Published online Feb 18, 2022. doi: 10.5312/wjo.v13.i2.178
Peer-review started: July 30, 2021
First decision: November 11, 2021
Revised: November 18, 2021
Accepted: January 6, 2022
Article in press: January 6, 2022
Published online: February 18, 2022
Processing time: 202 Days and 21.5 Hours
The Ankle Spacer was developed as a joint-sparing alternative to invasive end-stage surgeries. Currently, there are no clinical studies on the Ankle Spacer.
To describe the operative technique and the clinical efficacy of the Ankle Spacer for the treatment of multiple, cystic osteochondral lesions of the talus in patients with failed prior operative treatment.
This is a prospective study during which patients were assessed preoperatively, at 2- and 6 wk, and at 3, 6, 12 and 24 mo postoperatively. Patients with multiple, cystic or large (≥ 15 mm) osteochondral lesions of the talus after failed prior surgery were included. The primary outcome measure was the numeric rating scale (NRS) for pain during walking at 2 years postoperatively. Secondary outcome measures included the NRS in rest and during stair climbing, the American Orthopaedic Foot and Ankle Society Hindfoot Score, the Foot and Ankle Outcome Score, the Short- Form 36 physical and mental component scale, and the Range of Motion (ROM). Radiographic evaluations were conducted to evaluate prosthetic loosening and subsidence. Revision rates and complications were also assessed.
Two patients underwent an Ankle Spacer implantation on the talus. The NRS during walking improved from 6 and 7 preoperatively to 2 and 2 points postoperatively at 2 years, in patient 1 and 2, respectively. The other patient-reported outcome measures also improved substantially. There were no re-operations nor complications. Radiological imaging showed no loosening of the implant and no change of implant position.
The Ankle Spacer showed clinically relevant pain reduction during walking, improvement in clinical outcomes as assessed with PROMs, and no complications or re-operations. This treatment option may evolve as a joint-sparing alternative to invasive end-stage surgeries.
Core Tip: Our aim was to provide an overview of the clinical efficacy of a novel implant system that is a hemi-arthroplasty of the ankle. This device can be used for multiple, secondary osteochondral lesions of the talus and isolated osteo-arthritis of the talar dome. In this prospective case series, it was shown that this novel device showed promising clinical outcomes and can be considered safe.
- Citation: Dahmen J, Altink JN, Vuurberg G, Wijdicks CA, Stufkens SA, Kerkhoffs GM. Clinical efficacy of the Ankle Spacer for the treatment of multiple secondary osteochondral lesions of the talus. World J Orthop 2022; 13(2): 178-192
- URL: https://www.wjgnet.com/2218-5836/full/v13/i2/178.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i2.178
Osteochondral lesions of the Talus (OLT) are pathologic lesions of the talar cartilage and the subchondral bone. These lesions can occur in up to 50% of acute ankle fractures and sprains[1]. For OLTs of larger size (i.e., above 10 or 15 mm in diameter) and of non-fragmentous morphology, the ‘standard’ operative treatment options such as autologous chondrocyte implantation, osteochondral autograft transfer systems, and a Talar OsteoPeriostic grafting from the Iliac Crest procedure may result in satisfactory clinical outcomes[2-4]. However, in some patients, there are multiple secondary lesions present of large and cystic nature. For these lesions, it is not always possible to harvest an osteo(chondral) autograft that is large enough to replace all the diseased osteochondral tissue of the talus without damaging the donor site or compromising the congruency of the ankle joint. Allograft treatment could be considered for the treatment of these type of lesions. However, these contain the disadvantages of loss of viability and stability in one-third of the grafts, and possibly clinically fail due to immunological reactions[5,6]. However, when there are multiple secondary (i.e., failed prior surgery) lesions present on the talar articular surface in combination with a large and cystic nature, the above-described operative interventions are to be expected to result in relatively inferior outcomes.
To avoid inferior surgical outcomes in patients with persisting complaints after previous unsuccessful conservative management and operative therapy, other effective operative interventions are performed including an ankle arthrodesis or a total ankle prosthesis[7-9]. An (arthroscopic) ankle arthrodesis results in satisfactory clinical outcomes mostly concerning pain[10-12]. However, the operative intervention in question can result in functional limitations due to loss of the range of motion (ROM) of the ankle[13]. Furthermore, an ankle arthrodesis may potentially result in an increased long-term risk of arthritis in adjacent hindfoot joints, particularly the subtalar joint[14]. An alternative option is the placement of a total ankle prosthesis, for which a substantial amount of bone needs to be resected[15]. In order to address the problem of decreased joint motion (in the case of an ankle arthrodesis) and major bone resection (in the case of a prosthesis), a one-piece hemi-arthroplasty covering the talar surface, and leaving the tibia plafond untouched, can be a potential solution to the aforementioned challenges.
Although previous operative interventions similar to such a hemi-arthroplasty have been described in the literature, the studies applied operative techniques with resection of a substantial part of the talar cortical bone and cartilage in order to verify fitting of the implant thereby taking away a potential successful ankle arthrodesis in case of clinical failure[16-18]. Consequently, a novel bone-sparing hemi-prosthesis has been developed (The Ankle Spacer) to enable resurfacing of the talar dome and simultaneously preserving the ROM and potentially optimize physical functioning. Potential advantages include preservation of ankle motion, decreased stress on the midfoot and subtalar joints, and the possibility to perform an ankle fusion after failed hemi-arthroplasty. Up to now, no operative technique description combined with the first clinical outcomes has been described in the literature. It is therefore the purpose of the present study to describe the operative technique and the clinical efficacy of the Ankle Spacer for the treatment of multiple, cystic OLTs in patients with failed prior operative treatment. Despite the fact that no clinical trials have been published on this specific implant, it is hypothesized that the 2-year postoperative follow-up will show a clinically relevant pain reduction and prosthesis survival.
The study concerns a non-randomized, non-blinded, non-comparative prospective trial, with a 2-year follow-up period at the outpatient clinic aiming at assessing pain, function and implant survival, and thereby investigating the clinical efficacy of the Ankle Spacer. The study was approved by the local Medical Ethics Committee (Internal Review Board, IRB) of the Amsterdam University Medical Centre with reference number MEC 2017_175 and was performed in accordance with the principles of the Declaration of Helsinki and the medical Research Involving Human Subjects Act. The study was sponsored by Arthrex as a post market study with reference number EMEA-17037.
All patients eligible for operative implantation of the Ankle Spacer visiting the outpatient clinic between March 2017 and March 2019 for an OLT with a diameter of 15mm or more (anterior-posterior or medial-lateral), an OLT that failed prior operative intervention(s) or patients with multiple OLTs on the talar dome surface, were requested if they were willing to participate in the present clinical trial. If they were interested, patients were informed about this study and were given two wk to decide upon participation. In case patients provided their consent, they were screened for meeting the inclusion and exclusion criteria (Table 1).
| Patient | Gender | Age | BMI (kg/m2) | Prior procedures |
| 1 | M | 36 | 27 | 2013: Removal of anterior bony ankle impingement |
| 2013: Arthroscopic screw fixation of talar osteochondral lesion | ||||
| 2014: Screw removal | ||||
| 2015: Hyaluronic acid injections (multiple) | ||||
| 2016: Arthroscopic Bone Marrow Stimulation for talar osteochondral lesion | ||||
| 2 | F | 56 | 23 | 2005: Spongiosaplasty for talar osteochondral lesion |
| 2008: Arthroscopic bone marrow stimulation for talar osteochondral lesions | ||||
| 2014: Retrograde drilling for talar osteochondral lesion | ||||
| 2017: Hyaluronic acid injections (multiple) | ||||
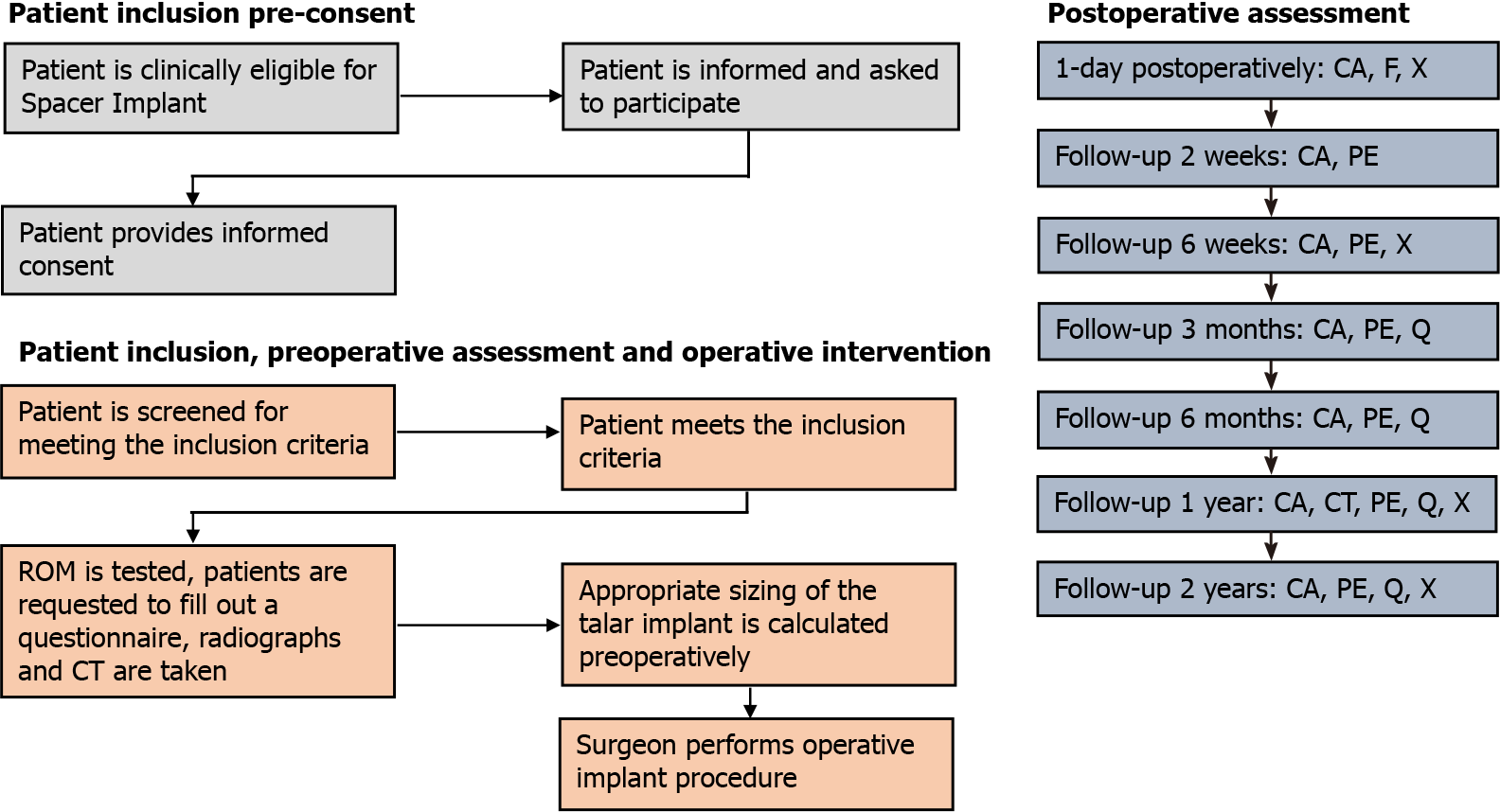
Preoperatively, standing weight bearing conventional radiographs and a computed tomography scan (CT) were taken. Preoperative patient-reported outcome measures (PROMs) consisted of the Numeric Rating Scale (NRS) of pain (during walking, in rest and during stairclimbing), patient satisfaction regarding complaints, activity and treatment, the American Orthopaedic Foot and Ankle Society (AOFAS) Hindfoot Score, Foot and Ankle Outcome Score (FAOS) and the Short-Form 36 (SF-36 physical and mental component scale). The AOFAS score physician subscale and ROM were assessed by an independent researcher who was not involved in the operative procedure[19,20]. Follow-up assessment was performed at two and six weeks, three and six months, one-year and two years postoperatively. At these follow-up moments the patients were requested to fill out a questionnaire consisting of the above-mentioned PROMs and a physical examination was performed to test the ROM (expect for the postoperative visits at 2 wk and 6 wk after the surgery). Radiographs were taken one day, 6 wk, one year and two years postoperatively. At one-year of follow-up, a CT-scan was made (Figure 1 describes the flow-chart outlining the study procedures). In addition, postoperative complications were recorded. Demographic data were also collected. The primary outcome measure in question was the NRS of pain during walking at 2-years follow-up and implant survival. All other outcome measures described above were secondary outcomes.
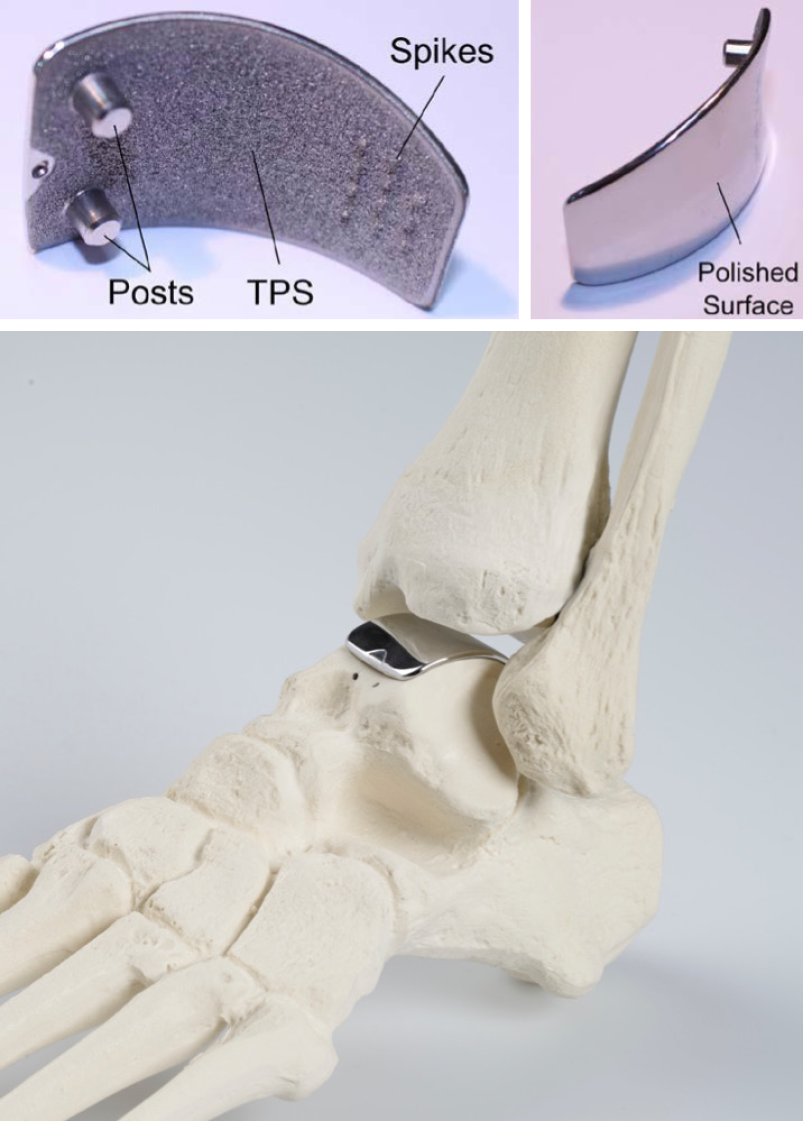
The Arthrex Ankle Spacer is a one-piece implant system that replaces the talar side of the tibio-talar joint (Figure 2). The Ankle Spacer replaces the articulating upper surface of the talus and offers several implant sizes in millimeters (18, 20, 22, 24, 26, 28) in order to fit to the different sizes of the talus. It is anatomically designed to the native talar dome to provide an optimal fit to the distal articular tibial surface. The implant is polished to a mirror effect on the articulating surface and has a rough titanium plasma spray (TPS) coated under surface with two posts and spikes for implant fixation (US10,350,079 B2). The rough surface enables secondary fixation due to bone ingrowth. Spikes at the posterior part of the prosthesis allow for further fixation to the bone. The weight of the ankle spacer weighs varies from 11 to 22 g, depending on the size of the spacer.
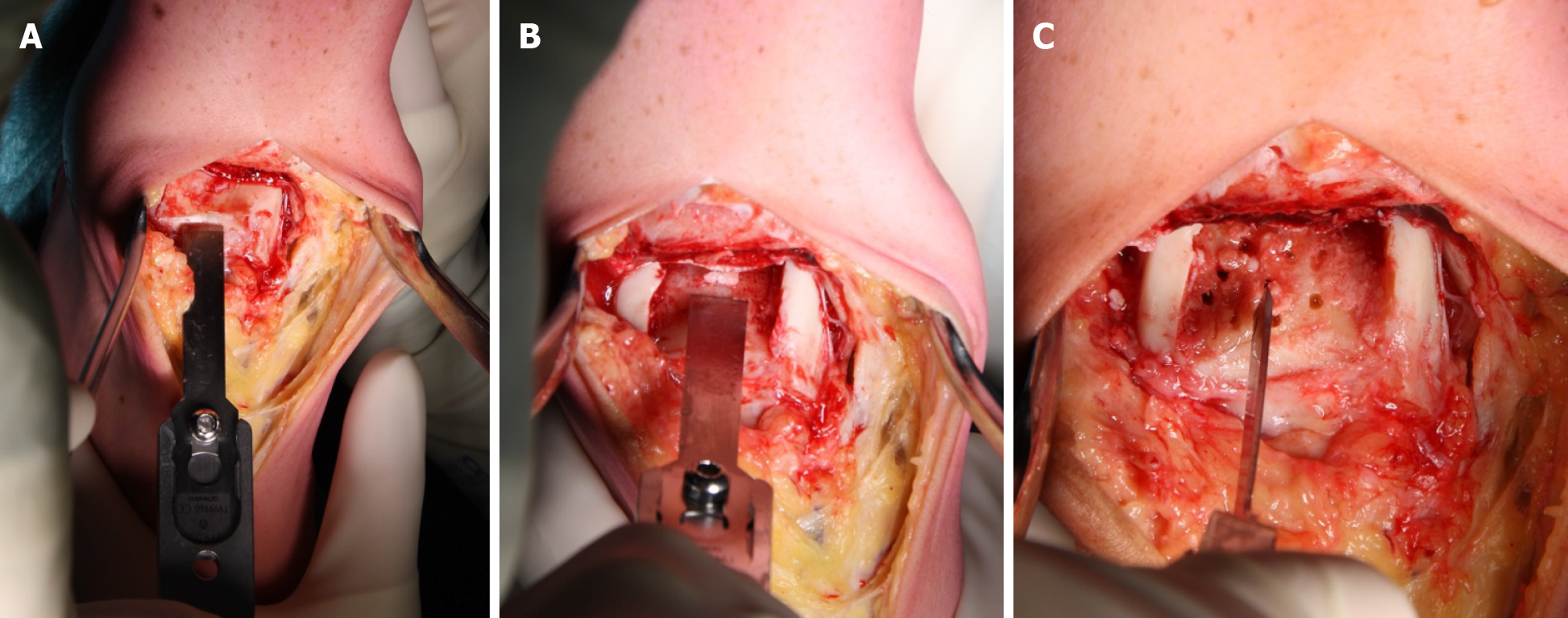
Before receiving the Ankle Spacer, the patient received 2 g of cefazoline pre-operatively. The procedure was carried out under general anesthesia. The patient was placed in the supine position with a tourniquet applied around the thigh and a rolled-up apron underneath the lateral malleolus to facilitate eversion of the foot to improve exposure of the talus. A longitudinal incision between 10 and 15 cm was centered over the ankle immediately lateral to the tibialis anterior tendon. The incision was deepened to the ankle joint while retracting the extensor hallucis longus and the neurovascular bundle laterally. If present, the osteophytes on the anterior tibia edge were removed. Subsequently, the cartilage was removed (Figure 3A) on the upper talar surface as well as part of the underlying subchondral bone (Figure 3B).
Additionally, microfracturing was performed to support ingrowth for secondary ankle spacer fixation (Figure 3C).
The tibiotalar joint was manually distracted and the appropriate trial Ankle Spacer was inserted into the joint. The trial Ankle Spacer was placed in a central position in the medial-lateral direction. The trial was fixated in the slotted hole with a K-wire and the trial inserter was removed afterwards.
Then, dynamic dorsi- and plantarflexion of the ankle was performed as this allowed self-alignment of the temporarily fixed trial. Afterwards, the trial spacer was fixated with a K-wire in the second hole in order to prevent dislocation, after which both K-wires were advanced bi-cortically in order to achieve proper fixation. Fluoroscopy was used to confirm proper sizing and positioning of the trial Ankle Spacer.
The double drill sleeve was aligned to the two holes on the Ankle Spacer and were maintained in a perpendicular angle relative to the surface of the ankle spacer. Subsequently, drilling was started with the first drill until the moment it stopped on the drill sleeve. This first drill was left for fixation in the drill hole, and then the second drill was to drill the second hole, in exactly the same manner.
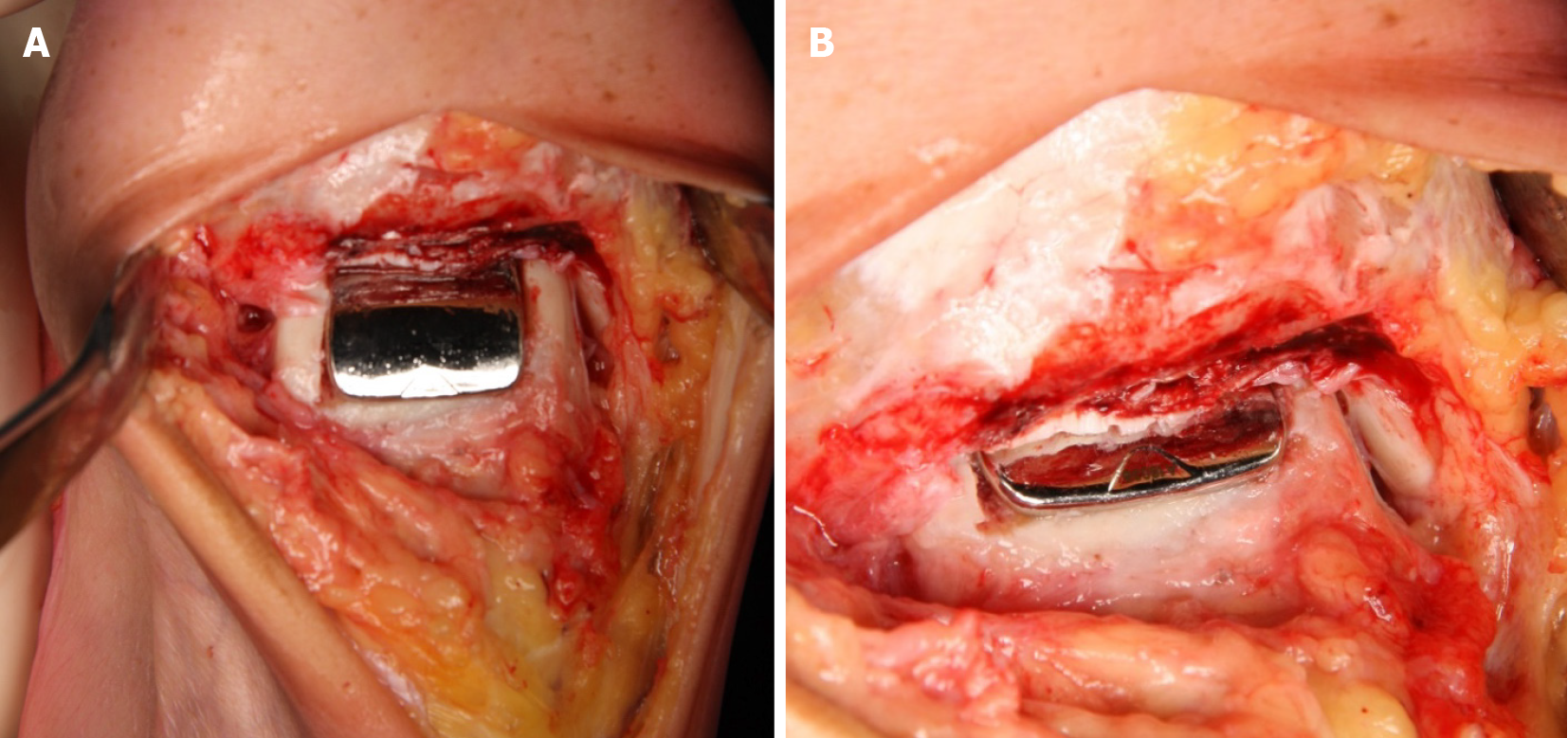
Then all instruments were removed. Prior to inserting the Ankle Spacer, the prepared bone bed was thoroughly cleaned and inspected. The Ankle Spacer was inserted on the talar dome using the ankle space inserter, while placing the two posts on the bottom of the Ankle Spacer into the prepared drill holes for fixation. The implant was impacted with an impactor until the posts were fully seated in the drill holes (Figure 4A and Figure 4B). The joint capsule was closed and the layers were closed subsequently.
Postoperatively, wound dressing with adequate padding was applied. At hospital discharge a removable lower leg splint was applied. Careful active and passive dorsi- and plantarflexion motion (flexion-extension exercises) without weight bearing monitored by a physiotherapist were started two days after surgery. After two wk of non-weight bearing, depending on the degree of wound and soft tissue healing, mobilization was started with progressive weight bearing to tolerance in a Walker. Flexion-extension exercises were continued. Six wk after surgery, intensive physiotherapy and rehabilitation was started for at least a period of 6 wk.
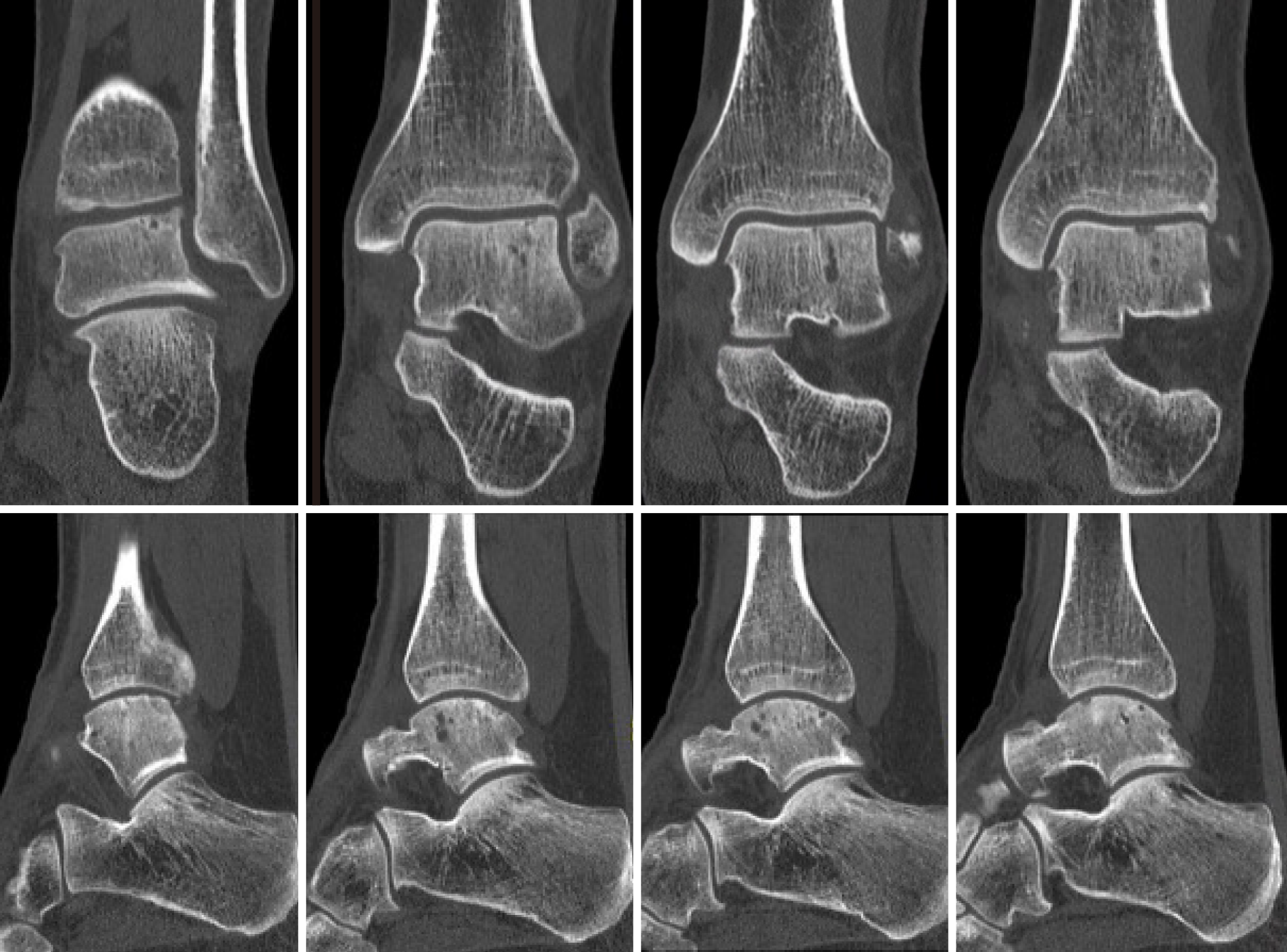
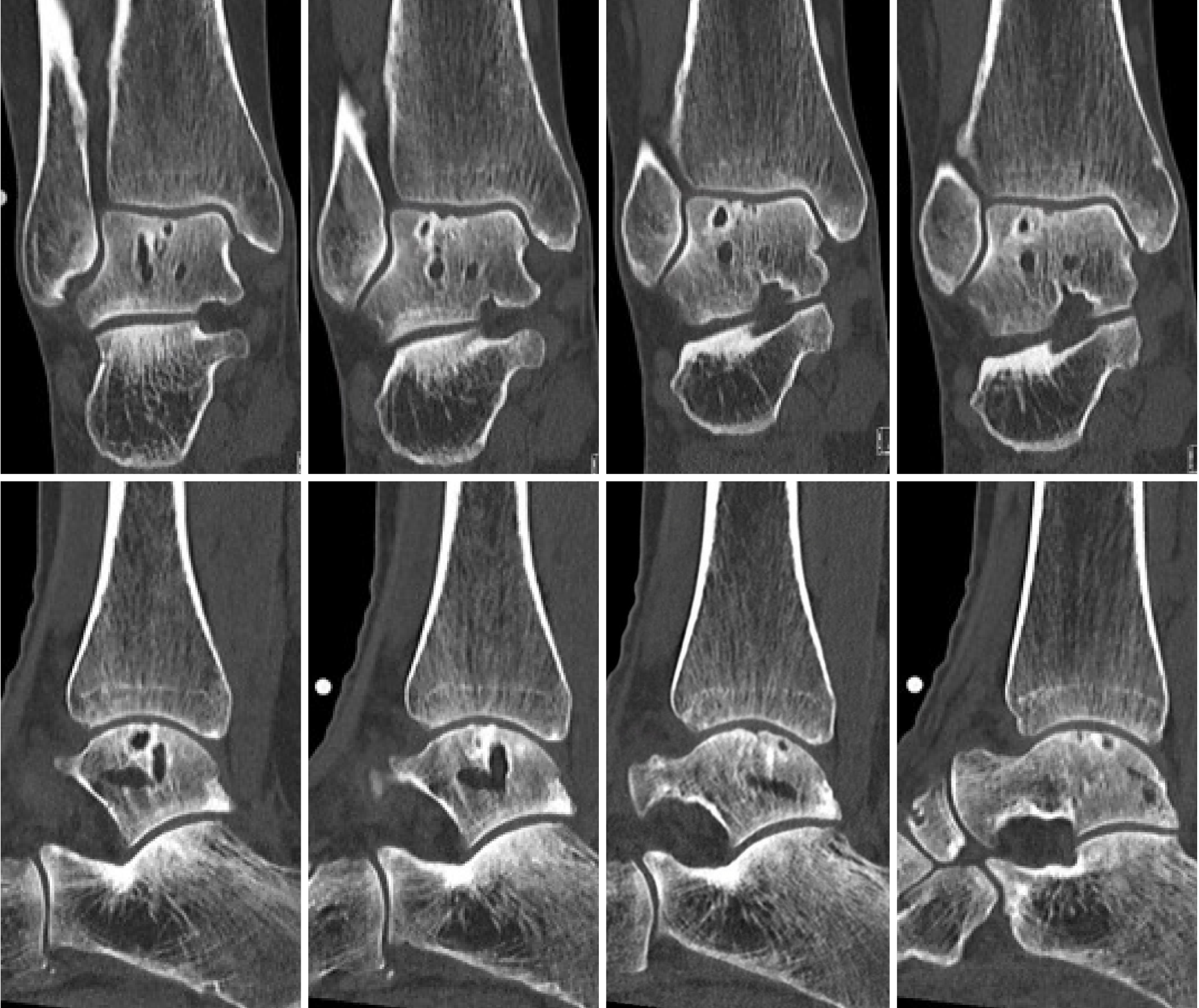
A power analysis was conducted to estimate the needed sample size for this prospective non-comparative trial using the NRS for pain as primary outcome measure with a clinical relevant difference of 1 point and a standard deviation of 1.5 points. Additionally, in this calculation a standard p-value of 0.05 (α = 0.05) and a power of 80% were used. This indicated that a total of 20 patients would need to be included in the trial in order to detect a clinically important difference in pain comparing pre- and postoperative NRS scores. Due to the relative rarity of patients presenting at our outpatient department with the exact above-described inclusion and exclusion criteria, two patients could be identified with fitting indications who were operated on with the implantation of the Ankle Spacer. The age of these patients was 36 and 56 years old (one male, one female). No patients were lost to follow-up. Prior procedures that were performed are listed in Table 2 alongside demographic factors. The pre-operative CT scans of the patients as presented in Figures 5 and 6.
| Inclusion criteria | Exclusion criteria |
| Age ranging from 18 to 80 yr | Severe ankle malalignment (more than 5° varus or valgus) |
| Failed previous conservative treatment | Suspicion of grade two or higher (Kellgren-Lawrence-Score) ankle joint degeneration on the tibia side |
| Complaints for at least 6 mo | Ankle Fracture less than 6 mo ago |
| Talar osteochondral lesions (multiple degenerative talar cysts present, and/or prior failed operative treatment and/or multiple defects and/or a diameter of 15mm or more) | Tendinitis |
| Advanced osteoporosis | |
| Adiposity grade I (BMI of 30 kg/m2 or more) | |
| Diabetes mellitus / reumathoid arthritis / severe neuro-arthropathy | |
| Blood supply limitations and active infections, which may retard healing | |
| Foreign-body sensitivity | |
| Currently participating in an investigational drug or another device study that clinically interferes with the current study endpoints |
All statistical analyses were performed by means of SPSS version 20.0 (SPSS Inc., Chicago, IL, United States). Data will be presented as descriptive analyses through qualitative comparisons in the different patients. Consequently, data will be presented per patient, aiming at presenting changes in scores preoperatively vs postoperatively.
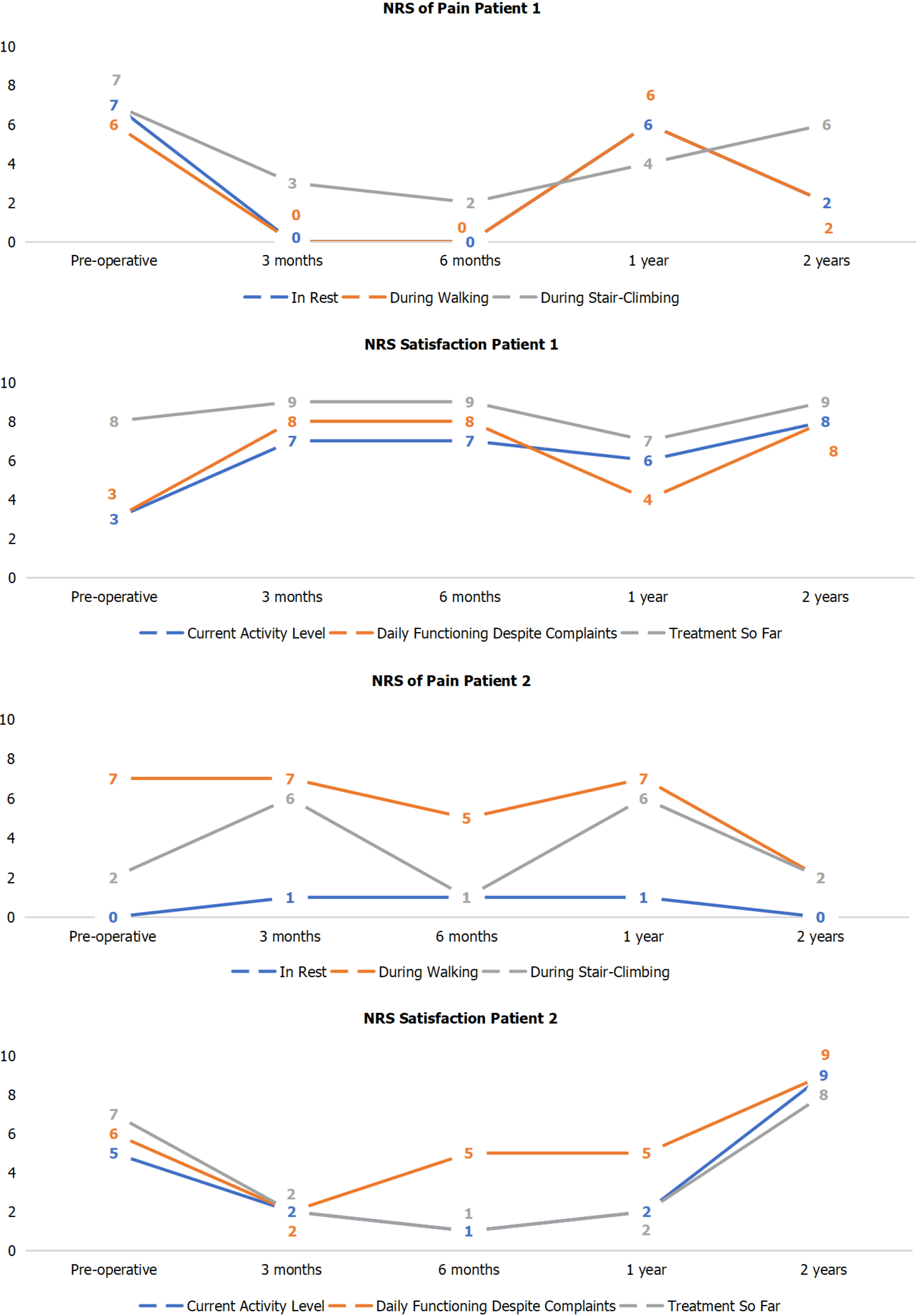
The primary outcome measure, the NRS of pain during walking improved from 6 preoperatively to 2 points at 2 years follow-up in patient 1, and from 7 preoperatively to 2 points postoperatively at 2 years follow-up in patient 2. This entails an improvement of 4 points and 5 points in patient 1 and 2, respectively (Figure 7).
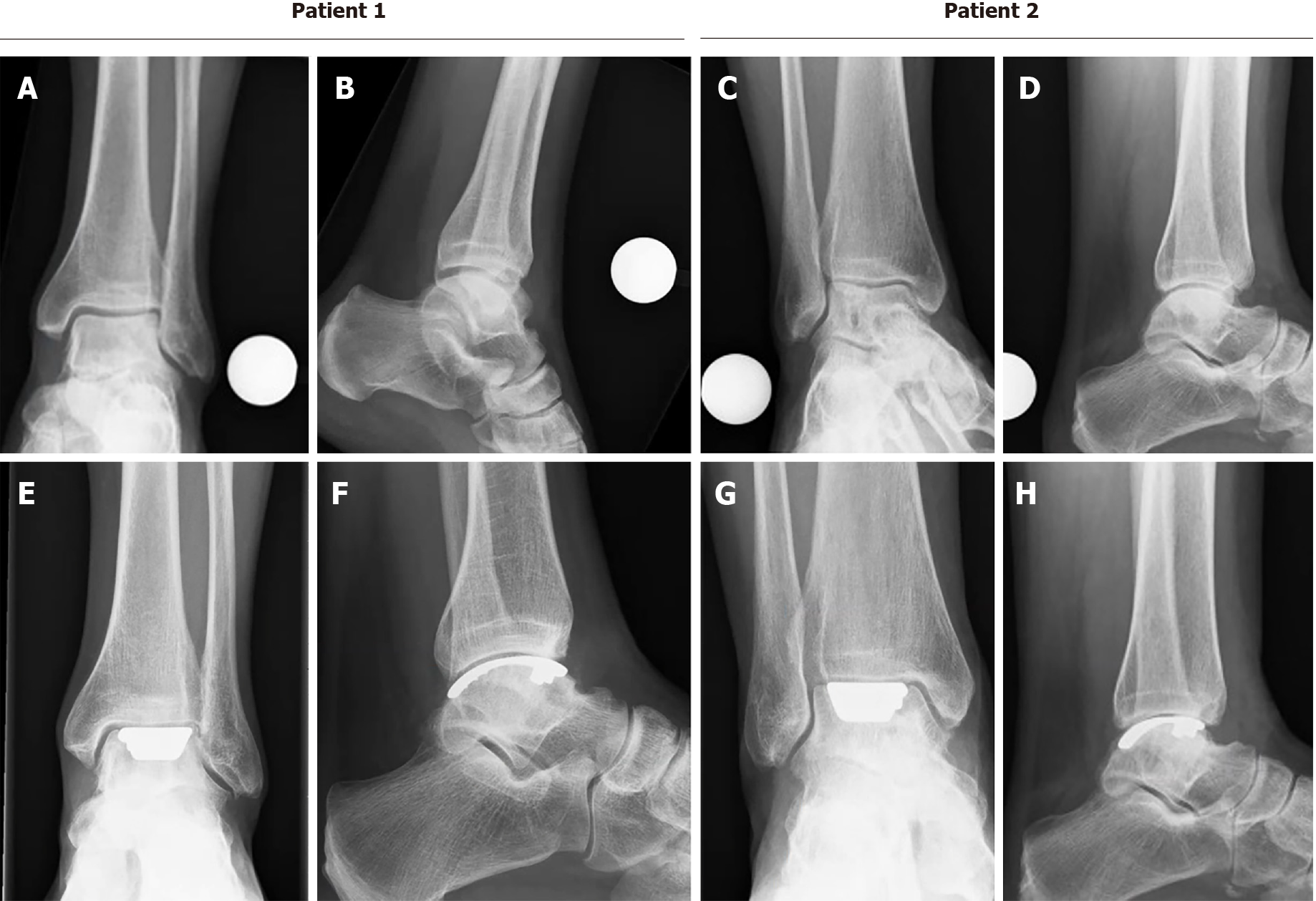
The other PROMs can be appreciated in Table 3. There were no complications and no reoperations. Radiologically, for all postoperative radiographs and CT-scans it was concluded that there were no indications of loosening of the implant, no implant migration, and no subsidence was noted (Figure 8). The ROM assessments were described in Table 4.
| Patient 1 | ||||||
| Time-Point | FAOS | SF-36 | AOFAS ankle hindfoot score | NRS satisfaction with current activity level | NRS satisfaction with daily functioning despite any complaints | NRS satisfaction with treatment so far |
| Preoperatively | Pain: 56; Symptoms: 46; ADL: 68; Sports: 20 QoL: 25 | PCS: 28; MCS: 61 | 61 | 3 | 3 | 8 |
| 3 mo postoperatively | Pain: 86; Symptoms: 82; ADL: 91; Sports: 40 QoL: 63 | PCS: 44; MCS: 55 | 82 | 7 | 8 | 9 |
| 6 mo postoperatively | Pain: 79; Symptoms: 86 ; ADL: 93; Sports: 60 QoL: 63 | PCS: 45; MCS: 53 | 75 | 7 | 8 | 9 |
| 1 year postoperatively | Pain: 46; Symptoms: 67; ADL: 79; Sports: 25 QoL: 44 | PCS: 39; MCS: 56 | 77 | 6 | 4 | 7 |
| 2 years postoperatively | Pain: 62; Symptoms: 81; ADL: 90; Sports: 55 QoL: 69 | PCS: 51; MCS: 52 | 100 | 8 | 8 | 9 |
| Patient 2 | ||||||
| Preoperative | Pain: 81; Symptoms: 79; ADL: 91; Sports: N.A. QoL: 25 | PCS: 44; MCS: 56 | 72 | 5 | 6 | 7 |
| 3 mo postoperatively | Pain: 64; Symptoms: 68; ADL: 66; Sports: 30 QoL: 0 | PCS: 33; MCS: 41 | 69 | 2 | 2 | 2 |
| 6 mo postoperatively | Pain: 68; Symptoms: 69 ; ADL: 72; Sports: 30 QoL: 13 | PCS: 29; MCS: 54 | 71 | 1 | 5 | 1 |
| 1 year postoperatively | Pain: 72; Symptoms: 50; ADL: 72; Sports: 30 QoL: 19 | PCS: 33; MCS: 51 | 68 | 2 | 5 | 2 |
| 2 years postoperatively | Pain: 81; Symptoms: 68; ADL: 78; Sports: 55 QoL: 50 | PCS: 43; MCS: 53 | 88 | 9 | 9 | 8 |
| Patient 1 | Patient 2 | |||
| Dorsiflexion in degrees (affected/unaffected side) | Plantarflexion in degrees (affected/unaffected side) | Dorsiflexion in degrees (affected/unaffected side) | Plantarflexion in degrees (affected/unaffected side) | |
| Preoperative | 5 / 10 | 35 / 40 | 5 / 5 | 40 / 40 |
| 2 wk postoperatively | 5 / 15 | 20 / 40 | 2 / 15 | 25 / 40 |
| 6 wk postoperatively | 7 / 10 | 35 / 40 | 5 / 10 | 35 / 40 |
| 3 mo postoperatively | 10 / 10 | 35 / 45 | 5 / 10 | 35 / 40 |
| 6 mo postoperatively | 10 / 10 | 35 / 45 | 10 / 10 | 35 / 40 |
| 1 yr postoperatively | 10 / 10 | 35 / 45 | 10 / 10 | 35 / 40 |
| 2 yr postoperatively | 10 / 10 | 35 / 45 | 7 / 10 | 35 / 40 |
This is the first study describing the results of the newly developed Ankle Spacer. The Ankle Spacer is a one-piece implant system that replaces the talar side of the tibiotalar joint, and can therefore be considered a hemi-arthroplasty procedure. The implant had been designed in order to overcome a number of downsides that are associated with the current treatment options for large, cystic, or multiple osteochondral lesions of the talus that have failed prior operative intervention(s). One of these treatment option being applied in the younger patient population is an arthroscopic ankle arthrodesis which results in highly satisfactory clinical outcomes mostly concerning pain outcomes[10-12]. The downside of this operative intervention is that it results in functional limitations due to loss of ROM of the ankle[13]. Furthermore, an ankle arthrodesis increases the long-term risk of (radiographic) osteoarthritis in adjacent hindfoot joints, particularly in the subtalar joint[14,21]. An alternative option is the placement of a total ankle prosthesis, for which a substantial amount of bone needs to be resected[15,22]. In order to tackle the downsides of these treatment options, develop a bone-sparing procedure and resurfacing the talar dome to preserve ROM and potentially optimize physical functioning, the Ankle Spacer, a novel bone-sparing hemi-prosthesis, has been developed. The outcomes of treatment with the Ankle Spacer were described in the present article. Potential advantages include preservation of ankle motion, decreased stress on the midfoot and subtalar joints, and the possibility to perform an ankle fusion after failed hemi-arthroplasty.
It is therefore clear that the Ankle Spacer is a new and innovative device. The current study is the first clinical trial performed investigating the efficacy of the Ankle Spacer, and therefore there is no clinical literature on this particular prosthetic device at this time to compare our clinical results to. Our current clinical results must therefore be compared to similar devices having been researched over the past decade. An articular and subchondral bone resurfacing implant procedure on one side of the joint (hemi-arthroplasty) can be considered a relatively novel operative intervention. Promising clinical results have been reported for similar treatment in the femoral and humeral head, as well as the first metatarsal and patellar surface. [16,23-27]. Van Bergen et al[17,18,28] showed that a focal resurfacing implant is a promising treatment for talar OLTs that have failed prior operative treatment[28]. They concluded that in a patient series including 38 patients the clinical results at a mean follow-up of three years were satisfactory. A different study by Holton et al[29] demonstrated that the use of a different resurfacing implant in the form of an articular resurfacing component maintained a good clinical improvement for patients at mid-term follow-up. A study by Brunner et al[30] which is less clinically comparable as it replaces the whole entity of the ankle, as opposed to the resurfacing of the talar surface as done by the Ankle Spacer or as opposed to the resurfacing implant as used by van Bergen et al[4,6,31], showed that short- term to mid-term results for patients treated with the Scandinavian Total Ankle Replacement (STAR) prosthesis have been encouraging. The long-term results were clinically inferior compared to the short-term and mid-term results. Additionally, studies on the Total Ankle Replacement (TAR), which is, to a certain though limited extent, comparable to the Ankle Spacer, have shown that long-term results demonstrated improved pain scores as it was observed to be a viable and durable operative implantation option[32,33]. Another implantation technique in the form of a Tornier Salto Talaris Anatomic Ankle has been described in the literature. Mid- term results showed that the survivorship of this implant was calculated to be around 95%[34,35]. A study by Queen et al[36] found that at a follow-up of 2 years investigating 51 patients after a fixed-bearing Total Ankle Replacement significant improvements in gait mechanics, pain reduction and functioning were observed.
As an objective conclusive remark on the evidence found on similar operative techniques, one can state that similar operative techniques yielded favorable clinical, radiological and safety outcomes. When comparing these three aspects to our results it can be concluded that in our treated patients, similar outcomes were found. Postoperative improvement was especially clear in the observed primary outcome measure which was the NRS pain during walking. This outcome measures typically describes the state and magnitude of pain in patients with OLTs of the talus as these patients experience pain especially when performing weight bearing activities, but often are unable to participate in sports due to complaints[4]. It was shown that this primary outcome measure improved from 6 and 7 preoperatively to 2 and 2 postoperatively at 2 years, in patient 1 and 2, respectively. As a result, we can conclude that for both patients a minimal clinically important difference was reached when comparing the preoperative state of pain to the postoperative state of pain at 2 years after the surgery. As an improvement of at least −1.0 on the NRS scale of 0 to 10 points is significantly associated with the concept of a “better improvement”, and an improvement of -2.0 on this scale is associated with a “much better improvement”[31]. The improvement was also reflected in the radiological outcomes and the subjective satisfaction scores that were taken both preoperatively and postoperatively at the different follow-up moments in which it was clear that the patients stated that they were satisfied with their level of activity. Moreover, this was also reflected in the postoperative AOFAS Hindfoot Scale, which ranged from 88 to 100, scores that can be considered to represent a successful surgery.
Furthermore, it was observed that there were no complications, no reoperations nor revisions, which implicates that, when extrapolating these results for a larger population based on our experience, the insertion of the Ankle Spacer can be regarded a safe procedure. This outcome should be interpreted in the light of the high re-intervention rates that are associated with Total Talar Prostheses, as some studies report that up to 38% of the patients need revision surgery within the first year after total ankle arthroplasty[37]. With regards to ROM, it was shown that the ROM did not decrease in the patients, potentially due to the fact that patients received adequate postoperative physiotherapy and because of the fact that the Ankle Spacer was inserted just below the level of the subchondral bone plater not affecting joint congruency. This is clinically relevant to take into account when comparing ROM outcomes after placement of the Ankle Spacer to outcomes after an ankle arthrodesis procedure[38].
This study should be interpreted in light of its strengths and weaknesses. Strengths of this study include its prospective and thorough methodology, completeness of follow-up, and the use of various validated outcome measures in the assessment of a not yet studied resurfacing implant. Limitations include the absence of a controlled group, lack of long-term follow-up outcomes ranging past the two years follow-up, and the small series of patients. However, we have found in our tertiary referral clinic that operates in an academic setting with the status of an official (inter)national expertise center for the treatment of OLTs in the ankle that the indication for an Ankle Spacer implantation is highly rare, even though we receive a high number of patients on a yearly basis. As a result, it was therefore noted that the Ankle Spacer has been removed from the market due to limited indications and strict regulatory requirements.
The clinical relevance of the present study can be interpreted in the light of the highly specific indication for the implantation of the Ankle Spacer. This device is particularly suited for patients with multiple OLTs of the talar dome having failed prior operative intervention(s) with an unaffected distal tibial part of the ankle joint. This study shows that the Ankle Spacer is effective for this particular and rare patient group, thereby functioning as a joint-sparing alternative to more invasive operative interventions, such as an ankle arthrodesis or total ankle arthroplasty.
The Ankle Spacer showed clinically relevant pain reduction during walking, improvement in clinical outcomes as assessed with PROMs, and no complications or re-operations. This treatment option may therefore evolve as a joint-sparing alternative to an ankle arthrodesis, a total talar implant or a total ankle arthroplasty/resurfacing.
Osteochondral lesions of the Talus (OLT) are pathologic lesions of the talar cartilage and the subchondral bone. These lesions can occur in up to 50% of acute ankle fractures and sprains. For OLTs of larger size (i.e., above 10 or 15 mm in diameter) and of non-fragmentous morphology, the ‘standard’ operative treatment options such as autologous chondrocyte implantation, osteochondral autograft transfer systems, and a Talar OsteoPeriostic grafting from the Iliac Crest procedure may result in satisfactory clinical outcomes. However, in some patients, there are multiple secondary lesions present of large and cystic nature. For these lesions, it is not always possible to harvest an osteo(chondral) autograft that is large enough to replace all the diseased osteochondral tissue of the talus without damaging the donor site or compromising the congruency of the ankle joint. Allograft treatment could be considered for the treatment of these type of lesions. However, these contain the disadvantages of loss of viability and stability in one-third of the grafts, and possibly clinically fail due to immunological reactions. However, when there are multiple secondary (i.e., failed prior surgery) lesions present on the talar articular surface in combination with a large and cystic nature, the above-described operative interventions are to be expected to result in relatively inferior outcomes.
Currently, it is difficult to treat patients with osteochondral lesions of the ankle that are of multiple, cystic and secondary nature. This is because the lesions are considered relatively large and difficult to treat. For this indication, it was usually performed to fuse the ankle joint. However, in the past 2 to 5 years, novel innovative surgical options have been developed, such as the Ankle Spacer, in order to overcome an ankle fusion or ankle prosthesis.
To describe the operative technique and the clinical efficacy of the Ankle Spacer for the treatment of multiple, cystic OLTs in patients with failed prior operative treatment.
In a prospective study including patients with multiple, cystic or large osteochondral lesions of the talus were included who failed previous surgical treatment. We looked at the numeric rating scale (NRS) for pain during walking at 2 years after implantation of the Ankle Spacer and we also assessed the NRS in rest and during stair-climbing, the American Orthopaedic Foot and Ankle Outcome Score, the Foot and Ankle Outcome Score, the Short-Form 36 and the range of motion of the ankles both pre-operatively as well as post-operatively. Radiographic evaluations were conducted to evaluate prosthetic loosening and subsidence. Revision rates and complications were also assessed.
In this prospective study, two patients underwent the implantation of an Ankle Spacer for osteochondral damage on the talar dome. We found that there were clinically relevant pain reductions during walking as well as important improvements in clinical outcomes as assessed with the patient-reported outcome measures. Furthermore, it was found that there were no complications nor re-operations.
The Ankle Spacer showed good clinical outcomes and clinically relevant pain reduction during walking, improvement in clinical outcomes as assessed with PROMs, and no complications or re-operations. This treatment option may therefore evolve as a joint-sparing alternative to an ankle arthrodesis, a total talar implant or a total ankle arthroplasty/resurfacing.
Future research should be focused at the development of a prospective, self-learning algorithm taking into account the individual patient factors influencing outcomes after conservative and surgical treatment so that we can assess which patients would benefit most from which treatment options.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Subramaniyan V S-Editor: Wang LL L-Editor: A P-Editor: Yuan YY
| 1. | Martijn HA, Lambers KTA, Dahmen J, Stufkens SAS, Kerkhoffs GMMJ. High incidence of (osteo)chondral lesions in ankle fractures. Knee Surg Sports Traumatol Arthrosc. 2021;29:1523-1534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 2. | Dahmen J, Lambers KT, Reilingh ML, van Bergen CJ, Stufkens SA, Kerkhoffs GM. No superior treatment for primary osteochondral defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2017;26:2142-2157. |
| 3. | Kerkhoffs GMMJ, Altink JN, Stufkens SAS, Dahmen J. Talar OsteoPeriostic grafting from the Iliac Crest (TOPIC) for large medial talar osteochondral defects : Operative technique. Oper Orthop Traumatol. 2021;33:160-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Rikken QGH, Kerkhoffs GMMJ. Osteochondral Lesions of the Talus: An Individualized Treatment Paradigm from the Amsterdam Perspective. Foot Ankle Clin. 2021;26:121-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 5. | Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am. 2012;94:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Pomajzl RJ, Baker EA, Baker KC, Fleischer MM, Salisbury MR, Phillips DM, Fortin PT. Case Series With Histopathologic and Radiographic Analyses Following Failure of Fresh Osteochondral Allografts of the Talus. Foot Ankle Int. 2016;37:958-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Brodsky JW, Scott DJ, Ford S, Coleman S, Daoud Y. Functional Outcomes of Total Ankle Arthroplasty at a Mean Follow-up of 7.6 Years: A Prospective, 3-Dimensional Gait Analysis. J Bone Joint Surg Am. 2021;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Usuelli FG, Indino C, Leardini A, Manzi L, Ortolani M, Caravaggi P. Range of motion of foot joints following total ankle replacement and subtalar fusion. Foot Ankle Surg. 2021;27:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Usuelli FG, Indino C, Maccario C, Manzi L, Romano F, Aiyer A, Kaplan JRM. A Modification of the Fibular Osteotomy for Total Ankle Replacement Through the Lateral Transfibular Approach. J Bone Joint Surg Am. 2019;101:2026-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | de Leeuw PA, Hendrickx RP, van Dijk CN, Stufkens SS, Kerkhoffs GM. Midterm results of posterior arthroscopic ankle fusion. Knee Surg Sports Traumatol Arthrosc. 2016;24:1326-1331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Hendrickx RP, Kerkhoffs GM, Stufkens SA, van Dijk CN, Marti RK. Ankle fusion using a 2-incision, 3-screw technique. Oper Orthop Traumatol. 2011;23:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Hendrickx RP, Stufkens SA, de Bruijn EE, Sierevelt IN, van Dijk CN, Kerkhoffs GM. Medium- to long-term outcome of ankle arthrodesis. Foot Ankle Int. 2011;32:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Sealey RJ, Myerson MS, Molloy A, Gamba C, Jeng C, Kalesan B. Sagittal plane motion of the hindfoot following ankle arthrodesis: a prospective analysis. Foot Ankle Int. 2009;30:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Wang Y, Wong DW, Tan Q, Li Z, Zhang M. Total ankle arthroplasty and ankle arthrodesis affect the biomechanics of the inner foot differently. Sci Rep. 2019;9:13334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Hintermann B, Zwicky L, Knupp M, Henninger HB, Barg A. HINTEGRA revision arthroplasty for failed total ankle prostheses. J Bone Joint Surg Am. 2013;95:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Reilingh ML, Van Bergen CJA, Van Dijk CN. Novel metal implantation technique for osteochondral defects of the medial talar dome. Techniques in Foot and Ankle Surgery. 2012;11:45-49. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | van Bergen CJ, van Eekeren IC, Reilingh ML, Sierevelt IN, van Dijk CN. Treatment of osteochondral defects of the talus with a metal resurfacing inlay implant after failed previous surgery: a prospective study. Bone Joint J. 2013;95-B:1650-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | van Bergen CJA, van Eekeren ICM, Reilingh ML, Gerards RM, Dijk CNV. The use of HemiCAP for the treatment of osteochondral lesions. Operative Techniques in Orthopaedics. 2014;24:190-194. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3199] [Cited by in RCA: 3113] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 20. | Reilingh ML, van Bergen CJ, Gerards RM, van Eekeren IC, de Haan RJ, Sierevelt IN, Kerkhoffs GM, Krips R, Meuffels DE, van Dijk CN, Blankevoort L. Effects of Pulsed Electromagnetic Fields on Return to Sports After Arthroscopic Debridement and Microfracture of Osteochondral Talar Defects: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Am J Sports Med. 2016;44:1292-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 547] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 22. | Hopgood P, Kumar R, Wood PL. Ankle arthrodesis for failed total ankle replacement. J Bone Joint Surg Br. 2006;88:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Anderson DD, Tochigi Y, Rudert MJ, Vaseenon T, Brown TD, Amendola A. Effect of implantation accuracy on ankle contact mechanics with a metallic focal resurfacing implant. J Bone Joint Surg Am. 2010;92:1490-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Davidson PA, Rivenburgh D. Focal anatomic patellofemoral inlay resurfacing: theoretic basis, surgical technique, and case reports. Orthop Clin North Am. 2008;39:337-346, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Hasselman CT, Shields N. Resurfacing of the first metatarsal head in the treatment of hallux rigidus. Techniques in Foot and Ankle Surgery. 2008;7:31-40. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Uribe JW, Botto-van Bemden A. Partial humeral head resurfacing for osteonecrosis. J Shoulder Elbow Surg. 2009;18:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Van Stralen RA, Haverkamp D, Van Bergen CJ, Eijer H. Partial resurfacing with varus osteotomy for an osteochondral defect of the femoral head. Hip Int. 2009;19:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Vuurberg G, Reilingh ML, van Bergen CJA, van Eekeren ICM, Gerards RM, van Dijk CN. Metal Resurfacing Inlay Implant for Osteochondral Talar Defects After Failed Previous Surgery: A Midterm Prospective Follow-up Study. Am J Sports Med. 2018;46:1685-1692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Holton C, Gudipati S, Budgen A. Mid-term follow-up of talar dome resurfacing surgery using the HemiCAP device for osteochondral lesions: review of 3 cases. Foot & ankle. 2013;. [DOI] [Full Text] |
| 30. | Brunner S, Barg A, Knupp M, Zwicky L, Kapron AL, Valderrabano V, Hintermann B. The Scandinavian total ankle replacement: long-term, eleven to fifteen-year, survivorship analysis of the prosthesis in seventy-two consecutive patients. J Bone Joint Surg Am. 2013;95:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 31. | Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 1138] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 32. | Alvine FG, Conti SF. [The AGILITY ankle: mid- and long-term results]. Orthopade. 2006;35:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Knecht SI, Estin M, Callaghan JJ, Zimmerman MB, Alliman KJ, Alvine FG, Saltzman CL. The Agility total ankle arthroplasty. Seven to sixteen-year follow-up. J Bone Joint Surg Am. 2004;86:1161-1171. [PubMed] |
| 34. | Bonnin M, Judet T, Colombier JA, Buscayret F, Graveleau N, Piriou P. Midterm results of the Salto Total Ankle Prosthesis. Clin Orthop Relat Res. 2004;6-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Schweitzer KM, Adams SB, Viens NA, Queen RM, Easley ME, Deorio JK, Nunley JA. Early prospective clinical results of a modern fixed-bearing total ankle arthroplasty. J Bone Joint Surg Am. 2013;95:1002-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Queen RM, De Biassio JC, Butler RJ, DeOrio JK, Easley ME, Nunley JA. J. Leonard Goldner Award 2011: changes in pain, function, and gait mechanics two years following total ankle arthroplasty performed with two modern fixed-bearing prostheses. Foot Ankle Int. 2012;33:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Criswell BJ, Douglas K, Naik R, Thomson AB. High revision and reoperation rates using the Agility™ Total Ankle System. Clin Orthop Relat Res. 2012;470:1980-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Wang Y, Li Z, Wong DW, Zhang M. Effects of Ankle Arthrodesis on Biomechanical Performance of the Entire Foot. PLoS One. 2015;10:e0134340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |