Published online Feb 18, 2022. doi: 10.5312/wjo.v13.i2.160
Peer-review started: May 9, 2021
First decision: June 16, 2021
Revised: July 1, 2021
Accepted: January 11, 2022
Article in press: January 11, 2022
Published online: February 18, 2022
Processing time: 284 Days and 16.6 Hours
The National Institute for Health and Care Excellence (NICE) guidelines have advised further research is required into investigating the added prognostic value of bone mineral density (BMD) in the assessment of fracture risk with the Fracture Risk Assessment Tool (FRAX) score.
To investigate the significance of BMD in fracture neck of femur patients and compare it to the outcome of the FRAX score.
Inclusion criteria for this study were all patients who underwent dual-energy X-ray absorptiometry (DXA) scan following fracture neck of femur between 2015 and 2017. Analysis of BMD, FRAX scores and patient demographic data was undertaken.
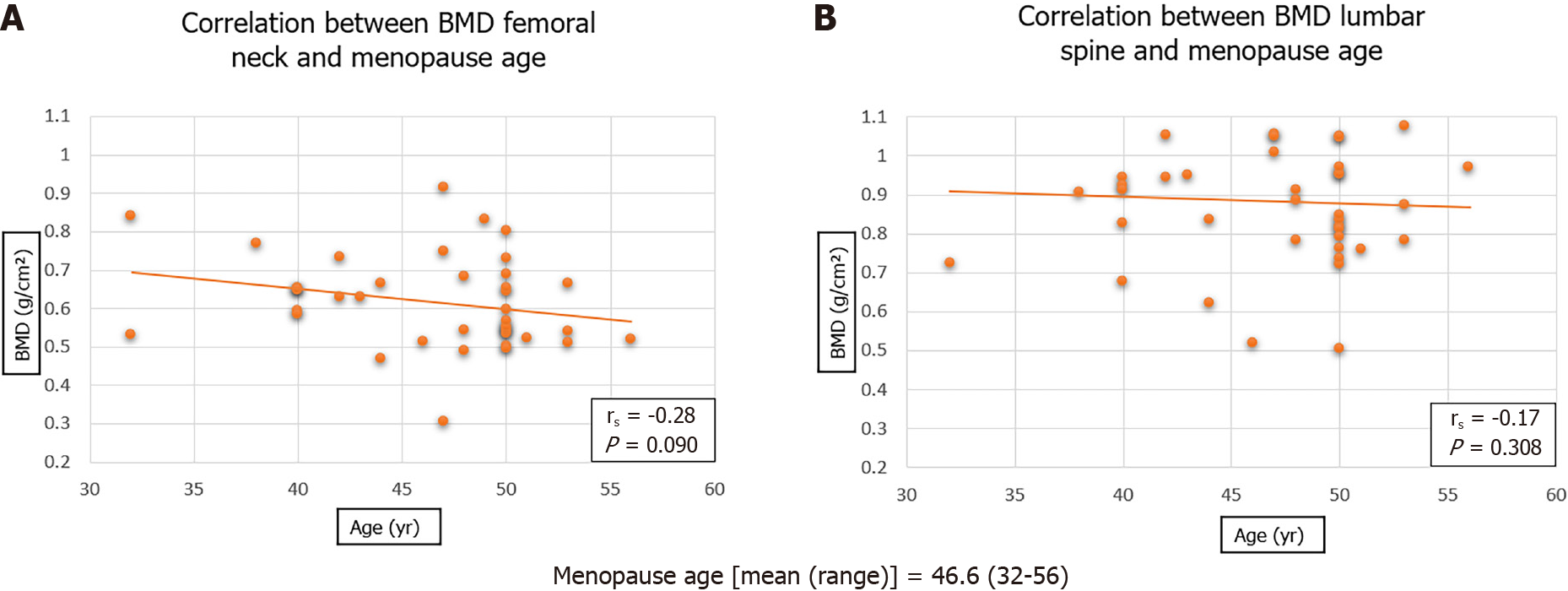
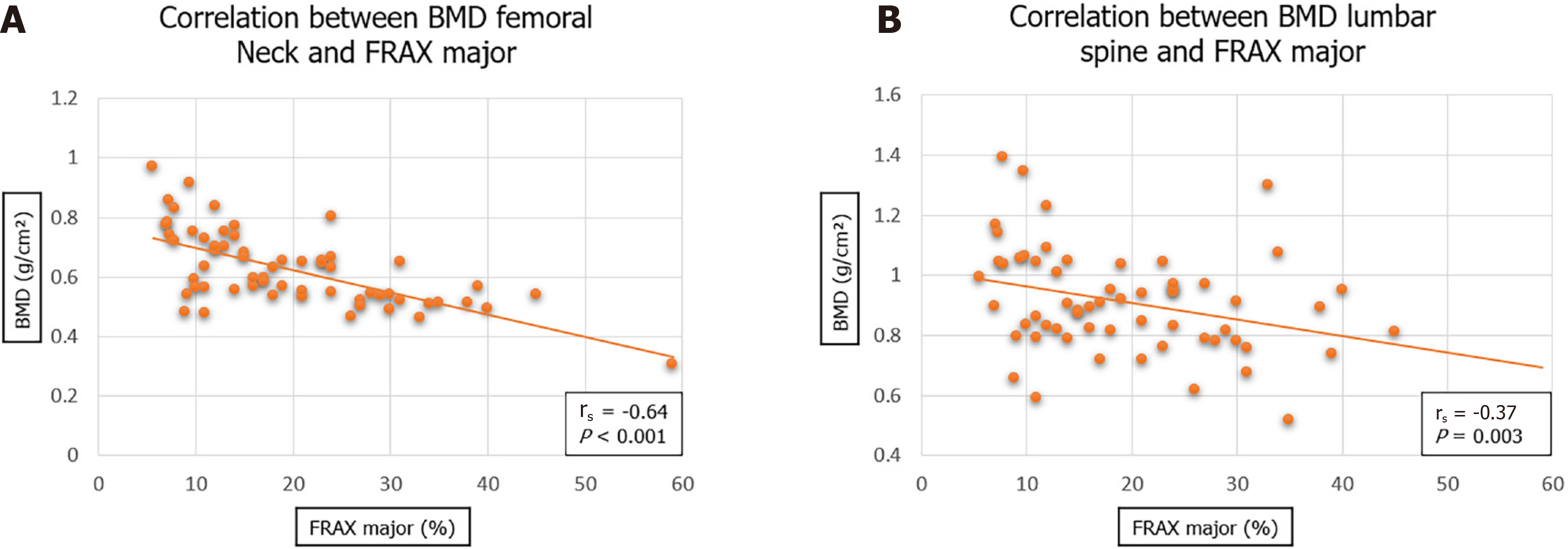
A total of 69 patients were included in the study, mean age 74.1 years. There was no significant difference between mean BMD of the femoral neck in males (0.65) as compared to females (0.61) (P = 0.364). Analyses showed no significant correlation between BMD and menopause age (rs = -0.28, P = 0.090). A significant difference was seen of the femoral neck BMD between the different fracture pattern types (P = 0.026). A stronger correlation was observed between BMD of femoral neck and FRAX major score (rs = -0.64, P < 0.001) than with BMD of lumbar spine and FRAX major score (rs = -0.37, P = 0.003).
This study demonstrated that BMD of the femoral neck measured by DXA scan is of added prognostic value when assessing patients for risk of fracture neck of femur in combination with the FRAX predictive scoring system.
Core Tip: The results in this study place more emphasis on bone mineral density (BMD) when assessing fracture risk, in comparison to key factors incorporated into the Fracture Risk Assessment Tool (FRAX) predictive score. Menopause age and female gender had an indeterminate influence on BMD, as well as World Health Organization classification of osteoporosis. Body mass index had a significant influence on BMD. Osteoporosis was more common in patients with extra-capsular hip fracture patterns. This study shows that BMD is significant in assessing risk of fracture neck of femur in comparison to the FRAX predictive score.
- Citation: Elamin Ahmed H, Al-Dadah O. Bone mineral density in fracture neck of femur patients: What's the significance? World J Orthop 2022; 13(2): 160-170
- URL: https://www.wjgnet.com/2218-5836/full/v13/i2/160.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i2.160
The National Institute for Health and Care Excellence (NICE) define osteoporosis as a disease characterised by low bone mass and structural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture[1]. Fractures secondary to osteoporosis may be referred to as fragility fractures; which are described as those resultant from a mechanical force that would not ordinarily be associated with a fracture[2]; further described by the World Health Organization (WHO) where a fracture occurs from forces equal to or less than a fall from a standing height[1].
Osteoporosis may be viewed as primary or secondary. Primary being characterised by age and sex hormone related progressive mineral loss[3]. Secondary osteoporosis occurs as a result of different pathologies and use of specific medication[4].
Osteoporosis is a disease that develops silently, only manifesting on occurrence of a fracture. It is for this reason that the increase in osteoporosis related pathology has been entitled by some as the “silent epidemic”[4]. The WHO considers osteoporosis to be only second to cardiovascular diseases as a crucial healthcare issue[5].
Prevalence of osteoporosis is known to increase with age, from 2% at age 50 years to 25% at age 80 years[1]. There are known to be over three million United Kingdom residents with osteoporosis at present[6], with this figure expected to continue to rise. Subsequently, the burden on healthcare services as a result of osteoporosis is only likely to rise with the ongoing increase in the world population. In the United Kingdom alone; projections suggest that the incidence of fragility fractures is likely to increase by 21% by the year 2020 (230000 per annum), with a resultant overall annual cost upwards of £2.1 billion[6]. In Europe it is estimated that direct costs resulting from osteoporotic fractures to have an overall cost of €76.7 billion[7].
Despite the above figures, it is almost impossible to truly predict the economic burden of osteoporosis and osteoporotic fractures. The consequences are not only that of economic cost to the healthcare system, but also of associated morbidity, impairment, reduced quality of life and mortality. It is therefore paramount that resources and research into fracture prediction and prevention continues, to ensure that efficient allocation of resources may continue in an already stretched healthcare system.
The International Osteoporosis Foundation and the WHO state that fracture risk should be advised as a short-term absolute risk[8]. A period of ten years is agreed upon as this comprises the probable duration of treatment as well as the benefits that may continue once treatment is ceased[4]. There are several different predicting tools used to identify risk of fragility fracture. The fracture risk assessment tool (FRAX®) is a predictive algorithm developed by the University of Sheffield that takes into account clinical risk factors, with or without the addition of bone mineral density (BMD)[9,10]. The FRAX tool provides a 10-year probability of hip or major osteoporotic fracture by means of a percentage likelihood[11]. The clinical risk factors utilised as part of the FRAX predictive tool are displayed in Table 1.
| Clinical risk factors |
| Age |
| Gender |
| Weight |
| Height |
| Previous fracture |
| Parent fractured hip |
| Current smoking |
| Glucocorticoid use |
| Rheumatoid arthritis |
| Secondary osteoporosis risk factors — Type I diabetes mellitus, osteogenesis imperfecta, hyperthyroidism, hypogonadism, premature menopause, chronic malnutrition or malabsorption, chronic liver failure |
| Alcohol — 3 or more units per day |
| Bone mineral density |
BMD is defined as grams per centimetre squared (g/cm2)[12]. This is measured by means of dual-energy X-ray absorptiometry (DXA) scan. A value of BMD (g/cm2) is therefore given for the area of bone scanned (most commonly proximal femur and lumbar spine)[12]. Alternatively, the value may be converted to a value in comparison (standard deviation) with the peak bone mass of a Caucasian female aged 30 (T-score), or for the peak bone mass adjusted for age, gender and race (z-score)[12]. It is the T-score that is used by the WHO as an objective means of defining osteoporosis in an individual as per DXA scan result; with Osteoporosis being defined as T-score less than or equal to -2.5 (Table 2)[13].
| Terminology | T-score definition |
| Normal | T ≥ -1.0 |
| Osteopenia | -2.5 < T < -1.0 |
| Osteoporosis | T ≤ -2.5 |
| Severe osteoporosis | T ≤ -2.5 in the presence of one or more fragility fracture |
Currently in the United Kingdom it is common practice for the FRAX tool to be used by a primary care physician to give 10-year probability of major osteoporotic fracture. NICE guidelines currently advise if the value is significant, then preventative management is to be initiated immediately, however if the risk is moderate, a DXA scan is advised to further quantify the probability.
A number of recent publications have focused on re-assessment of the importance of clinical risk factors associated with the progression to osteoporosis, including BMD. Trajanoska et al[14] conducted a meta-analysis of genome-wide association studies and a two-sample mendelian randomisation approach to assess the role of fifteen clinical risk factors on osteoporotic fracture risk. It was deduced that among clinical risk factors for fracture that were assessed, only BMD was shown to have a major causal effect on fracture. This mendelian randomisation study provides evidence against a causal effect of several proposed clinical risk factors for fractures (e.g., diabetes, glucose, rheumatoid arthritis, and vitamin D)[14].
Findings from the Foundation of the National Institutes of Health (FNIH) Bone Quality Project, a meta-analysis of twenty-two trials, have shown that a twenty-four percent change in BMD, as per DXA measurement, is responsible for a large proportion of the fracture risk reduction across a range of osteoporosis treatment options[15]. The regression analysis conducted as part of the study displayed percentage difference in increase in BMD (change in BMD with treatment minus the change in BMD with placebo) at the hip, femoral neck, and lumbar spine was significantly associated with relative risk reduction of having a fracture[15]. This, in turn, supports the notion that the BMD, as measured per DXA scan, be utilised as a surrogate biomarker in the measurement of disease progression and management of osteoporosis.
As part of recent guidelines, NICE have recommended research to be focused on the added prognostic value of BMD in the assessment of fracture risk with FRAX[1]. NICE state that there is currently not sufficient research, in primary or secondary care, evaluating if the addition of BMD to FRAX improves the accuracy of the predicted fracture risk[1]. More specifically, NICE advise further research is required to ensure that the addition of BMD results in the correct re-classification of risk, i.e., high to low risk and vice versa[1].
The authors hypothesise an increased significance of BMD in comparison to the individual components of the FRAX score. The aim of this study was to investigate the significance of BMD in fracture neck of femur patients and compare it to the outcome of the FRAX score.
This retrospective observational cohort study which did not require ethics committee approval. All patients had sustained a fracture neck of femur and managed as appropriate under the admitting on-call consultant orthopaedic surgeon between 2015 to 2017.
All patients underwent a DXA scan following completion of management of the fractured neck of femur. DXA images were taken of the contralateral femoral neck and the lumbar spine (L2, L3, L4), in line with published protocols[5,10,11]. Patients with contralateral hip prosthesis in-situ and with lumbar spine instrumentation were excluded from the study. FRAX scores were calculated for all the patients included in the study.
Neck of femur fractures were classified as intra-capsular or extra-capsular. Extra-capsular fractures were defined as those at the inter-trochanteric line (fracture line passes through the greater trochanter to the lesser trochanter) or distal to this as assessed on plain radiograph imaging (antero-posterior pelvis and lateral hip). Fractures proximal to the inter-trochanteric line were classified as intra-capsular fractures. This is a validated radiographic classification system of fracture neck of femur pattern[16].
The authors aim to investigate the association between BMD and the components of the FRAX score within the cohort.
All continuous data variables displayed a skewed distribution (verified by both plotted histograms and the Shapiro-Wilks test). The appropriate non-parametric statistical test was used in their analyses. This includes Mann Whitney U test and Kruskall-Wallis H test used in the appropriate manner. The Chi-squared test was used for the categorical data analysis of the fracture neck of femur pattern type and the Spearman Rank test was used for the correlation analyses. The level of statistical significance was set at P < 0.05. Statistical analysis was performed using SPSS for Windows version 25.0 (IBM Corp., Armonk, New York, United States).
Table 3 displays the demographic data of the study cohort. A total of 69 patients were included in the cohort, all undergoing DXA scan following fracture neck of femur and undergoing a complete FRAX score assessment.
| Patient demographic | |
| Number of patients (n) | 69 |
| Age yr (mean, range) | 74.1 (61-98) |
| Height cm (mean, range) | 161.9 (141-190) |
| Weight kg (mean, range) | 67.7 (31-150) |
| BMI (mean, range) | 25.7 (16-63) |
| Gender (male:female) | 25:44 |
| Menopause age (mean, range) | 46.6 (32-56) |
| Laterality (left:right) | 27:42 |
Table 4 shows the fracture neck of femur classification pattern. Table 5 shows the details of the surgical management.
| n (%) | |
| Intra-capsular fracture | 36 (52.2) |
| Extra-capsular fracture | 33 (47.8) |
| n (%) | |
| Total hip replacement | 24 (34.8) |
| Hemi-arthroplasty | 10 (14.5) |
| Dynamic hip screw | 31 (45.0) |
| Intra-medullary nail | 4 (5.7) |
The mean FRAX score for a major osteoporotic fracture amongst the study cohort was 19.87% (SD 11.01%); this score representing the percentage probability of occurrence of fracture of the distal radius, proximal humerus, lumbar spine or neck of femur over a ten-year period (Table 6). In addition, the mean FRAX score for fracture neck of femur amongst the study cohort was 7.75% (SD 8.09%); representing the ten-year percentage probability of fracture neck of femur only (Table 6). BMD of the femoral neck and lumbar spine where used to calculate the corresponding T-score and z-score. Table 2 shows the WHO classification of Osteoporosis when interpreting T-score values. None of the patients included in this study fitted the classification grade of osteoporosis as all of them had fragility fractures and so by definition were classified as severe osteoporosis when the T-score ≤ -2.5.
| Dual-energy X-ray absorptiometry scan | |
| FRAX major (mean ± SD) | 19.87 ± 11.01 |
| FRAX hip (mean ± SD) | 7.75 ± 8.09 |
| BMD hip (mean ± SD) | 0.62 ± 0.13 |
| T-score hip (mean ± SD) | -2.20 ± 1.05 |
| z-score hip (mean ± SD) | -0.60 ± 0.97 |
| BMD spine (mean ± SD) | 0.88 ± 0.30 |
| T-score spine (mean ± SD) | -1.36 ± 1.65 |
| z-score spine (mean ± SD) | 0.37 ± 1.72 |
Table 7 shows that there was no statistically significant difference for either BMD of femoral neck or lumbar spine between males and females.
| Male (n = 25) | Female (n = 44) | P value1 | |
| BMD femoral neck | 0.65 | 0.61 | 0.364 |
| BMD lumbar spine | 1.01 | 0.85 | 0.135 |
Figure 1 illustrates the scatter plot graph showing no significant correlation between the menopause age of female patients and the BMD of either the femoral neck or lumbar spine.
Tables 8 and 9 show no statistically significant difference between the mean menopause age of female patients and the WHO classification of the femoral neck BMD and the lumbar spine BMD respectively.
| n | Menopause age (mean ± SD) | P value1 | |
| Normal | 5 | 45.0 ± 7.4 | 0.086 |
| Osteopenia | 16 | 44.8 ± 5.1 | |
| Severe osteoporosis | 18 | 48.4 ± 5.1 |
| n | Menopause age (mean ± SD) | P value1 | |
| Normal | 15 | 47.2 ± 5.7 | 0.835 |
| Osteopenia | 15 | 46.1 ± 5.3 | |
| Severe osteoporosis | 10 | 46.3 ± 6.1 |
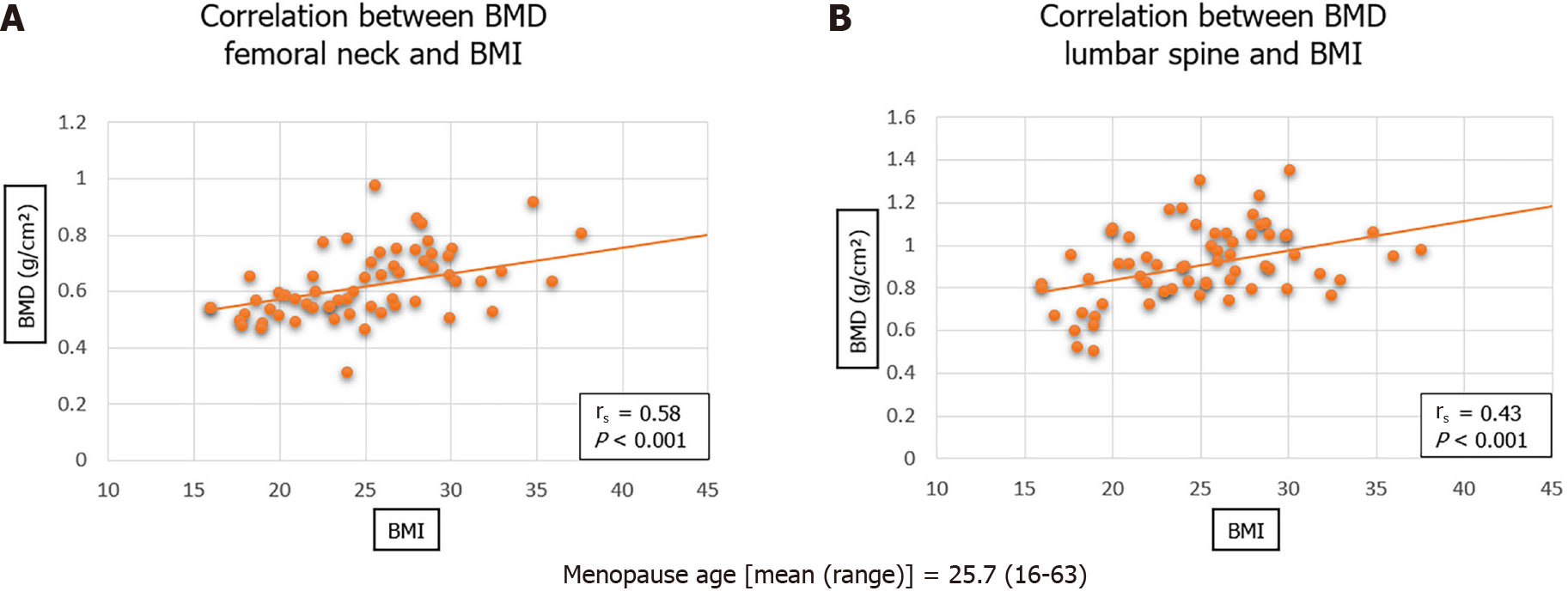
Figure 2 shows a significant direct correlation between body mass index (BMI) and BMD for both femoral neck (rs = 0.58; P < 0.001) and lumbar spine (rs = 0.43; P < 0.001).
Table 10 shows a statistically significant difference (P = 0.026) between the pattern of fracture neck of femur and the WHO Classification of femoral neck BMD. Patients with extra-capsular fractures where more likely to have severe osteoporosis than those with intra-capsular fractures.
| Intra-capsular (n) | Extra-capsular (n) | P value1 | |
| Normal | 6 | 2 | 0.026 |
| Osteopenia | 17 | 7 | |
| Severe osteoporosis | 10 | 20 |
Figure 3 illustrates a statistically significant inverse correlation between the femoral neck BMD and the lumbar spine BMD with the FRAX major score (percentage likelihood of major osteoporotic fracture in the next ten years). The correlation was stronger with the femoral neck BMD (rs = -0.64; P < 0.001) than that of the lumbar spine BMD (rs = -0.37; P < 0.001).
The main findings of this study were that BMD had a significant correlation with the FRAX score and also with BMI. Neither female gender or early menopause age had a significant influence on BMD. Osteoporosis was more common in patients with extra-capsular hip fracture patterns.
The aetiological factors associated with development of both primary and secondary osteoporosis have been looked at in numerous studies. With the incidence of osteoporosis continuing to rise[17], it is important that new research continues to advance and build upon previous findings.
The clinical risk factors associated with development of osteoporosis have long been agreed upon, with emerging contributory risk factors to secondary osteoporosis being a constant subject of further research[18]. However, the significance of the contribution of certain clinical risk factors to the pathophysiology of the development of primary osteoporosis is less understood. This study aimed to investigate the significance of BMD in fracture neck of femur patients and compare it to the outcome of the FRAX score.
This study addressed a relatively unique cohort, all patients had sustained a major osteoporotic fracture (i.e., fracture neck of femur). This allowed for research into arguably the most at risk group of individuals in the community. This contrasts to previous literature, whereby major osteoporotic fracture is used as an end-point on patient follow-up[10].
Local clinical guidelines allow for the discretion of the referring clinician with regards to whether DXA scan is appropriate following surgical management and rehabilitation of fracture neck of femur patients. In general, this would be all patients under the age of 75, as well as those patients felt to be independent in all aspects of daily living. This ensures that those included in this cohort will potentially benefit from preventative medical treatments to avert further major osteoporotic fractures. In addition, this ensures that 10-year percentage probability of major osteoporotic fracture data generated is relevant for the cohort.
The majority of patients with intra-capsular fracture neck of femur were managed with total hip arthroplasty, as opposed to hemi-arthroplasty, as displayed in Table 5. This reflects the relatively low mean age of the patients in the cohort. Current National Institute for Health and Care Excellence (NICE) guidelines advise that fracture neck of femur patients be managed surgically by total hip replacement if they are independently mobile and independent in activities of daily living[18]. This, in turn, portrays that the cohort in this study represents a group of individuals who are expected to continue with an independent lifestyle after surgical management, and therefore it is important that further fracture prevention is prioritised by all members of the healthcare team. Osteoporosis was more common in patients with extra-capsular hip fracture patterns in this study. Dynamic hip screw (DHS) is a common internal fixation device used for this fracture pattern. The biomechanical pull-out strength of locking plates is superior to that of conventional plates particularly in osteoporotic bone. It may be more prudent to use DHS implants which have locking plates for screw fixation in the surgical management of patients with an extra-capsular fracture configuration.
The significant (inverse) relationship between femoral neck BMD and lumbar spine BMD with the FRAX score (10-year percentage probability of major osteoporotic fracture) is in agreement with previous literature[17,20]. This supports the ongoing understanding that measures to increase BMD will result in reduced incidence in major osteoporotic fractures[15,21].
The correlation between BMD (both femoral neck and lumbar spine) and BMI is also in agreement with previous literature[22]. This is likely to be explained by a combination of factors, including nutritional status, mechanical load and hormones. It has previously been hypothesised that increased weight, and subsequently increased mechanical load, results in decreased osteoclast activity and increased osteoblast activity, increasing bone strength and bone mineral content[23]. This supports current emphasis on nutritional status of fracture neck of femur patients, not only in aiding rehabilitation, but also in prevention of further fracture.
BMD was noted to have a significant influence on the fracture pattern at the neck of femur. This is also in agreement with previous literature[16], whereby extra-capsular fracture patterns are associated with a lower BMD. However, it is important to emphasis that literature has shown there to be other factors that will have an influence on fracture pattern at the neck of femur[24], most notably inter-trochanteric outer diameter and buckling ratio.
Gender difference has long been known to have an influence on both osteoporosis fracture risk and BMD. This is variable is included in the FRAX questionnaire[25]. Interestingly, there was no significant difference between males and females for BMD within the cohort studied. It is important to note that it is not the intention to disprove the influence of gender on BMD, and ultimately osteoporosis. However, it may be argued that the effect of gender is not in isolation, and that other factors that affect BMD (nutrition, mechanical force, genetic predisposition, ethnicity, etc.) may outweigh gender’s overall role.
It was shown that menopause age did not have a significant association with either BMD or WHO osteoporosis classification. This is in contrast to the majority of published literature[26], whereby it is understood that earlier menopause age is associated with lower BMD, and therefore increased incidence of osteoporosis and fragility fractures[27]. This study does not conclude that age of menopause has no effect on BMD, osteoporosis and fracture risk. However, it emphasises that a multi-faceted approach must be taken when attempting to address the field in question.
Limitations of the study included that much of the presented data focuses on the influence of gender on BMD, and consequently the influence of gender on osteoporosis and fracture risk. One must make note of the differences in frequency between males (n = 25) and females (n = 44). This was appropriately adjusted for in the statistical analyses conducted. This gender discrepancy is explained by the disproportionately higher incidence of fracture neck of femur in females as compared to males[28].
Recommendations for future research include a greater focus on how BMD may help reclassify patient fracture risk when added to the already calculated FRAX score based on 10-year probability of both major osteoporotic fracture and fracture neck of femur. This will aid to ensure osteoporosis preventative management is appropriately prioritised to reduce the incidence of fragility fractures. The findings in this paper also support the requirement for further research into the use of BMD as a surrogate biomarker for both fracture risk and osteoporosis prevention and management. This may be in the context of a cross-sectional study of fragility fractures at differing ages group and further appropriate stratification as per age, with confounding factors adjusted for.
The results in this study place more emphasis on BMD when assessing fracture risk, in comparison to key factors incorporated into the FRAX predictive score. Menopause age and female gender had an indeterminate influence on BMD, as well as WHO classification of osteoporosis. BMI had a significant influence on BMD. Osteoporosis was more common in patients with extra-capsular hip fracture patterns. This study shows that BMD is significant in assessing risk of fracture neck of femur in comparison to the FRAX predictive score.
There has been a steady increase in fragility fractures in the United Kingdom and worldwide. This has been seen in the increased number of patients admitted with fracture neck of femur. It is essential to gain a further understanding of the aetiology to understand preventative measures.
Increased prevalence of osteoporosis fragility fractures in the NHS, causing an increased economic burden.
The aim of this study was to investigate the significance of bone mineral density (BMD) in fracture neck of femur patients and compare it to the outcome of the Fracture Risk Assessment Tool (FRAX) score.
Statistical analyses undertaken to ascertain the relationship between BMD and the individual factors included in the FRAX score.
The results in this study place more emphasis on BMD when assessing fracture risk, in comparison to key factors incorporated into the FRAX predictive score. Menopause age and female gender had an indeterminate influence on BMD, as well as World Health Organization classification of osteoporosis. BMI had a significant influence on BMD. Osteoporosis was more common in patients with extra-capsular hip fracture patterns. This study shows that BMD is significant in assessing risk of fracture neck of femur in comparison to the FRAX predictive score.
This study demonstrated that BMD of the femoral neck measured by dual-energy X-ray absorptiometry scan is of added prognostic value when assessing patients for risk of fracture neck of femur in combination with the FRAX predictive scoring system.
The findings in this paper also support the requirement for further research into the use of BMD as a surrogate biomarker for both fracture risk and osteoporosis prevention and management. This may be in the context of a cross-sectional study of fragility fractures at differing ages group and further appropriate stratification as per age, with confounding factors adjusted for.
The authors wish to thank David Donohue (clinical technologist) from the South Tyneside Medical Physics Department for his technical assistance and insights.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheungpasitporn W S-Editor: Gao CC L-Editor: A P-Editor: Yu HG
| 1. | The National Institute for Health and Care Excellence. Osteoporosis - prevention of fragility fractures. [cited 11th November 2020]. In: The National Institute for Health and Care Excellence [Internet]. Available from: https://cks.nice.org.uk/osteoporosis-prevention-of-fragility-fractures#.XYihz9zxKPo.mendeley. |
| 2. | Curtis EM, Moon RJ, Harvey NC, Cooper C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone. 2017;104:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 192] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 3. | Albright F. Annals of internal medicine, Volume 27, 1947: Osteoporosis. Nutr Rev. 1989;47:85-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Pisani P, Renna MD, Conversano F, Casciaro E, Di Paola M, Quarta E, Muratore M, Casciaro S. Major osteoporotic fragility fractures: Risk factor updates and societal impact. World J Orthop. 2016;7:171-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 317] [Cited by in RCA: 327] [Article Influence: 36.3] [Reference Citation Analysis (5)] |
| 5. | Kanis JA, Delmas P, Burckhardt P, Cooper C, Torgerson D. Guidelines for diagnosis and management of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporos Int. 1997;7:390-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 495] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Burge R, Worley D, Johansen A, Bhattacharyya S, Bose U. The cost of osteoporotic fractures in the UK: Projections for 2000-2020. J Med Econ. 2008;4:51-62. |
| 7. | Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2849] [Cited by in RCA: 3085] [Article Influence: 162.4] [Reference Citation Analysis (0)] |
| 8. | Kanis JA, Johnell O, Oden A, De Laet C, Jonsson B, Dawson A. Ten-year risk of osteoporotic fracture and the effect of risk factors on screening strategies. Bone. 2002;30:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, Jönsson B. Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2011;6:59-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 407] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 10. | Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, Burckhardt P, Cooper C, Christiansen C, Cummings S, Eisman JA, Fujiwara S, Glüer C, Goltzman D, Hans D, Krieg MA, La Croix A, McCloskey E, Mellstrom D, Melton LJ 3rd, Pols H, Reeve J, Sanders K, Schott AM, Silman A, Torgerson D, van Staa T, Watts NB, Yoshimura N. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 847] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 11. | Watts NB. The Fracture Risk Assessment Tool (FRAX®): applications in clinical practice. J Womens Health (Larchmt). 2011;20:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Berger A. Bone mineral density scans. BMJ. 2002;325:484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 2370] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 14. | Trajanoska K, Morris JA, Oei L, Zheng HF, Evans DM, Kiel DP, Ohlsson C, Richards JB, Rivadeneira F; GEFOS/GENOMOS consortium and the 23andMe research team. Assessment of the genetic and clinical determinants of fracture risk: genome wide association and mendelian randomisation study. BMJ. 2018;362:k3225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 15. | Busko M. Study Finds BMD Can Stand in for Fractures. Will the FDA Agree? [cited 11th November 2020 In: Medscape [Internet]. Available from: https://www.medscape.com/viewarticle/902884. |
| 16. | Nakamura N, Kyou T, Takaoka K, Ohzono K, Ono K. Bone mineral density in the proximal femur and hip fracture type in the elderly. J Bone Miner Res. 1992;7:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Kanis JA. Diagnosis and Clinical Aspects of Osteoporosis. In: Ferrari SL, Roux C. Pocket Reference to Osteoporosis. Cham: Springer International Publishing, 2019: 11-20. |
| 18. | Mirza F, Canalis E. Management of endocrine disease: Secondary osteoporosis: pathophysiology and management. Eur J Endocrinol. 2015;173:R131-R151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 19. | Ftouh S, Morga A, Swift C; Guideline Development Group. Management of hip fracture in adults: summary of NICE guidance. BMJ. 2011;342:d3304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Imerci A, Yalın Kılınç C, Aydogan NH, Karalezli MN, Savran A. Fracture Risk Assessment Tool (FRAX®) Results Calculated With and Without Bone Mineral Density Values for the Evaluation of Fracture Risk in Postmenopausal Women With Osteopenia. J Clin Densitom. 2018;21:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Ferrari S, Libanati C, Lin CJF, Brown JP, Cosman F, Czerwiński E, de Gregόrio LH, Malouf-Sierra J, Reginster JY, Wang A, Wagman RB, Lewiecki EM. Relationship Between Bone Mineral Density T-Score and Nonvertebral Fracture Risk Over 10 Years of Denosumab Treatment. J Bone Miner Res. 2019;34:1033-1040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Wu SF, Du XJ. Body Mass Index May Positively Correlate with Bone Mineral Density of Lumbar Vertebra and Femoral Neck in Postmenopausal Females. Med Sci Monit. 2016;22:145-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Vincent HK, Heywood K, Connelly J, Hurley RW. Obesity and weight loss in the treatment and prevention of osteoarthritis. PM R. 2012;4:S59-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Rivadeneira F, Zillikens MC, De Laet CE, Hofman A, Uitterlinden AG, Beck TJ, Pols HA. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam Study. J Bone Miner Res. 2007;22:1781-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 25. | Moberg LME, Nilsson PM, Holmberg AH, Samsioe G, Borgfeldt C. Primary screening for increased fracture risk by the FRAX® questionnaire-uptake rates in relation to invitation method. Arch Osteoporos. 2019;14:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Heidari B, Hosseini R, Javadian Y, Bijani A, Sateri MH, Nouroddini HG. Factors affecting bone mineral density in postmenopausal women. Arch Osteoporos. 2015;10:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Mishra S, Manju M, Toora BD, Mohan S, Venkatesh BP. Comparison of bone mineral density and serum minerals in pre and post-menopausal women. Int J Clin Trials. 2015;2. [DOI] [Full Text] |
| 28. | Hedlund R, Lindgren U. Trauma type, age, and gender as determinants of hip fracture. J Orthop Res. 1987;5:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 1703] [Article Influence: 100.2] [Reference Citation Analysis (0)] |