone 5 and 5 cases (2%) at zone 4. There was grade 1 stress shielding (calcar round-off) in 5 cases (2%). No hypertrophy at zones 8-14 (lateral view) were observed. Stem subsidence > 3 mm, in addition to the one symptomatic case revised, was observed 4 more times (4 mm, 7mm, 6 mm and 10 mm respectively). In all these 4 cases, the absence of pain and the tolerated leg length discrepancy did not compromise the good final functional result.
Finally, 445 (86.4%) surgeries (11 bilateral) were performed via the DAA using a standard operating room table and 54 (19.4%) surgeries (7 bilateral) were performed using the Amis Mobile Leg. Comparison between demographic characteristics and the incidence of complications in the two patient groups (anatomical and straight stem) did not reveal significant differences (Table 2).
| Complications | Anatomical stem (n = 238) | Straight stem (n = 161) |
| Major complication (with stem revision) | 6 | 4 |
| Acute mobilization | 1 | 1 |
| Trochanter fracture | - | 2 |
| Diaphyseal fracture | 1 | - |
| Aseptic loosening | 1 | - |
| Subidence | 1 | - |
| Periprosthetic fractures | 2 | 1 |
| Minor complication | 18 | 9 |
| Calcar fractures (Intraoperative cerclage) | 5 | 1 |
| Ossification | - | 1 |
| Subsidence | ||
| Conservative treatment ≤ 3 mm | 9 | 5 |
| Conservative treatment > 3 mm | 3 | 1 |
| Dislocation | - | 1 |
| Early infection | 1 | - |
| LCFN dysesthesia | 4 | 2 |
This study was undertaken to evaluate DAA-THA complication rates. For this reason, we retrospectively evaluated clinical and radiographic complications at a mean follow-up of 64.8 mo (range 12-120 mo) in 381 patients (399 hips). All patients received a cementless anatomical short stem or a tapered wedged short stem according to inclusion criteria.
Incidence of dislocation is certainly the most relevant result of our study. Commonly considered to be lower than that of others approaches, the small dislocation rate of the DAA is one of the reasons for the great popularity of this approach, although a widely accepted consensus has not been reached yet and some authors report higher complication rates[25].
We found one case of hip dislocation on 399 hips (0.2%) (Figure 3), which represents the one of the lower dislocation incidences reported in literature in case of large study group[26], with the latest works reporting dislocation rate from 0.23% to 2.5%[27,28].
Unexpectedly, the case of hip dislocation found in our study was a posterior dislocation occurred after hip hyper-flexion and adduction, factually suggesting an inadequate cup anteversion, since the dislocation after THA performed with the DAA is usually anterior also due to the preservation of the musculo-tendinous attachments of the short external rotators which are important for hip stabilization after arthroplasty. Nevertheless, also Barnett et al[27] reported a prevalence of posterior dislocation.
Acetabular cup anteversion and inclination are a key point for the long-term success of THA and seen as a challenge in DAA-THA. There are some studies suggesting alternative solutions on this issue; Hu et al[29] described satisfactory clinical and radiographic outcomes achieved by DAA-THA performed in the lateral position. Fluoroscopic guidance is reported to be used to improve component positioning during anterior approach THA, but with still debated results; for example, Rathod et al[30] depicted a reduction of variability of acetabular cup anteversion using fluoroscopy with the patient in the supine position during direct anterior THA, while Kobayashi et al[31] revealed potential excessive cup anteversion and flexion implantation of the stem obtained from fluoroscopic assistance by surgeons in their early experience with DAA. In our practice, we do use intraoperative imaging, but especially to check stem size and alignment in order to mitigate the tendency to undersize the femoral implant associated to DAA-THA, as described in our previous work[32].
Although the use of different femoral heads size has been described in many studies[33,34], it’s known that the use of larger femoral heads increases the head-neck ratio and consequently increases the range of movement before reaching the point of primary impingement thus reducing dislocation rates, as reported in the published literature[35-37]. Also aware of this, in our series we used only 32 mm or 36 mm diameter heads.
An analysis of the Norwegian Arthroplasty Register found overall similar revision rates between hip approaches, but the posterior approach was associated with more than twice the risk of revision due to dislocation when compared with alternate approaches[38].
In the literature are reported various revision rates, rising from 0%[39] to 3.3%[28] with different follow up time. In our series, the global revision rate was 1.8% and revision rate for aseptic loosening was 1% at a mean follow up of 65 mo, with particular attention to three clinically symptomatic early subsidence revised respectively 5, 6 and 7 mo after surgery; especially in these cases it is critical, in our opinion, the intraoperative imaging to mitigate the tendency to undersize the femoral implant associated to DAA-THA[32].
The risk of intraoperative fractures is due to the difficult exposure of the femur. Early studies reported a high complication rate with the use of a fracture table[40]. Most of these were avulsions of the greater trochanter. The evolution of specialized traction tables for DAA, promoting greater patient hip positioning than patient hip traction, has reduced the incidence of complications. Although the differences between complications during the traction table or the standard table are still debated in the current literature, the incidence of intraoperative fracture in DAA is reported between 1.3% and 2.4%[41-43]. The use of short femoral stems is described as a reduction in the risk of intraoperative fractures[44-46]. Dietrich et al[44] reported a significantly reduced fracture rate of 1.6% vs 6.8% in 457 DAA with conventional straight stems. Luger et al[45] reported 0.9% of intraoperative fractures after 1052 DAA- THA with the same straight stem used in our study. In our opinion, the use of short stems with specific instruments for DAA favors the introduction into the femoral canal, decreases the points of conflict with cortices during introduction and decreases the incidence of complications. We observed 3 major complications (0.7%) related to intraoperative fractures (Figure 4). Our rate of intraoperative fracture increases to 2% including minor complications (intraoperative cerclages due to calcar incomplete fractures). Our results encourage us to continue our experience with short stems, both with the use of the standard table and the traction table[8]. Surprisingly, however, Greco et al[42] report an opposite experience. They observed higher femoral complications (1.27%) with short stem standard profile as compared to full length standard profile (0.77%). The Authors concluded that short stem may impart greater stress concentration in the proximal femur during broaching and stem insertion which could increase the risk of fracture. On the contrary, a longer stem aids with the direction of broaching and may prevent inappropriate contact against the femoral cortices. One of the Authors has decided to avoid use of the short stem option in elderly female patients given the compounded risk of femoral complication.
Despite DAA utilizes an internervous plane between Tensor fasciae lata and Gluteus medius muscles (Superior gluteal nerve) and Sartorius and Rectus femoris muscles (Femoral nerve), nerve complications are however possible.
Injury to lateral cutaneous femoral nerve (LCFN) is a not rare minor complication. LCFN is a pure sensory nerve is a sensory nerve that forms from the roots of L2 and L3; travels along the posterolateral aspect of the psoas e iliac muscles through the anterosuperior iliac spine, ending superficially at the sartorius muscle. Then LCFN pierces the fascia lata beneath the inguinal ligament and runs laterally and distally within the subcutaneous tissue of the anterolateral region of the thigh. Some authors describe a division of the LFCN into an anterior (femoral) and posterior (gluteal) branch after passing behind or through the inguinal ligament. Rudin et al[47] classified the branching pattern of the LFCN in three as Sartorius-type (dominant anterior branch on the lateral border of the Sartorius muscle and further branches in the anterior aspect of the thigh), posterior-type [strong posterior branch equal in thickness to, or thicker than, the anterior branch. It runs laterally and crosses the medial border of the tensor fascia lata (TFL) muscle distal to the anterior superior iliac spine (ASIS)] or fan-type (multiple nerve branches of equal thickness on the anterolateral region of the proximal aspect of the thigh, crossing over the TFL and the lateral border of the Sartorius). They reported 36% of sartorius-type, 32% of posterior-type and 32 of fan-type after dissection of twenty-eight cadaveric hemipelves from 18 donors. In contrast to this data, Thaler et al[48], after a study on 44 Limbs and hemipelves from 22 formalin-preserved cadavers, showed a Sartorius-type branch pattern (70.5%) of the LFCN in the majority of cases, while a posterior-type and a fan-type were detected in 13.6% and 15.9% of cases, respectively. If these data were confirmed, injury to branches of the LFCN should be avoided in most cases by a skin incision 2 cm lateral to the ASIS end its distal extension as lateral as possible.
The true incidence of this complication remains unclear. The reported incidence in literature ranges from 0.1% to 81%. This is due to both the diagnostic difficulty and the degree of accuracy of the clinical examination at follow up. In fact, symptoms ranging from hypoesthesia to painful paresthesias and there is no validated diagnostic tool for LFCN neuropraxia. Patients with LFCN injury often report numbness, paresthesias, or even dysesthesias analogous to meralgia paresthetica within the cutaneous distribution of the anterolateral thigh. In our experience, patients often do not report dysesthesia during postoperative follow-up. The disorder is in fact reported only if the patient is directly questioned about the problem of skin sensitivity. Only in 6 cases patients did report dysesthesia or numbness as a symptom that occasionally caused discomfort. We did not include direct skin sensitivity assessment forms in our follow-up, this is a possible reason of our low incidence (1.5%) of LCFN injury. Homma et al[49] investigating skin sensitivity problems using a dedicated questionnaire, showed 31.9% of LFCN injury. In the same way Patton et al[50] reported numbness in 37% of patients with decreasing of incidence to 11% of patients from 6-8 years post op. The fact that symptoms related to LCFN lesion are often reported only after a specific question further suggests that it is a minor complication. Furthermore, in most cases, the presence of LCFN lesions appears to be independent of hip function scores and does not affect final result.
The superior gluteal nerve is a motor nerve, which is formed from the roots of L4 and L5 and the first sacral spinal nerves that supply the gluteus medius, gluteus minimus, and TFL muscles. It runs sideways between the gluteus medius and minimus and then divides into upper and lower branches. Both the upper and lower branches innervate the gluteus medius and minimal muscles. Furthermore, the terminal branches of the inferior branch run anteriorly and supply the tensor of the fascia lata. Precisely these terminal branches represent the anatomical structure at risk. Overstretching the TFL muscle using retractors during surgery or coagulation of the near ascending branch of the lateral circumflex femoral artery can be causes of Injury. There is little information in the literature regarding injury to the TFL with respect to the DAA[51]. The consequences of atrophy of the tensor fasciae are cosmetic, but potential functional deficit is unknow.
Even if in our study we did not consider blood loss, it is reported that DAA THA is related to lower intraoperative blood loss and smaller changes in pre- and postoperative hemoglobin values, less blood drained, and lower volumes of blood transfusions required[52]. Moreover, Zhao et al[53] described no statistically significant differences in the rate of blood transfusion, hematoma, or re-bleeding between patients undergone ligation of the branches of the lateral circumflex femoral artery pedicle vs those treated with electrocautery.
There are some uncommon complications that are still to be taken into account; Hogerzeil et al[6] reported the case of a 69-year-old male patient who developed acute compartment syndrome of the thigh after elective DAA THA while using therapeutic low molecular weight heparin as bridging for regular oral anticoagulation. Also the risk of excessive radiation exposure to both the patients and the surgeons has to be considered; Jinnai et al[54] compared the intraoperative fluoroscopy time of DAA-THA with that of osteosynthesis for proximal femoral fracture to determine if the level of radiation exposure exceeded safety limits and concluding that the intraoperative exposure level was significantly lower and the fluoroscopy time was significantly shorter in DAA-THA than in osteosynthesis.
There are some limitations in this paper. First, the main weakness of our work is the retrospective design of the study. Second, we did not include direct skin sensitivity assessment forms in our follow-up controls and this could be a possible reason of our low incidence of LCFN injury. Third, the exclusion of ages of more than 80 could be considered a selection bias, improving clinical outcome and reducing the revision rate. Fourth, we did not use radiostereometric analysis to evaluate for stem subsidence, but only manual techniques of measurement.
DAA is associated to less surgical trauma, minimal dissection of soft tissues, lower blood loss, shorter rehabilitation times and lower incidence of dislocation. Complication rate can be reduced by mindful patient selection, preoperative planning with proper implant choice, sufficient learning curve, use of intraoperative imaging to check cup and stem orientation and to mitigate the tendency to undersize the femoral implant.
The direct anterior approach for total hip arthroplasty (DAA-THA) is increasing in popularity due to some advantages such as less surgical trauma, minimal dissection of soft tissues, shorter rehabilitation times, faster return to daily activities, lower incidence of dislocation with different reports in the published papers.
Some literature reports a high rate of perioperative complications, with many different rates and complication types among the published papers without reaching a clear consensus.
Objectives of our study are to analyze our complications and comparing results with the literature reports and to report measures that we have taken to reduce complications rate.
We retrospectively collected data of all DAA-THA patients with one year minimum follow up who were operated at a single high-volume centre, between January 2010 and December 2019. All surgeries were performed using cementless short anatomical or straight stems and press fit cups. Patients’ follow-up was performed with clinical and radiological evaluation. Primary outcomes were stem revision for aseptic loosening and all-cause stem revision. Second outcome was intra-operative and post-operative complications identification.
The authors collected 394 patients underwent DDA-THA from January 2010 and December 2019, for a total of 412 hips; twelve patients lost to follow-up and one patient who died from causes not related to surgery were excluded from the study. Mean follow-up time was 64.8 mo (range 12–120 mo). Seven stems were revised. One cortical perforation, one trochanteric and lateral cortical wall intraoperative fracture, one diaphyseal fracture, three clinically symptomatic early subsidence and one late aseptic loosening. We also observed 3 periprosthetic fractures B1 according to the Vancouver Classification. Other minor complications not requiring stem revision were 5 undisplaced fractures of the calcar region treated with preventive cerclage, one early infection, one case of late posterior dislocation, 18 case of asymptomatic stem subsidence, 6 cases of lateral cutaneous femoral nerve dysesthesia.
In our experience DAA is associated to good outcomes and lower incidence of dislocation. According to our results complication rate can be reduced by mindful patient selection, thorough preoperative planning, sufficient learning curve and use of intraoperative imaging.
Despite these good results, the choice of the ideal surgical approach of THA is still controversial and studies on larger samples are needed.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lass R, Austria S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Judet J, Judet H. [Anterior approach in total hip arthroplasty]. Presse Med. 1985;14:1031-1033. [PubMed] |
| 2. | Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;441:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 444] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 3. | Connolly KP, Kamath AF. Direct anterior total hip arthroplasty: Literature review of variations in surgical technique. World J Orthop. 2016;7:38-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Aggarwal VK, Weintraub S, Klock J, Stachel A, Phillips M, Schwarzkopf R, Iorio R, Bosco J, Zuckerman JD, Vigdorchik JM, Long WJ. 2019 Frank Stinchfield Award: A comparison of prosthetic joint infection rates between direct anterior and non-anterior approach total hip arthroplasty. Bone Joint J. 2019;101-B:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Post ZD, Orozco F, Diaz-Ledezma C, Hozack WJ, Ong A. Direct anterior approach for total hip arthroplasty: indications, technique, and results. J Am Acad Orthop Surg. 2014;22:595-603. [PubMed] |
| 6. | Hogerzeil DP, Muradin I, Zwitser EW, Jansen JA. Acute compartment syndrome of the thigh following hip replacement by anterior approach in a patient using oral anticoagulants. World J Orthop. 2017;8:964-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Alvarez-Pinzon AM, Mutnal A, Suarez JC, Jack M, Friedman D, Barsoum WK, Patel PD. Evaluation of wound healing after direct anterior total hip arthroplasty with use of a novel retraction device. Am J Orthop (Belle Mead NJ). 2015;44:E17-E24. [PubMed] |
| 8. | Rivera F, Bardelli A, Giolitti A. Promising medium-term results of anterior approach with an anatomical short stem in primary hip arthroplasty. J Orthop Traumatol. 2021;22:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Goulding K, Beaulé PE, Kim PR, Fazekas A. Incidence of lateral femoral cutaneous nerve neuropraxia after anterior approach hip arthroplasty. Clin Orthop Relat Res. 2010;468:2397-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Woolson ST, Pouliot MA, Huddleston JI. Primary total hip arthroplasty using an anterior approach and a fracture table: short-term results from a community hospital. J Arthroplasty. 2009;24:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | De Geest T, Vansintjan P, De Loore G. Direct anterior total hip arthroplasty: complications and early outcome in a series of 300 cases. Acta Orthop Belg. 2013;79:166-173. [PubMed] |
| 12. | Ukai T, Ebihara G, Watanabe M. Comparison of short-term outcomes of anterolateral supine approach and posterolateral approach for primary total hip arthroplasty: a retrospective study. J Orthop Traumatol. 2021;22:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Dall'Oca C, Ceccato A, Cresceri M, Scaglia M, Guglielmini M, Pelizzari G, Valentini R, Magnan B. Facing complications of direct anterior approach in total hip arthroplasty during the learning curve. Acta Biomed. 2020;91:103-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Migliorini F, Trivellas A, Eschweiler J, El Mansy Y, Mazzanti MC, Tingart M, Aretini P. Hospitalization length, surgical duration, and blood lost among the approaches for total hip arthroplasty: a Bayesian network meta-analysis. Musculoskelet Surg. 2020;104:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Huang XT, Liu DG, Jia B, Xu YX. Comparisons between Direct Anterior Approach and Lateral Approach for Primary Total Hip Arthroplasty in Postoperative Orthopaedic Complications: A Systematic Review and Meta-Analysis. Orthop Surg. 2021;13:1707-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Vles GF, Corten K, Driesen R, van Elst C, Ghijselings SG. Hidden blood loss in direct anterior total hip arthroplasty: a prospective, double blind, randomized controlled trial on topical versus intravenous tranexamic acid. Musculoskelet Surg. 2021;105:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Bendich I, Landy DC, Do H, Krell E, Diane A, Boettner F, Rodriguez J, Alexiades M, Gonzalez Della Valle A. Intraoperative Complications and Early Return to the Operating Room in Total Hip Arthroplasty Performed Through the Direct Anterior and Posterior Approaches. An Institutional Experience of Surgeons After Their Learning Curve. J Arthroplasty. 2021;36:2829-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Khanuja HS, Vakil JJ, Goddard MS, Mont MA. Cementless femoral fixation in total hip arthroplasty. J Bone Joint Surg Am. 2011;93:500-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 348] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 19. | Khanuja HS, Banerjee S, Jain D, Pivec R, Mont MA. Short bone-conserving stems in cementless hip arthroplasty. J Bone Joint Surg Am. 2014;96:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 20. | von Engelhardt LV, Breil-Wirth A, Kothny C, Seeger JB, Grasselli C, Jerosch J. Long-term results of an anatomically implanted hip arthroplasty with a short stem prosthesis (MiniHipTM). World J Orthop. 2018;9:210-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Tootsi K, Lees L, Geiko B, Märtson A. Intraoperative complications in total hip arthroplasty using a new cementless femoral implant (SP-CL®). J Orthop Traumatol. 2020;21:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Dorr LD, Faugere MC, Mackel AM, Gruen TA, Bognar B, Malluche HH. Structural and cellular assessment of bone quality of proximal femur. Bone. 1993;14:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 666] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 23. | Summers S, Nigh E, Sabeh K, Robinson R. Clinical and radiographic outcomes of total hip replacement with a 3-part metaphyseal osseointegrated titanium alloy stem enhanced with low plasticity burnishing: a mean 5-year follow-up study. Arthroplast Today. 2019;5:352-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Solarino G, Vicenti G, Piazzolla A, Maruccia F, Notarnicola A, Moretti B. Total hip arthroplasty for dysplastic coxarthrosis using a cementless Wagner Cone stem. J Orthop Traumatol. 2021;22:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Pincus D, Jenkinson R, Paterson M, Leroux T, Ravi B. Association Between Surgical Approach and Major Surgical Complications in Patients Undergoing Total Hip Arthroplasty. JAMA. 2020;323:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 26. | Chen W, Sun JN, Zhang Y, Chen XY, Feng S. Direct anterior versus posterolateral approaches for clinical outcomes after total hip arthroplasty: a systematic review and meta-analysis. J Orthop Surg Res. 2020;15:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Barnett SL, Peters DJ, Hamilton WG, Ziran NM, Gorab RS, Matta JM. Is the Anterior Approach Safe? J Arthroplasty. 2016;31:2291-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Rahm S, Tondelli T, Steinmetz S, Schenk P, Dora C, Zingg PO. Uncemented Total Hip Arthroplasty Through the Direct Anterior Approach: Analysis of a Consecutive Series of 275 Hips With a Minimum Follow-Up of 10 Years. J Arthroplasty. 2019;34:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Hu F, Shang X, Zhang X, Chen M. Direct anterior approach in lateral position achieves superior cup orientation in total hip arthroplasty: a radiological comparative study of two consecutive series. Int Orthop. 2020;44:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Rathod PA, Bhalla S, Deshmukh AJ, Rodriguez JA. Does fluoroscopy with anterior hip arthroplasty decrease acetabular cup variability compared with a nonguided posterior approach? Clin Orthop Relat Res. 2014;472:1877-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 31. | Kobayashi H, Homma Y, Baba T, Ochi H, Matsumoto M, Yuasa T, Kaneko K. Surgeons changing the approach for total hip arthroplasty from posterior to direct anterior with fluoroscopy should consider potential excessive cup anteversion and flexion implantation of the stem in their early experience. Int Orthop. 2016;40:1813-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Rivera F, Leonardi F, Evangelista A, Pierannunzii L. Risk of stem undersizing with direct anterior approach for total hip arthroplasty. Hip Int. 2016;26:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Barrett WP, Turner SE, Murphy JA, Flener JL, Alton TB. Prospective, Randomized Study of Direct Anterior Approach vs Posterolateral Approach Total Hip Arthroplasty: A Concise 5-Year Follow-Up Evaluation. J Arthroplasty. 2019;34:1139-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Klasan A, Neri T, Oberkircher L, Malcherczyk D, Heyse TJ, Bliemel C. Complications after direct anterior versus Watson-Jones approach in total hip arthroplasty: results from a matched pair analysis on 1408 patients. BMC Musculoskelet Disord. 2019;20:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Ho KW, Whitwell GS, Young SK. Reducing the rate of early primary hip dislocation by combining a change in surgical technique and an increase in femoral head diameter to 36 mm. Arch Orthop Trauma Surg. 2012;132:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87:2456-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 210] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 37. | van Loon J, Hoornenborg D, van der Vis HM, Sierevelt IN, Opdam KT, Kerkhoffs GM, Haverkamp D. Ceramic-on-ceramic vs ceramic-on-polyethylene, a comparative study with 10-year follow-up. World J Orthop. 2021;12:14-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Mjaaland KE, Svenningsen S, Fenstad AM, Havelin LI, Furnes O, Nordsletten L. Implant Survival After Minimally Invasive Anterior or Anterolateral Vs. Conventional Posterior or Direct Lateral Approach: An Analysis of 21,860 Total Hip Arthroplasties from the Norwegian Arthroplasty Register (2008 to 2013). J Bone Joint Surg Am. 2017;99:840-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Henri Bauwens P, Fary C, Servien E, Lustig S, Batailler C. Early low complication rate of ceramic-on-ceramic total hip arthroplasty by direct anterior approach. SICOT J. 2020;6:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop Relat Res. 2011;469:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 41. | Sarraj M, Chen A, Ekhtiari S, Rubinger L. Traction table versus standard table total hip arthroplasty through the direct anterior approach: a systematic review. Hip Int. 2020;30:662-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Greco NJ, Lombardi AV Jr, Morris MJ, Hobbs GR, Berend KR. Direct Anterior Approach and Perioperative Fracture With a Single-Taper Wedge Femoral Component. J Arthroplasty. 2019;34:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Cohen EM, Vaughn JJ, Ritterman SA, Eisenson DL, Rubin LE. Intraoperative Femur Fracture Risk During Primary Direct Anterior Approach Cementless Total Hip Arthroplasty With and Without a Fracture Table. J Arthroplasty. 2017;32:2847-2851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Dietrich M, Kabelitz M, Dora C, Zingg PO. Perioperative Fractures in Cementless Total Hip Arthroplasty Using the Direct Anterior Minimally Invasive Approach: Reduced Risk With Short Stems. J Arthroplasty. 2018;33:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Luger M, Hipmair G, Schopper C, Schauer B, Hochgatterer R, Allerstorfer J, Gotterbarm T, Klasan A. Low rate of early periprosthetic fractures in cementless short-stem total hip arthroplasty using a minimally invasive anterolateral approach. J Orthop Traumatol. 2021;22:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Drosos GI, Tottas S, Kougioumtzis I, Tilkeridis K, Chatzipapas C, Ververidis A. Total hip replacement using MINIMA® short stem: A short-term follow-up study. World J Orthop. 2020;11:232-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Rudin D, Manestar M, Ullrich O, Erhardt J, Grob K. The Anatomical Course of the Lateral Femoral Cutaneous Nerve with Special Attention to the Anterior Approach to the Hip Joint. J Bone Joint Surg Am. 2016;98:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 48. | Thaler M, Dammerer D, Hechenberger F, Hörmann R, Van Beeck A, Stofferin H. The Anatomical Course of the Lateral Femoral Cutaneous Nerve in Relation to Various Skin Incisions Used for Primary and Revision Total Hip Arthroplasty With the Direct Anterior Approach. J Arthroplasty. 2021;36:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Homma Y, Baba T, Sano K, Ochi H, Matsumoto M, Kobayashi H, Yuasa T, Maruyama Y, Kaneko K. Lateral femoral cutaneous nerve injury with the direct anterior approach for total hip arthroplasty. Int Orthop. 2016;40:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 50. | Patton RS, Runner RP, Lyons RJ, Bradbury TL. Clinical Outcomes of Patients With Lateral Femoral Cutaneous Nerve Injury After Direct Anterior Total Hip Arthroplasty. J Arthroplasty. 2018;33:2919-2926.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Zhao G, Zhu R, Jiang S, Xu N, Bao H, Wang Y. Using the anterior capsule of the hip joint to protect the tensor fascia lata muscle during direct anterior total hip arthroplasty: a randomized prospective trial. BMC Musculoskelet Disord. 2020;21:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Alecci V, Valente M, Crucil M, Minerva M, Pellegrino CM, Sabbadini DD. Comparison of primary total hip replacements performed with a direct anterior approach versus the standard lateral approach: perioperative findings. J Orthop Traumatol. 2011;12:123-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 53. | Zhao GY, Wang YJ, Xu NW, Liu F. Dissection and ligation of the lateral circumflex femoral artery is not necessary when using the direct anterior approach for total hip arthroplasty. World J Clin Cases. 2019;7:4226-4233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Jinnai Y, Baba T, Zhuang X, Tanabe H, Banno S, Watari T, Homma Y, Kaneko K. Does a fluoro-assisted direct anterior approach for total hip arthroplasty pose an excessive risk of radiation exposure to the surgeon? SICOT J. 2020;6:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |