Published online Feb 18, 2022. doi: 10.5312/wjo.v13.i2.150
Peer-review started: June 16, 2021
First decision: July 28, 2021
Revised: August 16, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 18, 2022
Processing time: 246 Days and 15.3 Hours
Hemiarthroplasty is the most common treatment in elderly patients with displaced intra-capsular femoral neck fracture (FNF). Prosthetic joint infection (PJI) is one of the most feared and frequent complications post-surgery because of the frail health status of these patients and the need for fast track surgery. Therefore, priorities should lie in effective preventive strategies to mitigate this burden.
To determine how much the implementation of the routine use of antibiotic-loaded bone cement (ALBC) as a relatively easy-to-apply amendment to the surgical practice reduces the infection rate in our hemiarthroplasty cohort.
We retrospectively assessed all demographic, health status and treatment-related data of our FNF patients undergoing cemented hemiarthroplasty in the period from 2011 to 2017; 241 patients were further analyzed after exclusion of patients with cancer-related sequelae and those who died before the end of the 1-year observation period. The PJI rate as diagnosed on basis of the Musculoskeletal Infection Society (MSIS) criteria 2011 was determined for each included patient and compared in function of the bone cement used for hip stem fixation. Patients were split into a group receiving a plain bone cement in the period from January 2011 to June 2013 (non-ALBC group) and into a group receiving an ALBC in the period July 2013 to December 2017 (ALBC group). Data analysis was performed with statistical software. We further calculated the cost-efficacy of the implementation of routine use of ALBC in the second group balancing the in-hospital infection related treatment costs with the extra costs of use of ALBC.
In total 241 FNF patients who received cemented hemiarthroplasty in the period from January 2011 to January 2017 were eligible for inclusion in this retrospective study. There were 8 PJI cases identified in the ALBC group among n = 94 patients, whereas 28 PJI cases were observed in the non-ALBC group among n = 147 patients. The statistical analysis showed an infection risk reduction of 55.3% (in particular due to the avoidance of chronic delayed infections) in the ALBC group (95%CI: 6.2%-78.7%; P = 0.0025). The cost-evaluation analysis demonstrated a considerable cost saving of 3.500 € per patient, related to the implementation of routine use of ALBC in this group.
Use of ALBC is a potent infection preventive factor in FNF patients receiving cemented hemiarthroplasties. It was further found to be highly cost-effective.
Core Tip: Routine use of antibiotic-loaded bone cement in cemented hemiarthroplasties of femoral neck fracture patients has the potential of reducing the infection risk to a significant degree. This measure should be considered on top of the implementation of strict protocols of special pre-, peri- and postoperative orthogeriatric care
- Citation: Crego-Vita D, Aedo-Martín D, Garcia-Cañas R, Espigares-Correa A, Sánchez-Pérez C, Berberich CE. Periprosthetic joint infections in femoral neck fracture patients treated with hemiarthroplasty – should we use antibiotic-loaded bone cement? World J Orthop 2022; 13(2): 150-159
- URL: https://www.wjgnet.com/2218-5836/full/v13/i2/150.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i2.150
Prosthetic joint infection (PJI) is a rare but very dreadful complication of arthroplasty. The incidence is typically in the range of 1%-2% after primary elective joint replacement[1], but easily surpasses 5% in the elderly population of femoral neck fracture (FNF) patients undergoing hemi- or total hip arthroplasty[2]. This high infection rate reflects the fragile health conditions of such patients being on an emergency surgery track and their often suboptimal pre-, peri- and postoperative care. It is expected that the FNF patient numbers will grow significantly in our ageing societies causing high socioeconomic costs and a high burden of morbidity and mortality[3]. In order to mitigate the high risk of complications occurring in this patient cohort, it is therefore mandatory to consider amendments to the routine surgical protocols. This also includes more potent preventive strategies for infections.
The prophylactic use of antibiotic-loaded bone cement (ALBC) is a frequent surgical practice in cemented hip and knee joint replacement aiming at reducing the risk of procedure related infections. The idea behind delivering antibiotics directly into the joint compartment was originally pioneered by Buchholz and Engelbrecht in 1969. They reported high initial peak concentrations of the antibiotic eluted in situ from bone cement which was found to exceed 100-1000 fold the minimum inhibitory concentration (MIC) of the pathogen in the first days without exposing the patient to major risks of side effects[4,5]. Systemically applied antibiotics, by contrast, often do not reach effective concentration levels in the osteoarticular compartment as a consequence of reduced blood flow in inflamed tissue and limited bone penetration of many antibiotics[6]. Indeed, by implementing this additional prevention measure Buchholz achieved an impressive reduction in deep infections in both, primary and revision hip replacements[7]. Subsequently, the Scandinavian registries and – most recently - the National Joint Registry of UK have demonstrated that the additional use of ALBC to perioperative systemic prophylaxis reduces the revision risk in cemented joint replacement[8-10]. It can be further speculated that this effect might vary if specific cement brands were compared due to brand-specific differences in antibiotic elution as a function of their special polymer contents and porosities[11,12]. Although one would expect from these experiences in elective procedures that hemiarthroplasty patients might benefit equally or even more from additional local antibiotic prophylaxis, data in this patient group are still sparse. To the best of our knowledge there is also no cost evaluation available in fracture patients which calculated the treatment costs of hemiarthroplasty related infections against the extra costs of ALBC use instead of plain cement.
In view of the very high numbers of PJI cases which we observed in the past in our hemiarthroplasty patients we decided to assess the infection preventive effect of the implementation of routine ALBC use for our cemented hemiarthroplasties. By comparing the infection rate before and after this surgical protocol modification we wanted to address the following questions in form of a retrospective clinical study: (1) (Primary endpoint of the study) Does the implementation of ALBC instead of plain cement as standard for hip stem fixation reduce the infection rates in this frail patient cohort? (2) Do we observe the highest protective effect, if we switch to an ALBC brand which has been described as a superior antibiotic eluting polymer matrix[13,14]? (3) What is the influence of individual patient risk factors and parameters on the occurrence and course of PJI cases; and (4) (Secondary endpoint of the study) Is the implementation of routine use of ALBC cost-effective?
For this purpose we retrospectively analyzed the patients and compared the PJI rate in those hemiarthroplasty procedures which had been cemented in the period from 2011 to 2013 with the plain bone cement Cemex with the PJI rate of those procedures which had been cemented in the subsequent period 2013 to 2017 with the gentamicin loaded bone cement Palacos R+G.
This study (study approval number 35/17 NoEPA by Hospital Ethical Committee) analyzed all hip fracture patients treated at the University Hospital Central de la Defensa Gómez Ulla in Madrid, Spain, in the period from 2011 to 2017. Patient data and clinical histories were available on paper or in electronic form in the hospital IT program Balmis® (Hewlett Packard, Spain). In total 427 patients with diagnosis of hip fractures were reported. Of these 72 were excluded because of osteosynthesis treatment, 114 patients were further excluded because of periprosthetic or oncologic fractures or because of premature death before completing the 1-year observation period. Finally, 241 patients with intracapsular neck of femur fractures who went on to have cemented hemiarthroplasty either with a mono- or with a bipolar prosthesis in this period were found eligible for study inclusion. All interventions were performed by the same team of surgeons and the same surgical access route to the femur (direct lateral or Hardinge access route). The preoperative parental antibiotic prophylaxis was administered in the following way: either administration of 2 g of cefazolin, initiated 60 min before incision, or administration of 1 g of vancomycin, respectively, in case of contraindications or allergy against cephalosporins. In total 3 additional shots of 1 g of cefazolin, each one in an interval of 8 h, or 1 g of vancomycin, each one in an interval of 12 h, respectively, were administered postoperatively.
The hemiarthroplasty prosthesis used in all patients of this study was the brand Multifit Integrated System® with monopolar Ellittica® head or the bipolar prosthesis head SBA® (both Samobiomedical, Italy). The bone cement used for stem fixation in the period from January 2011 until June 2013 was the antibiotic-free brand Cemex (Tecres Spa, Italy) of high viscosity, mixed under atmospheric conditions and retrogradely injected with aid of a gun. The bone cement subsequently used in the period from July 2013 until the end of the study in December 2017 was the 0.5 g gentamicin containing high viscous cement Palacos R+G (Heraeus-Medical, Germany), prepared in a vacuum-mixing system and retrogradely injected under pressure with aid of a gun.
The following patient data were collected from the files: age, sex, type of intracapsular femur fracture according to the Garden classification, type of trauma (low or high energy impact), form of arrival to the emergency department at the hospital (self-walking, in vehicle of relatives, by ambulance or transfer from another health institution). Presence of risk factors for infections were recorded including inflammatory arthropathies, degree of immunosuppression, diabetes mellitus, previous articular infection, malnutrition, hemophilia and presence of tumors. The health status of all patients was assessed using the American Society of Anesthesiologists (ASA) score, the degree of mobility was assessed according to the Functional Ambulation Category (FAC) score and the Barthel Functional (BF) index for activities of daily living was used to evaluate the level of functional independence. The diagnosis of acute or delayed periprosthetic infections within the observation period of 1 year was done on basis of the MSIS criteria 2011[15] (see Table 1). All data were collected in a Microsoft Excel® speadsheet in strict anonymous form and analyzed using the statistical software package of SPSS®, version 15 (SPSS Inc., Chicago, IL, United States).
| PJI exists when |
| (1) There is a sinus tract communicating with the prosthesis; or (2) A pathogen is isolated by culture from at least two separate tissue or fluid samples obtained from the affected prosthetic joint; or (3) Four of the following six criteria exist: (a) Elevated serum erythrocyte sedimentation rate (ESR) and serum C-reactive protein (CRP) concentration; (b) Elevated synovial leukocyte count; (c) Elevated synovial neutrophil percentage (PMN%); (d) Presence of purulence in the affected joint; (e) Isolation of a microorganism in one culture of periprosthetic tissue or fluid; or (f) Greater than five neutrophils per high-power field in five high-power fields observed from histologic analysis of periprosthetic tissue at 9400 magnification. |
We further performed a cost-evaluation in which we compared across both study arms the overall product related costs as well as the costs related to the surgical interventions and to the days of in-hospital care according to the 2014 updated DRG (diagnosis related groups) based hospital reimbursement regulation DEF/2277/2014 for medical services and products in institutions of the Ministry of Defense of Spain. These costs include in detail: (1) Routine and special diagnostic and therapeutic examinations prior to the surgical procedure; (2) All expenses related to complications during the pre-, peri- and post-operative phase; (3) All expenses of surgical reinterventions, if required, because of bad surgical practice and within a period of 2 mo from day of hospital discharge; (4) All medication during the treatment including blood supply and hemoderivates; (5) Nutritional assistance including parenteral or enteral products; (6) Expenses related to the medical team (specialized doctors, nurses and auxillary staff); (7) Expenses for use of theatre and anesthesia; (8) Expenses for all required medical consumables and pre- and postoperative controls including the early ambulatory phase; (9) Expenses related to the days in an individual or shared hospital room; (10) Expenses within the intensive care unit, if required; and (11) Expenses for revision-related procedures after hospital discharge.
In total 241 patients with intracapsular neck of femur fractures who went on to receive cemented hemiarthroplasty in the period from January 2011 to December 2017 were eligible for inclusion in this retrospective study. They were stratified into 2 study groups according to the bone cement used for the fixation of the hip stem, 94 patients received the gentamicin-loaded bone cement (ALBC group) and 147 patients received the plain cement without any added antibiotic (non-ALBC group). The demographic and health status related data for all patients were analyzed in detail and compared between both groups with focus on risk factors for infections (see Table 2). There were 38 patients (25.9%) in the non-ALBC group who had one or several risk factors including inflammatory arthropathies, malnutrition, immunosuppression, diabetes, prior articular infection, tumors or hemophilia. The percentage of risk for infection patients was higher in the ALBC group with 39.4% (n = 47). Use of oral anticoagulants (acenocumarol) was also assessed and compared between the groups (6.8% in the non-ALBC group vs 8.5% in the ALBC group, data not shown). The predominant overall ASA status of the patients in both groups was 3 (52.9%) and the predominant FAC mobility score was 4. Distribution of sex and average of patient age were comparable in both groups.
| Items | Plain cement group (n = 147), n (%) | ALBC group (n = 94), n (%) | Both groups, (n = 241), n (%) |
| Sex | |||
| Male | 42 (28.6) | 29 (30.9) | 71 (29.5) |
| Female | 105 (71.4) | 65 (69.1) | 170 (70.5) |
| Age | |||
| < 80 | 27 (18.4) | 25 (26.6) | 52 (21.6) |
| > 80 | 120 (81.6) | 69 (73.4) | 189 (78.4) |
| Garden classification | |||
| I | 4 (2.7) | 10 (10.6) | 14 (5.8) |
| II | 19 (12.9) | 8 (8.5) | 27 (11.2) |
| III | 47 (32.0) | 35 (37.2) | 82 (34.0) |
| IV | 77 (52.4) | 41 (43.6) | 118 (49.0) |
| Type of trauma | |||
| High energy | 1 (0.7) | 0 (0) | 1 (0.4) |
| Low energy | 146 (99.3) | 94 (100) | 240 (99.6) |
| Arrival at hospital | |||
| Walking | 2 (1.4) | 5 (5.3) | 7 (2.9) |
| Transferral by relatives | 1 (0.7) | 1 (1.1) | 2 (0.8) |
| Ambulance | 95 (64.6) | 75 (79.8) | 170 (70.5) |
| Transferral from other centres | 49 (33.3) | 13 (13.8) | 62 (25.7) |
| Risk factors for infections | |||
| Yes | 38 (25.9) | 37 (39.4) | 75 (31.1) |
| No | 109 (74.1) | 57 (60.6) | 166 (68.9) |
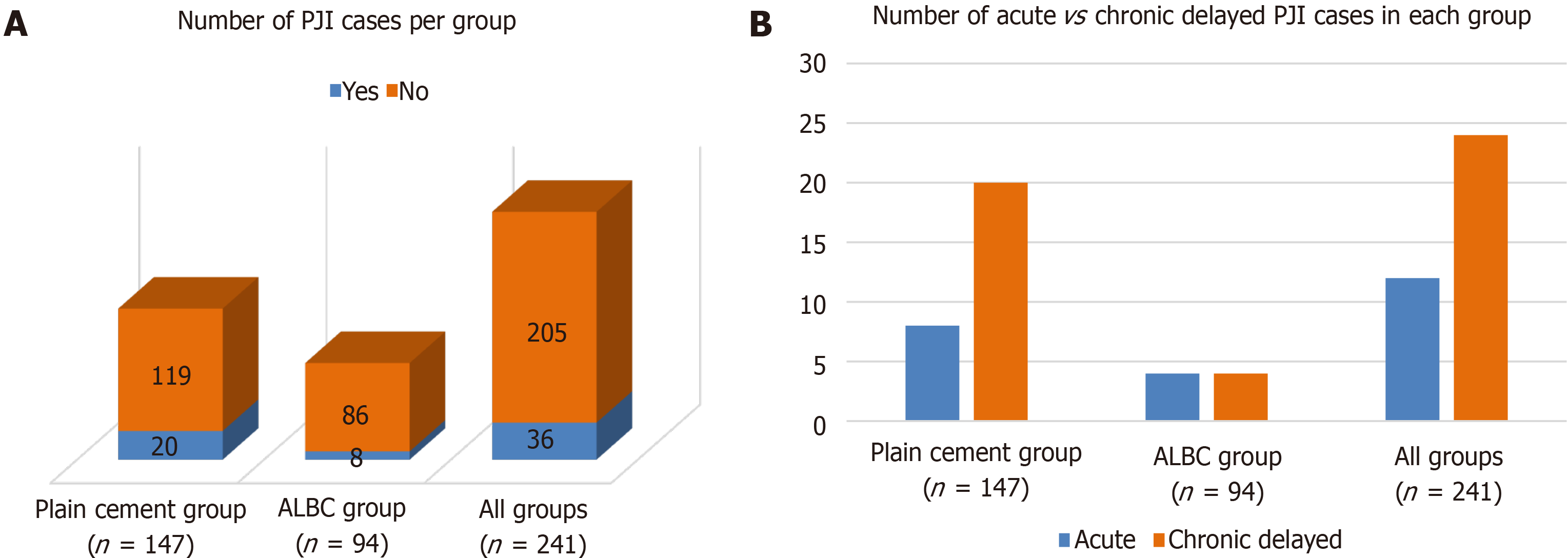
The overall incidence of PJI was compared between both groups. The number of infection cases was found to be with 8 cases among 94 patients (8.5%) significantly lower in the ALBC group as opposed to 28 infections among 147 patients in the non-ALBC group (19%) within the observation period of 1 year (see Figure 1A). From the total number of 8 infections occurring in the ALBC group, 4 cases were classified as acute (evolution of symptoms before 3 mo) and 4 cases as chronic delayed (evolution of symptoms after three months). In the non-ALBC group, 8 infections were of acute nature while 20 were delayed (see Figure 1B). This means in the statistical analysis that the use of the gentamicin-loaded bone cement Palacos R+G instead of the plain cement Cemex led to an overall reduction of the infection risk of 55.3% (95%CI: 6.2%-78.7%; P = 0.0025). The number needed to treat was 1.8.
The implementation of routine use of the antibiotic-loaded bone cement Palacos R+G instead of the plain cement Cemex resulted in additional treatment costs. In order to justify these extra costs, we calculated the cost-benefit ratio of this new measure on basis of our routine hemiarthroplasty cost figures and the additional treatment expenses related to the management of the infection cases. The documented in-hospital costs for the implantation of a primary cemented hemiprosthesis in our institution are 12.665 €. In case of complications these costs increase significantly to 24.205 € for treatment of acute infections or to 35.746 € for the treatment of chronic delayed infections, reflecting in particular length of stay in the hospital and additional surgical intervention costs. The added costs for each patient receiving ALBC instead of plain cement (+20 € per package of 40 g) were subsequently put in relation to the number of avoided infections in the ALBC group. Based on the above shown numbers of infections occurring in each group with their subsequent treatment costs for both, acute and chronic cases, we calculated an average treatment cost of 14.127 € per patient in the ALBC group and of 17.632 € per patient in the non-ALBC group. These figures represent a cost saving of approximately 3.505 € for each patient after switching to the ALBC Palacos R+G.
The occurrence of PJI in the cohort of FNF patients treated with hemiarthroplasty is much more frequent and an even more catastrophic complication than in elective hip replacement patients. The high infection rates reflect the general frailty of the patients presenting at an advanced age, with an acute trauma event and with a high burden of comorbidities[16]. Further risk factors for infections have to be added to this including the operative conditions (junior surgeon, uncemented stems, duration of surgery) and the post-operative management regimen (length of hospitalization, hematoma, prolonged wound drainage and urinary catheterizations) as shown in a recent literature review[17]. The surgical and antibiotic treatment of the infection cases together with the extended immobility period further weakens the organism leading to 1-year mortality rates in the range of 40% to 50%[18,19].
Staphylococcus is the most commonly found microorganism in acute and chronic infections. While the prevalence of S. aureus as culprit of PJI is gradually decreasing, coagulase-negative Staphylococci are being found more and more in acute and particularly chronic infections[20]. These bacteria are either directly inoculated through contaminated implants during surgery or postoperatively through contiguous spread from slow healing wounds or through hematogeneous dissemination from more remote bacteremias. Among the anti-straphylococcal weapons available the bactericidal antibiotic gentamicin has still largely retained its antimicrobial efficacy – at least if it is present at high concentrations. Such high peak levels can be expected after initial release of the antibiotic from a well eluting bone cement. Indeed, the analysis of major European arthroplasty registries has shown that the combination of systemic perioperative and local antibiotic prophylaxis via gentamicin-loaded bone cement lowers the overall revision risk and protects the implants from early infections[8-10] In a similar way Sanz-Ruiz et al[21] recently observed an impressive reduction in their PJI rate (-57% for all cemented primary prostheses and -72.6% for cemented hip replacements) after switching from plain cement to ALBC. Interestingly, the PJI incidence in all their uncemented procedures did not change in the entire observation period, thus suggesting that the outcome was, in fact, due to the change of the bone cement category[21].
One might speculate that the particularly frail population of FNF patients treated with cemented hemiarthroplasty would equally or benefit even more from the presence of an additional antimicrobial “frontline” in situ. However, this hypothesis has not been systematically evaluated in our country. Our present study provides evidence that the implementation of the routine use of ALBC instead of plain cement is a powerful anti-infective measure in this patient cohort. We show here that the number of PJI cases could be significantly reduced by 55% if stems were cemented with ALBC. This effect was even more remarkable in light of the higher percentage of patients with well described risk factors for infections in the ALBC group compared to the non-ALBC group.
We are aware that this finding may not only reflect the switch from an unloaded to an antibiotic-loaded cement category, but may also depend on individual cement brand characteristics. This fits to prior observations showing that the ALBC brand PALACOS R+G has a superior antibiotic elution behaviour compared to other bone cement brands[13,14].
The PJI rate in our hemiarthroplasty patients was found to be much higher than described in the literature, probably reflecting “real world” experiences in our hospital until recently. This refers to the absence of a special orthogeriatric optimization protocol prior and post-surgery as well as to the absence of a routine follow-up of possible complications. Patients were transferred back to their residences or homes and followed by a general practitioner without further hospital consultations in the orthopedic unit where the surgery had been performed. This led to practices where patients with infections were re-admitted to our hospital in an already chronic state, with strong wound dehiscence or even fistula formation and oral antibiotic therapy already initiated. The lessons which we learned from these observations have now triggered the implementation of new protocols. We now routinely improve the pre-operative control of our FNF patients (focus on nutritional status, glycemic control, treatment of urinary tract infections etc.), we strictly avoid intraoperative hypothermia and implement postoperative follow-up consultations in the hospital with early diagnosis of possible infection cases. By following these new protocols in combination with the use of ALBC we hope to further decrease the PJI incidence in our FNF patients.
Inspired by recent observations that the antibiotic combinations gentamicin and clindamycin or gentamicin and vancomycin in bone cement exert a stronger and more sustained growth inhibition on many bacteria compared to the gentamicin mono-cement[22,23], we will soon start to test the hypothesis of an even more efficient local antibiotic prophylaxis with dual ALBC in our FNF patients. We therefore want to repeat the here described clinical study comparing in the future PJI incidence between a group receiving the single ALBC Palacos R+G vs the PJI rate in a group receiving the dual ALBC Copal G+C (containing 1 g of gentamicin and 1 g of clindamycin). Indeed, Sanz-Ruiz et al[24] have recently provided some proof of concept by showing that the routine use of Copal G+C decreased the number of PJI cases in procedures associated with a higher infection risk (aseptic knee revision procedures). The observed risk reduction in the Copal G+C group was 53% compared to the Palacos R+G group. Similarly, Sprowson et al[25] also reported a lower infection rate in their highly standardized hemiarthroplasty patient cohorts in the United Kingdom, if allocated to the dual ALBC group. However, it has to be pointed out that the United Kingdom in contrast to many other countries follows a high standard of care for fracture patients by imposing orthogeriatric guidelines on the basis of regular assessments of their national fracture database[26]. Therefore, clinical treatment experiences in the United Kingdom may not be fully transferable to countries with less rigorous orthogeriatric care standards.
Routine use of ALBC is still associated with concerns regarding possible systemic side effects and the risk of resistance development. While caution with high dose ALBC (exceeding concentrations of 3.6 g antibiotic per 40 g of cement – e.g., in spacers during staged septic treatment) appears justified for patients with pre-existing acute kidney injuries[27], there is no evidence for such concerns with low dose ALBC. In fact, we did not observe a higher rate of complications attributed to the use of ALBC in our FNF patients nor a higher rate of Clostridium difficile infections. Because of the local mode of antibiotic action there is also no proof so far that ALBC use triggers clinically relevant antibiotic resistance development[28].
Most health systems have come under progressive economic pressure. It is therefore of interest to conclude from our simple cost-benefit evaluations that the relatively easily applicable measure of routine ALBC use in hemiarthroplasty patients has such a cost saving potential in our institution. We could show that approximately 3.500 € could be saved for each patient in the ALBC group. Although this figure will vary from country to country and even from hospital to hospital depending on the price differences between the cements and the hospital-specific PJI treatment costs, we believe that they can provide an indication of what can be achieved.
Our study has several limitations. These refer in the first instance to the retrospective nature of the study and to the limited and not equally balanced number of patients in each study arm. We can also not exclude a cement material related effect because of choosing the plain cement brand from one manufacturer and the ALBC brand from another. However, we would rule out a major patient, procedure and surgical skill related bias between both groups, since patient characteristics, prosthesis use and duration of surgeries as well as the pool of operating surgeons were comparable in the observation periods.
The present pilot study has demonstrated that in the absence of good clinical protocols for the frail cohort of FNF patients the relatively easy-to-apply implementation of routine use of ALBC significantly reduces the high number of PJI cases in cemented hemiarthroplasty. This measure was found to be highly cost-effective and leads to considerable savings of treatment costs. The study also brings to attention how important it is to implement orthogeriatric guidelines in a hospital in order to achieve a better pre-, peri- and postoperative care of FNF patients.
Further studies are needed to truly elucidate the effect of ALBC – even loaded with two antibiotics - on a larger scale in FNF patients. Based on our experiences so far we strongly recommend the use of ALBC for cemented arthroplasty in FNF patients.
Use of antibiotic-loaded bone cement (ALBC) for fixation of cemented hip stems in the context of hemiarthroplasties may establish an additional antimicrobial “frontline” in the vulnerable joint compartment.
Given the high periprosthetic joint infection (PJI) rates in this frail patient group of femur neck fracture (FNF), it appears mandatory to consider further effective and easy-to-apply infection preventive measures.
The aim of this study was to compare the PJI rate between patients receiving cemented hip stems with plain cement (non-ALBC group) and patients receiving cemented hip stems with gentamicin-loaded bone cement (ALBC group) in one of the biggest Military hospitals in Spain. The treatment costs of PJI cases in each group were subsequently put in relation to the extra costs related to the routine use of ALBC instead of plain cement.
In total 241 FNF patients who went on to receive cemented hemiarthroplasty during the period from January 2011 to December 2017 were eligible for inclusion in this retrospective study. Patients were stratified into 2 study groups according to the bone cement used for the fixation of the hip stem. The number of PJI cases were analyzed and compared between both groups. Infections were further differentiated between early or chronic delayed infections by the onset of symptoms. Treatment costs in each group were compared with the extra costs related to the use of gentamicin-loaded bone cement in the ALBC group.
Use of ALBC in our hospital setting and in the absence of strict guidelines regulating pre-, peri- and postoperative care of FNF patients has been found to reduce the infection rate by 55%. Despite the extra costs of ALBC use instead of plain cement, this change of surgical practice led to savings of approximately 3500 € per patient.
Use of ALBC was found to be a potent infection prevention factor in FNF patients receiving cemented hemiarthroplasties. It was further found to be highly cost-effective.
Further studies validating the generalizability of our findings under different pre-, peri- and postoperative conditions of FNF patient care are warranted. This does also include the use of dual ALBC to further reduce the still high infection rates.
We are grateful to Dr. Nada Pezic for her support as native English speaker in editing the grammar and syntax of the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodriguez-Pardo D S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev. 2019;4:482-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 394] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 2. | Gallardo-Calero I, Larrainzar-Coghen T, Rodriguez-Pardo D, Pigrau C, Sánchez-Raya J, Amat C, Lung M, Carrera L, Corona PS. Increased infection risk after hip hemiarthroplasty in institutionalized patients with proximal femur fracture. Injury. 2016;47:872-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Baker PN, Salar O, Ollivere BJ, Forward DP, Weerasuriya N, Moppett IK, Moran CG. Evolution of the hip fracture population: time to consider the future? BMJ Open. 2014;4:e004405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Buchholz HW, Elson RA, Heinert K. Antibiotic-loaded acrylic cement: current concepts. Clin Orthop Relat Res. 1984;96-108. [PubMed] |
| 5. | Neut D, Kluin OS, Thompson J, van der Mei HC, Busscher HJ. Gentamicin release from commercially-available gentamicin-loaded PMMA bone cements in a prosthesis-related interfacial gap model and their antibacterial efficacy. BMC Musculoskelet Disord. 2010;11:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sörgel F. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin Pharmacokinet. 2009;48:89-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63-B:342-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 403] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Engesaeter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 262] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 327] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 10. | Leong JW, Cook MJ, O'Neill TW, Board TN. Is the use of antibiotic-loaded bone cement associated with a lower risk of revision after primary total hip arthroplasty? Bone Joint J. 2020;102-B:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Kuehn KD, Ege W, Gopp U. Acrylic bone cements: composition and properties. Orthop Clin North Am. 2005;36:17-28, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Vaishya R, Chauhan M, Vaish A. Bone cement. J Clin Orthop Trauma. 2013;4:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 13. | Meeker DG, Cooper KB, Renard RL, Mears SC, Smeltzer MS, Barnes CL. Comparative Study of Antibiotic Elution Profiles From Alternative Formulations of Polymethylmethacrylate Bone Cement. J Arthroplasty. 2019;34:1458-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Hendriks JG, van Horn JR, van der Mei HC, Busscher HJ. Backgrounds of antibiotic-loaded bone cement and prosthesis-related infection. Biomaterials. 2004;25:545-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 181] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992-2994. [PubMed] |
| 16. | Crego-Vita D, Sanchez-Perez C, Gomez-Rico JA, de Arriba CC. Intracapsular hip fractures in the elderly. Do we know what is important? Injury. 2017;48:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Noailles T, Brulefert K, Chalopin A, Longis PM, Gouin F. What are the risk factors for post-operative infection after hip hemiarthroplasty? Int Orthop. 2016;40:1843-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Guren E, Figved W, Frihagen F, Watne LO, Westberg M. Prosthetic joint infection-a devastating complication of hemiarthroplasty for hip fracture. Acta Orthop. 2017;88:383-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Edwards C, Counsell A, Boulton C, Moran CG. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br. 2008;90:770-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Osmon DR. Microbiology and Antimicrobial Challenges of Prosthetic Joint Infection. J Am Acad Orthop Surg. 2017;25 Suppl 1:S17-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Sanz-Ruiz P, Matas-Diez JA, Sanchez-Somolinos M, Villanueva-Martinez M, Vaquero-Martín J. Is the Commercial Antibiotic-Loaded Bone Cement Useful in Prophylaxis and Cost Saving After Knee and Hip Joint Arthroplasty? J Arthroplasty. 2017;32:1095-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Ensing GT, van Horn JR, van der Mei HC, Busscher HJ, Neut D. Copal bone cement is more effective in preventing biofilm formation than Palacos R-G. Clin Orthop Relat Res. 2008;466:1492-1498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Cara A, Ballet M, Hemery C, Ferry T, Laurent F, Josse J. Antibiotics in Bone Cements Used for Prosthesis Fixation: An Efficient Way to Prevent Staphylococcus aureus and Staphylococcus epidermidis Prosthetic Joint Infection. Front Med (Lausanne). 2020;7:576231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Sanz-Ruiz P, Matas-Diez JA, Villanueva-Martínez M, Santos-Vaquinha Blanco AD, Vaquero J. Is Dual Antibiotic-Loaded Bone Cement More Effective and Cost-Efficient Than a Single Antibiotic-Loaded Bone Cement to Reduce the Risk of Prosthetic Joint Infection in Aseptic Revision Knee Arthroplasty? J Arthroplasty. 2020;35:3724-3729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 25. | Sprowson AP, Jensen C, Chambers S, Parsons NR, Aradhyula NM, Carluke I, Inman D, Reed MR. The use of high-dose dual-impregnated antibiotic-laden cement with hemiarthroplasty for the treatment of a fracture of the hip: The Fractured Hip Infection trial. Bone Joint J. 2016;98-B:1534-1541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Ftouh S, Morga A, Swift C; Guideline Development Group. Management of hip fracture in adults: summary of NICE guidance. BMJ. 2011;342:d3304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Dagneaux L, Limberg AK, Osmon DR, Leung N, Berry DJ, Abdel MP. Acute Kidney Injury When Treating Periprosthetic Joint Infections After Total Knee Arthroplasties with Antibiotic-Loaded Spacers: Incidence, Risks, and Outcomes. J Bone Joint Surg Am. 2021;103:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Sanz-Ruiz P, Berberich C. Infection Risk-Adjusted Antibiotic Prophylaxis Strategies in Arthroplasty: Short Review of Evidence and Experiences of a Tertiary Center in Spain. Orthop Res Rev. 2020;12:89-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |