Peer-review started: March 18, 2021
First decision: July 18, 2021
Revised: July 29, 2021
Accepted: December 21, 2021
Article in press: December 21, 2021
Published online: January 18, 2022
Processing time: 304 Days and 23.8 Hours
Three-dimensional (3D) printing is a rapidly evolving and promising field to improve outcomes of orthopaedic surgery. The use of patient-specific 3D-printed models is specifically interesting in paediatric orthopaedic surgery, as limb deformity corrections often require an individual 3D treatment. In this editorial, various operative applications of 3D printing in paediatric orthopaedic surgery are discussed. The technical aspects and the imaging acquisition with computed tomography and magnetic resonance imaging are outlined. Next, there is a focus on the intraoperative applications of 3D printing during paediatric orthopaedic surgical procedures. An overview of various upper and lower limb deformities in paediatrics is given, in which 3D printing is already implemented, including post-traumatic forearm corrections and proximal femoral osteotomies. The use of patient-specific instrumentation (PSI) or guiding templates during the surgical procedure shows to be promising in reducing operation time, intraoperative haemorrhage and radiation exposure. Moreover, 3D-printed models for the use of PSI or patient-specific navigation templates are promising in improving the accuracy of complex limb deformity surgery in children. Lastly, the future of 3D printing in paediatric orthopaedics extends beyond the intraoperative applications; various other medical applications include 3D casting and prosthetic limb replacement. In conclusion, 3D printing opportunities are numerous, and the fast developments are exciting, but more evidence is required to prove its superiority over conventional paediatric orthopaedic surgery.
Core Tip: Three-dimensional (3D) printing for intraoperative use in paediatric orthopaedic surgery is a relatively novel field. Research has shown that 3D anatomic models can be used for patient-specific instrumentation and patient-specific templates, that possibly allow the orthopedic surgeon to perform complex surgery more accurately. Based on the latest scientific evidence, this editorial provides an overview of the overall role of 3D printing in intraoperative applications of upper and lower limb surgery in paediatric orthopaedics.
- Citation: Goetstouwers S, Kempink D, The B, Eygendaal D, van Oirschot B, van Bergen CJ. Three-dimensional printing in paediatric orthopaedic surgery. World J Orthop 2022; 13(1): 1-10
- URL: https://www.wjgnet.com/2218-5836/full/v13/i1/1.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i1.1
Over the last decade, three-dimensional (3D) printing–or additive/layer manufacturing-has become a more and more interesting application in medicine. It has been used in various surgical specialties, including neurosurgery, oral and maxillofacial surgery, plastic surgery and cardiothoracic surgery[1-3]. Also in the field of orthopaedic surgery the interest and use of 3D printing has grown over the years, since it started in 1999 in a case of complex spinal surgery[4].
In the orthopaedic field, 3D technology can be used in three different sections: Including preoperative planning, 3D-printed models and 3D printing for patient-specific instrumentation (PSI). A recent meta-analysis reported that the use of 3D-printed models in preoperative planning significantly reduced the operative time, intraoperative blood loss and fluoroscopy use during orthopedic trauma surgery[5]. These outcomes may be the result of a better understanding of the pathological anatomy in complex cases. Moreover, 3D printing helps the surgeon in preoperative planning of the surgical procedure by means of fracture reduction and the sizes and positioning of plates and screws for internal fixation. This could possibly reduce operation time and the amount of dissection of surrounding tissues and therefore blood loss[5]. In addition to trauma surgical applications, PSI allows the surgeon to perform precise osteotomies for deformity correction[5-8], which is specifically interesting for paediatric patients[9].
Correcting limb deformities in children is a challenging and complex type of surgery. Therefore, novel technologies such as 3D printing are increasingly applied, aiming to achieve more accurate corrections and improved outcomes[9]. The use of patient-specific 3D-printed models is specifically interesting and promising in paediatric orthopaedic surgery, as limb deformity corrections require [sh6] an individual 3D treatment. For example, the use of 3D printing in preoperative planning of hip preservation surgery created an improvement in trainee and patient education for understanding the abnormality of the patient’s disorder[6]. However, due to its novelty, evidence on the use of 3D printing in pediatric orthopedics is still limited.
This editorial provides an overview of the various intraoperative applications of 3D printing in pediatric orthopedic surgery and assesses the overall role, challenges and future of this relatively novel technique.
3D printing, or additive layer manufacturing, is a technique to create a 3D object from a digital model. It is an advanced, computer-controlled technology that deposits successive layers of materials (e.g., metals or plastic) to create an object. In contrast to traditional subtractive manufacturing processes that take away or shape material, 3D printing has the advantage to create complex structures by adding hundreds of miniscule layers that are fused together. Another advantage of 3D printing is the possibility to create shapes of different materials including plastic, rubber, metals or ceramics[7,10].
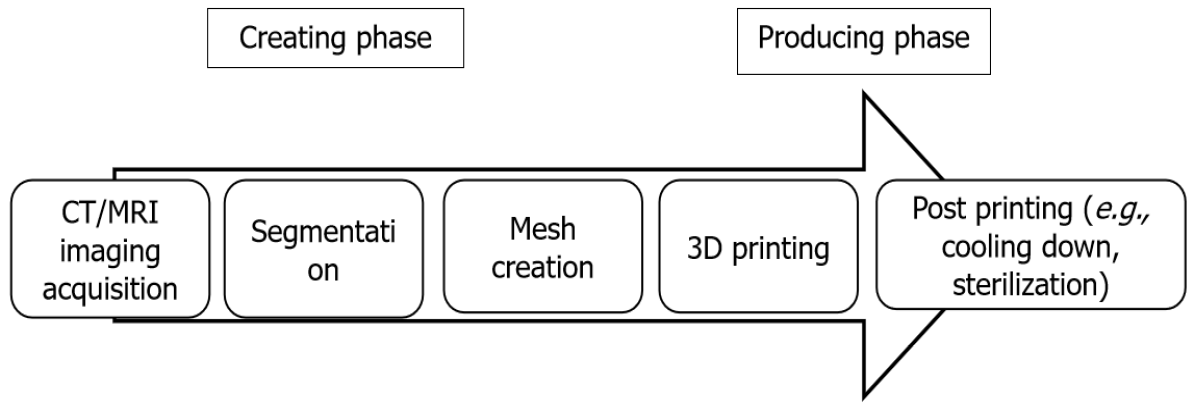
The process of creating 3D-printed models for medical applications starts with high-resolution imaging (Figure 1)[7,8,10-12]. Multi-row detecting computed tomography (MDCT) and magnetic resonance imaging (MRI) are frequently used in orthopedics for diagnostics of complex anatomy or severe deformities[7,11-13]. MDCT is a high-contrast computed tomography (CT) that produces thin-section slices of less than 1 mm and therefore highly suitable for analysing bony structures. After acquiring the clinical dataset, MDCT images are stored according to a universal data format; standardized digital imaging and communications in medicine (DICOM). Post-processing software extracts these DICOM files[7,8,14]. This extraction process is called segmentation. It separates the outlines of different anatomical structures in each individual 2D image (slice) by using colour contrast to create separate objects. In the next phase, computer-aided design (CAD) software (e.g., MIMICS or InPrint) combines all the individual 2D images (slices) and creates a virtual 3D initial object (Mesh creation)[7,8,10,14]. This makes it possible to see depth, angulation and diameters of the anatomical structure or pathology. Next, the 3D object is transformed into a file that is ready for printing. In some cases, the resolution of the radiology is suboptimal, or the 3D object has no clear boundaries. In these cases, a manual reconstruction of a 3D object can be performed (i.e., ReplicatorG software) and anatomical corrections of the model can be made in this CAD software[7,8,14]. It also provides control of the filling of the model, with possibilities between 0% filling (shell alone) to 100% filling and makes the model more suitable for 3D printing or material of preference[7,8,10,14]. Once the CAD model is finalised, it is converted into a common 3D file format, stereolithography (SLA) file and sent to the appropriate 3D printer[2,7,8,14]. Post process, the materials first need to cool down before they can be used and consequently sterilization is required for intraoperative use[12].
Different types of 3D printing techniques have been used over the last years, including printers that use powder, melted polymers, gel, liquids, or a combination of these substances[7,8]. In the past, Fused Deposition Modeling (FDM) was used as a 3D printing technique, in which a movable nozzle places long, thin wire of thermoplastic material on top of each other. A 3D object is created layer by layer. It is a relatively cheap and fast production method to create anatomical models. However, the shape of a FDM print differs greatly in quality from a professional 3D printer[7,8,15,16]. Therefore, different 3D printing procedures are used for the production of patient-specific models and patient-specific surgical guides at this moment. Selective laser sintering (SLS) is a powder-based fusion technology that uses a laser beam to locally sinter polymer powder to build 3D objects layer by layer[7,8,15,16]. SLS uses bio-based polyamide materials and metals for 3D printing. Other 3D printing techniques are SLA and digital light processing (DLP) that use UV laser and a liquid bath containing a UV-sensitive liquid polymer[8,10,12,17]. This liquid is illuminated layer by layer by the laser where the liquid has to cure. The surface cures into a solid state and subsequently the surface raises one layer. The next layer is then exposed and cured. 3D-printed objects that are generated SLS, DLP and SLA can be sterilized and therefore can be used in the operating room.
Intraoperative applications of 3D printing in pediatric orthopedics involve the creation of PSI to perform more accurate complex surgery or correct deformities. There are increasingly interesting and promising applications in both upper limb and lower limb deformities.
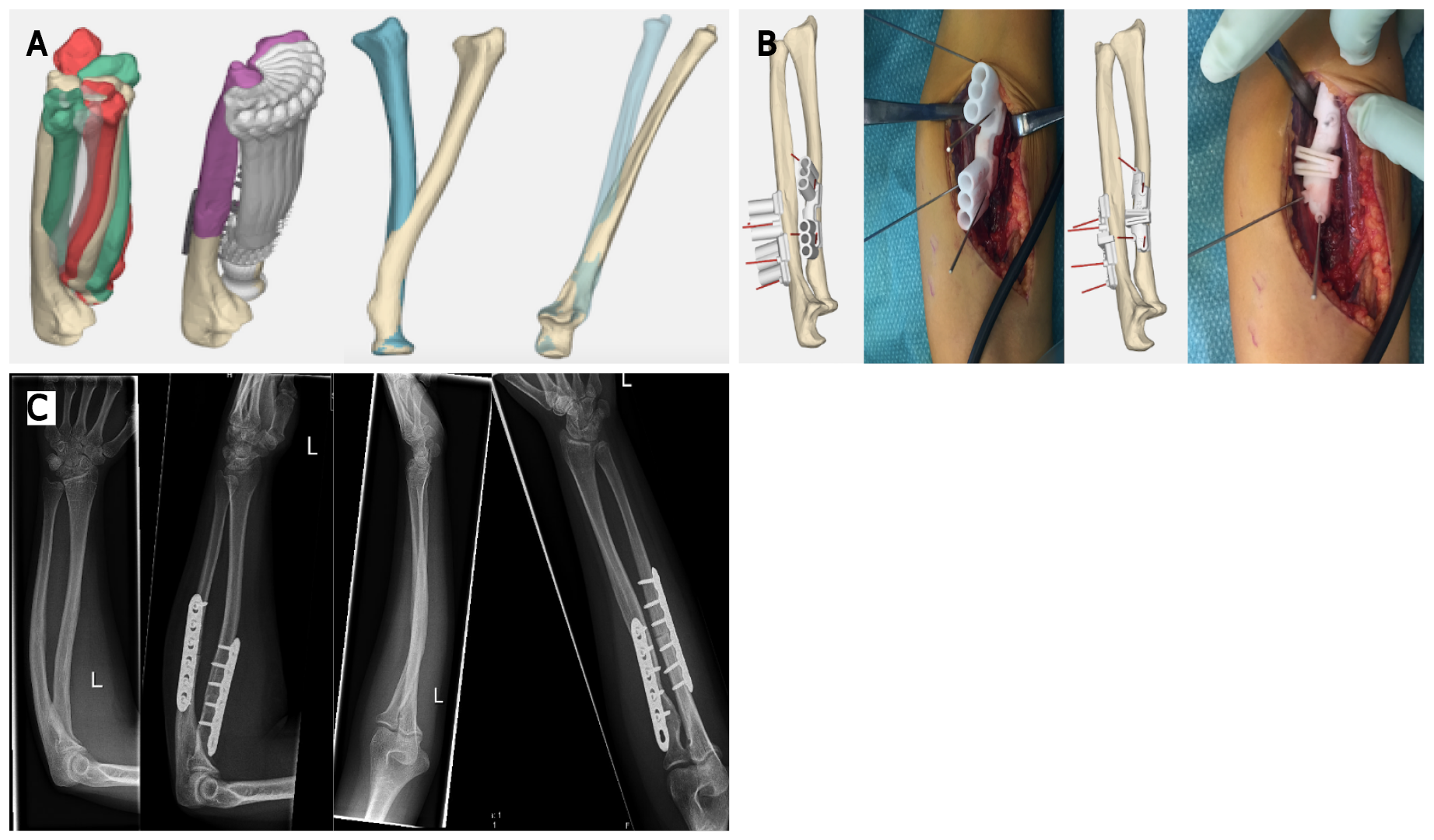
Currently, the most commonly described application of 3D printing in the upper extremity is the forearm, usually rotational impairment after malunited forearm fractures. Byrne et al[18] used 3D-printed patient-specific osteotomy guides and custom-made plates for multiplanar corrective osteotomies in 5 patients with posttraumatic malunion of the forearm. An angular correction of the ulna and radius of 9.9° and 10.0° was planned, respectively. They reported mean postoperative corrections of 10.1° and 10.8°, respectively. Forearm pronation improved from 68° to 87° and supination improved from 47° to 89°. Furthermore, a significant improvement in pain relief and grip strength was seen. Another prospective study enrolled 16 patients with a total of 17 bone deformities, including distal radial malunion, distal humeral malunion and forearm diaphyseal malunion[19]. They reported that the use of 3D patient-matched instruments for corrective osteotomies showed a significant deformity improvement of 22.2°. Also, in patients treated for distal radial malunion and diaphyseal malunion, the flexion, extension and pronation of the forearm were significantly improved. Clinical implications as pain, range of motion, and grip strength were also significantly improved compared to the preoperative situation. Another study used 3D-printed templates to guide the osteotomy orientation in a posttraumatic forearm malunion of a 15-year-old female[3]. The authors reported that 3D-printed templates made it possible to achieve near-anatomical reduction close to 1° residual deformity in all three planes and a recovery to full function within 3 mo. One of our own cases presented with decreased rotational range of motion after sustaining a forearm fracture as a child, without improvement after extensive rehabilitation. 3D analysis determined the deformity and optimal planes of correction (Figure 2). Patient-specific osteotomy guides with predrilling of the screw holes were designed, and hardware for fixation was selected (Figure 2). The surgical procedure was then completed according to plan, which resulted in a vast improvement in range of motion and high patient satisfaction. In the period 2014-2020, 42 cases were operated using this technique, of which 16 were malunited forearms with rotational impairment. Most patients had a severe supination deficit (mean -10 degrees), which improved to a mean supination of +60 degrees. Pronation limitation was much less severe (mean +45 degrees) with a mean improvement to +55 degrees. We experienced one complication due to a transient posterior interosseous nerve paralysis, which recovered spontaneously within the first 6 wk after surgery. Thus, in deformities of the forearm due to malunion, the use of 3D PSI shows improvement in correction angles as well as in clinical outcomes as grip strength and pain relief[1,18,19]. Nevertheless, it is important to realize that the studies mentioned above are low-grade evidence and therefore the results need to be analysed with a critical view.
Cubitus varus deformities are sometimes seen after elbow fractures in children. Correction of this deformity is a complex surgical procedure and requires a 3D approach. Hu et al[20] included 35 patients and assigned them into two different groups comparing traditional surgery to surgery using an intraoperative patient-specific 3D-printed navigation template. All patients underwent similar surgery with wedge osteotomy of the lateral distal humerus. The 3D-printed patient-specific template significantly reduced the operation time with a mean of 11 min and significantly improves the accuracy of the correction by a mean of 3°. However, the question remains whether and accuracy of 3° is clinically relevant. Another study analysed 25 patients with cubitus varus deformity and compared a group of patient-specific 3D-printed osteotomy guides with a traditional group[21]. The 3D guiding template procedure resulted in a significant decrease of the operation time (almost 30 min), less intraoperative blood loss (17.5 mL) and higher satisfaction. However, the most important achievement of correcting deformities in pediatric orthopedics is recovery of function.
For lower limb paediatrics, 3D printing has been used for various complex techniques, including femoral and pelvic osteotomies and tarsal coalition resection[15,22]. Femoral and pelvic osteotomies with use of 3D guides have been applied for late sequelae of developmental dysplasia of the hip (DDH), slipped capital femoral epiphysis (SCFE) and Legg-Calvé-Parthes (LCP) disease[15].
Severe DDH can lead to hip deformity that may require surgical correction. Zheng et al[23] compared 12 cases of femoral corrective osteotomy after DDH using patient-specific 3D navigation templates with 13 cases using conventional approaches[23]. No differences in varus and angles were reported. However, significantly decreased operation time (26 min) and fluoroscopy were reported in favour of the 3D-printed model group.
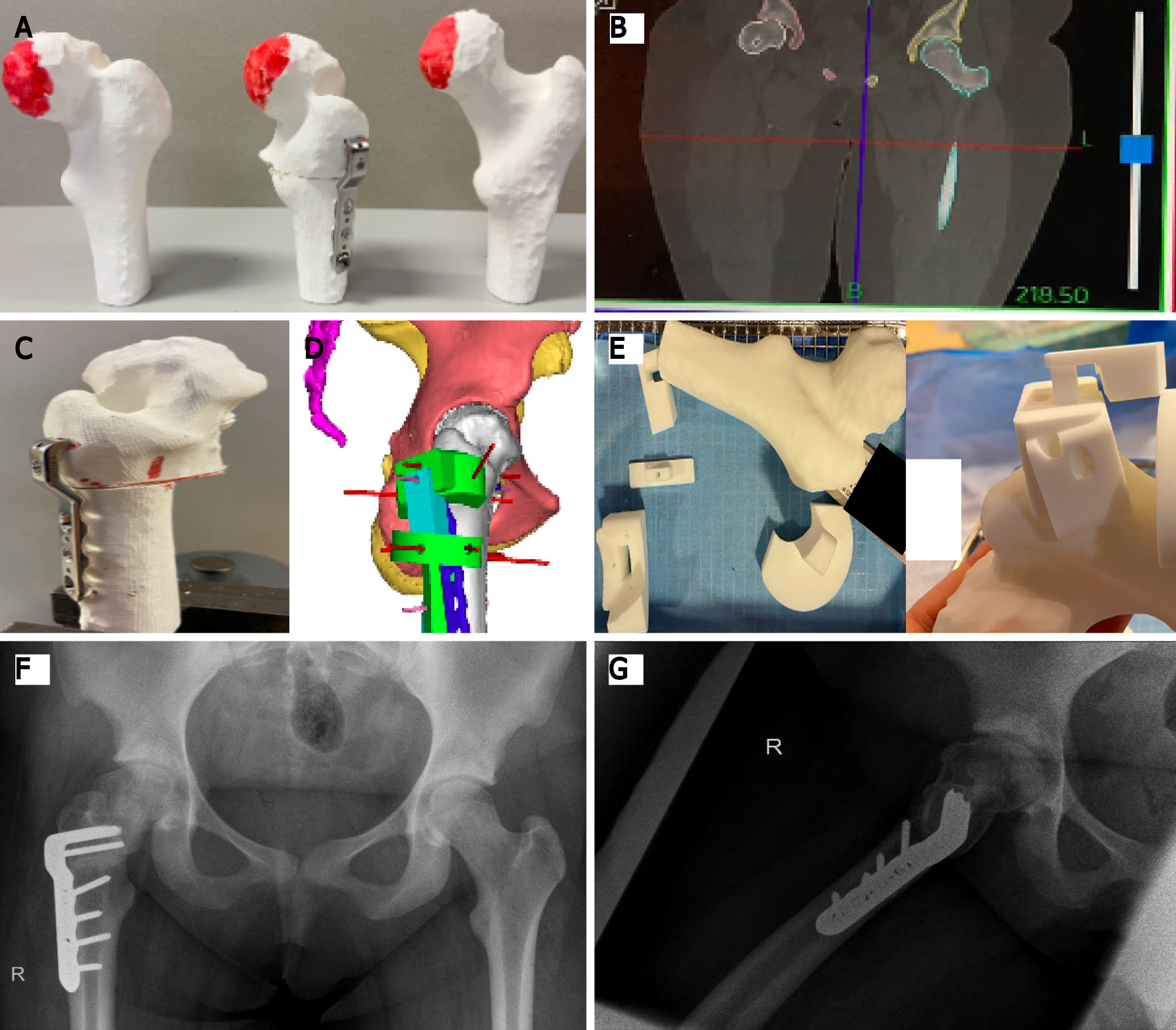
SCFE leads to a posterior and inferior displacement of the femoral head, giving an altered mobility of the hip joint and a syphon-shaped femoral neck after consolidation. A 3D sub- or intertrochanteric osteotomy can be performed for correction of the varus, internal rotation and flexion of the hip and thereby restoring its function. Cherkasskiy et al[24] used 3D models for proximal femoral osteotomy following SCFE and also found decreased operation and fluoroscopy times. We have used a CT-based 3D-printed model to plan and perform a complex osteotomy in a previously pinned SCFE case, with favourable results with regard to osteotomy precision, positioning of the implant, surgical time and use of the image intensifier (Figure 3). In this case the patient had a pre-operative externally rotated right hip of 40 degrees. Post-operatively, she was able to internally rotate the hip 10 degrees, compared to 20 degrees of internal rotation on the contralateral left side (Figure 3).
LCP disease can lead to deformity of the femoral head and an adaptive deformity of the acetabulum. Six patients with LCP disease were treated with a 3D-printed patient-specific osteotomy model[25]. The model allowed the surgeon to correct the femoral head almost identical to the contralateral healthy side[25].
A recent review confirmed that use of PSI for the above indications has led to improved accuracy and precision, decreased procedure times, and decreased intra-operative imaging requirements, compared to conventional methods of performing femoral or pelvic osteotomy[15].
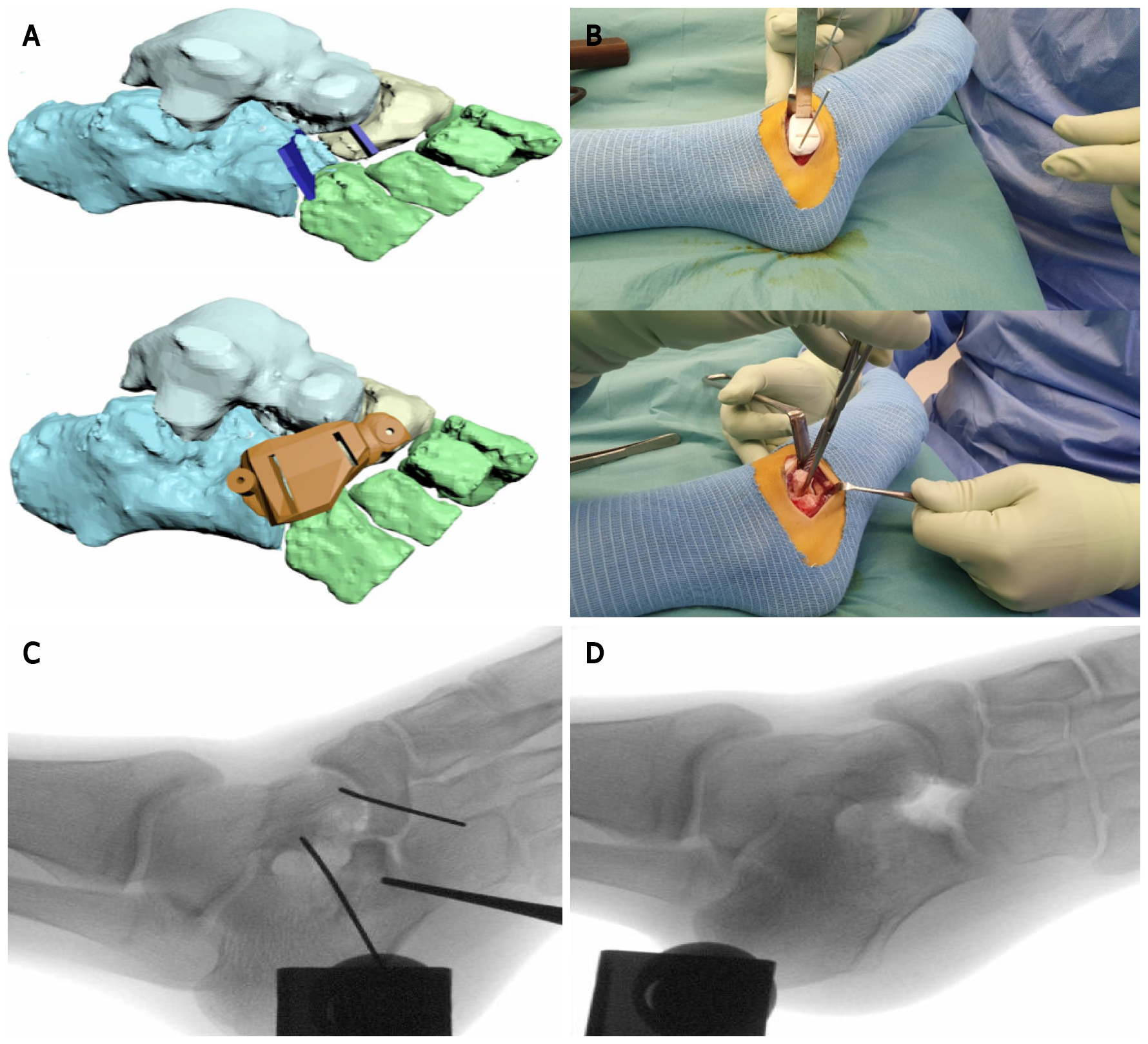
3D techniques have also been used in pediatric foot orthopaedics. De Wouters et al[22] used 3D-printed PSI to guide the surgeon in removal of talocalcaneal and calcaneonavicular coalitions. It helped to orientate the saw blade for the resection of the bone bridge at the correct depth, which resulted in complete resections with no recurrence after a mean follow-up period of 18 mo. We have promising experience with 3D-guided resection of a calcaneonavicular coalition based on preoperative MRI (Figure 4).
Over the last years, 3D printing has been successfully introduced in pediatric orthopaedics. Because of its seemingly endless possibilities, this relatively novel technique expands further than surgical applications alone.
In the technology of prosthetic limbs, 3D printing is increasingly used. Traditional prostheses for children with upper extremity amputees have been considered to be too heavy or too expensive to be a true benefit for a child[22]. In addition, children outgrow prostheses and may damage them[26]. In 3D printed prostheses, there is the possibility to replace a part of the prosthesis instead of the complete device[13]. Children are also allowed to choose the design and colour schemes, which make 3D-printed model prostheses more tailored to a child’s choices. Therefore, children may be more self-confident, as described in various studies of 3D-printed upper limb replacement[13,27-29].
In the conservative treatment of paediatric fractures, two studies described the treatment of nondisplaced forearm fractures with a 3D-printed device compared to a traditional plaster cast[30,31]. The results showed an improvement of wrist function after immobilization with a 3D-printed device. Moreover, activities of everyday life, patient satisfaction and patient comfort during the immobilization were improved compared to the traditional cast group. In addition, the 3D-printed devices were reported to be lighter than traditional casts and removable, which make them more patient-friendly (e.g. when taking a shower). This suggests that a 3D device can also be an effective alternative approach in the conservative treatment of fractures in paediatric orthopaedics.
As outlined, 3D printing seems to have great potential in numerous paediatric orthopaedic applications. However, there are several challenges in this field that need further investigation and improvement.
Although the production time of a 3D product has decreased since it was invented, preparation and production of PSI still take at least several days[32]. Therefore, the application in the acute setting is challenging (e.g., for fractures). However, research and development in 3D printing is a growing field of interest, resulting in new upcoming materials with better biomechanical and biocompatible characteristics. Furthermore, the development of 3D printers that can create models within hours is very promising.
Another challenge is reduction of radiation exposure. Although the use of fluoroscopy during the surgical procedure is reduced by using 3D PSI or 3D-printed model guiding templates[23,33], the total dose of radiation might not be decreased per case. A preoperative high-resolution CT scan is usually obtained to produce a 3D image, which is additional radiation exposure to the child[16,32]. Instead, the use of MRI would help reduce the radiation exposure, with the possible additional advantage of a more detailed image of the paediatric anatomic structures (e.g., physeal bar, periosteum and soft tissue)[10]. However, the process of undergoing an MRI scan is more difficult for very young children (under the age of 5), because of the necessity of sedation, motion reduction and/or accelerated imaging[7,8,17,34,35]. Moreover, studies using MRI for preoperative imaging acquisition in 3D processes are scarce. Our case shows the potential of MRI to produce surgical 3D guides (Figure 4).
In addition, 3D printing requires advanced technology and financial resources, which may not be available in developing countries[32]. However, printing costs seem to decrease over time[36] and costs of 3D printing may be outweighed by saving operation time. A cost analysis showed that using 3D-printed models saved a mean operating time of 62 min, translated to $3720 per case compared to conventional techniques[37]. Due to the ambiguity in evidence on the cost-savings of using 3D-printed models in paediatric orthopaedic surgery, an in-depth cost analysis is required of production costs vs potential savings obtained by improved intraoperative results
Finally, more scientific evidence is required on the use of 3D techniques in paediatric orthopaedic surgery. Despite the fact that the current literature shows promising results for various indications as discussed, randomized trials on 3D printing compared to conventional methods are still lacking. It is likely to expect that 3D printing will be mainly beneficial for complex surgical cases.
3D printing is a promising technique for numerous upper and lower limb surgical applications in paediatric orthopaedics. In upper limb surgery, 3D has been most frequently used in posttraumatic deformities. In lower limb surgery, 3D-printed models are mostly used to correct congenital and developmental deformities of the hip. Other applications of 3D-printed models include limb prostheses and non-surgical treatment of fractures. Future possibilities of this exciting technique are numerous.
The affordability of 3D printers has increased over the years, and literature shows that using 3D-printed models for PSI or intraoperative guiding reduces the operating time and radiation exposure. Moreover, an improved accuracy of deformity correction is attained. However, most studies have a low level of evidence. Moreover, using 3D-printed models in pediatric orthopaedic surgery is complex due to growth of children and therefore, the moment of planning vs the timing of the surgery is also a challenge to overcome. All in all, more research, preferably randomized controlled studies, is required to compare conventional approaches and the intraoperative use of 3D-printed models. Nevertheless, the use of 3D-printed models as an intraoperative tool seems to have great future potential in complex pediatric orthopaedic surgical procedures.
The authors wish to thank Christa Niehot and Wichor Bramer from the Erasmus Medical Centre Medical Library for assistance with the literature search.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Keltz E S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Chae MP, Rozen WM, McMenamin PG, Findlay MW, Spychal RT, Hunter-Smith DJ. Emerging Applications of Bedside 3D Printing in Plastic Surgery. Front Surg. 2015;2:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 196] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 2. | Giannopoulos AA, Steigner ML, George E, Barile M, Hunsaker AR, Rybicki FJ, Mitsouras D. Cardiothoracic Applications of 3-dimensional Printing. J Thorac Imaging. 2016;31:253-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Jeuken RM, Hendrickx RPM, Schotanus MGM, Jansen EJ. Near-anatomical correction using a CT-guided technique of a forearm malunion in a 15-year-old girl: A case report including surgical technique. Orthop Traumatol Surg Res. 2017;103:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | D'Urso PS, Askin G, Earwaker JS, Merry GS, Thompson RG, Barker TM, Effeney DJ. Spinal biomodeling. Spine (Phila Pa 1976). 1999;24:1247-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Morgan C, Khatri C, Hanna SA, Ashrafian H, Sarraf KM. Use of three-dimensional printing in preoperative planning in orthopaedic trauma surgery: A systematic review and meta-analysis. World J Orthop. 2020;11:57-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (2)] |
| 6. | Bockhorn L, Gardner SS, Dong D, Karmonik C, Elias S, Gwathmey FW, Harris JD. Application of three-dimensional printing for pre-operative planning in hip preservation surgery. J Hip Preserv Surg. 2019;6:164-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Garcia J, Yang Z, Mongrain R, Leask RL, Lachapelle K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn. 2018;4:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Wong KC. 3D-printed patient-specific applications in orthopedics. Orthop Res Rev. 2016;8:57-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | Ma L, Zhou Y, Zhu Y, Lin Z, Wang Y, Zhang Y, Xia H, Mao C. 3D-printed guiding templates for improved osteosarcoma resection. Sci Rep. 2016;6:23335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Starosolski ZA, Kan JH, Rosenfeld SD, Krishnamurthy R, Annapragada A. Application of 3-D printing (rapid prototyping) for creating physical models of pediatric orthopedic disorders. Pediatr Radiol. 2014;44:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Jovičić MŠ, Vuletić F, Ribičić T, Šimunić S, Petrović T, Kolundžić R. Implementation of the three-dimensional printing technology in treatment of bone tumours: a case series. Int Orthop. 2021;45:1079-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Parthasarathy J, Krishnamurthy R, Ostendorf A, Shinoka T. 3D printing with MRI in pediatric applications. J Magn Reson Imaging. 2020;51:1641-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Zuniga JM, Young KJ, Peck JL, Srivastava R, Pierce JE, Dudley DR, Salazar DA, Bergmann J. Remote fitting procedures for upper limb 3d printed prostheses. Expert Rev Med Devices. 2019;16:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Tack P, Victor J, Gemmel P, Annemans L. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online. 2016;15:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 811] [Cited by in RCA: 602] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 15. | Baraza N, Chapman C, Zakani S, Mulpuri K. 3D - Printed Patient Specific Instrumentation in Corrective Osteotomy of the Femur and Pelvis: A Review of the Literature. 3D Print Med. 2020;6:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Furlow B. Radiation protection in pediatric imaging. Radiol Technol. 2011;82:421-439. [PubMed] |
| 17. | Jaimes C, Gee MS. Strategies to minimize sedation in pediatric body magnetic resonance imaging. Pediatr Radiol. 2016;46:916-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | Byrne AM, Impelmans B, Bertrand V, Van Haver A, Verstreken F. Corrective Osteotomy for Malunited Diaphyseal Forearm Fractures Using Preoperative 3-Dimensional Planning and Patient-Specific Surgical Guides and Implants. J Hand Surg Am. 2017;42:836.e1-836.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Oka K, Tanaka H, Okada K, Sahara W, Myoui A, Yamada T, Yamamoto M, Kurimoto S, Hirata H, Murase T. Three-Dimensional Corrective Osteotomy for Malunited Fractures of the Upper Extremity Using Patient-Matched Instruments: A Prospective, Multicenter, Open-Label, Single-Arm Trial. J Bone Joint Surg Am. 2019;101:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Hu X, Zhong M, Lou Y, Xu P, Jiang B, Mao F, Chen D, Zheng P. Clinical application of individualized 3D-printed navigation template to children with cubitus varus deformity. J Orthop Surg Res. 2020;15:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Zhang YW, Xiao X, Gao WC, Xiao Y, Zhang SL, Ni WY, Deng L. Efficacy evaluation of three-dimensional printing assisted osteotomy guide plate in accurate osteotomy of adolescent cubitus varus deformity. J Orthop Surg Res. 2019;14:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | de Wouters S, Tran Duy K, Docquier PL. Patient-specific instruments for surgical resection of painful tarsal coalition in adolescents. Orthop Traumatol Surg Res. 2014;100:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Zheng P, Yao Q, Xu P, Wang L. Application of computer-aided design and 3D-printed navigation template in Locking Compression Pediatric Hip PlateΤΜ placement for pediatric hip disease. Int J Comput Assist Radiol Surg. 2017;12:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Cherkasskiy L, Caffrey JP, Szewczyk AF, Cory E, Bomar JD, Farnsworth CL, Jeffords M, Wenger DR, Sah RL, Upasani VV. Patient-specific 3D models aid planning for triplane proximal femoral osteotomy in slipped capital femoral epiphysis. J Child Orthop. 2017;11:147-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Fürnstahl P, Casari FA, Ackermann J, Marcon M, Leunig M, Ganz R. Computer-assisted femoral head reduction osteotomies: an approach for anatomic reconstruction of severely deformed Legg-Calvé-Perthes hips. A pilot study of six patients. BMC Musculoskelet Disord. 2020;21:759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Aimar A, Palermo A, Innocenti B. The Role of 3D Printing in Medical Applications: A State of the Art. J Healthc Eng. 2019;2019:5340616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 264] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 27. | Gálvez JA, Gralewski K, McAndrew C, Rehman MA, Chang B, Levin LS. Assessment and Planning for a Pediatric Bilateral Hand Transplant Using 3-Dimensional Modeling: Case Report. J Hand Surg Am. 2016;41:341-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Gurnaney HG, Fiadjoe JE, Levin LS, Chang B, Delvalle H, Gálvez J, Rehman MA. Anesthetic management of the first pediatric bilateral hand transplant. Can J Anaesth. 2016;63:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Tanaka KS, Lightdale-Miric N. Advances in 3D-Printed Pediatric Prostheses for Upper Extremity Differences. J Bone Joint Surg Am. 2016;98:1320-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Chen Y, Lin H, Yu Q, Zhang X, Wang D, Shi L, Huang W, Zhong S. Application of 3D-Printed Orthopedic Cast for the Treatment of Forearm Fractures: Finite Element Analysis and Comparative Clinical Assessment. Biomed Res Int. 2020;2020:9569530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Guida P, Casaburi A, Busiello T, Lamberti D, Sorrentino A, Iuppariello L, D'Albore M, Colella F, Clemente F. An alternative to plaster cast treatment in a pediatric trauma center using the CAD/CAM technology to manufacture customized three-dimensional-printed orthoses in a totally hospital context: a feasibility study. J Pediatr Orthop B. 2019;28:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Iobst CA. New Technologies in Pediatric Deformity Correction. Orthop Clin North Am. 2019;50:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Zheng P, Xu P, Yao Q, Tang K, Lou Y. 3D-printed navigation template in proximal femoral osteotomy for older children with developmental dysplasia of the hip. Sci Rep. 2017;7:44993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Barnea-Goraly N, Weinzimer SA, Ruedy KJ, Mauras N, Beck RW, Marzelli MJ, Mazaika PK, Aye T, White NH, Tsalikian E, Fox L, Kollman C, Cheng P, Reiss AL; Diabetes Research in Children Network (DirecNet). High success rates of sedation-free brain MRI scanning in young children using simple subject preparation protocols with and without a commercial mock scanner--the Diabetes Research in Children Network (DirecNet) experience. Pediatr Radiol. 2014;44:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 35. | de Bie HM, Boersma M, Wattjes MP, Adriaanse S, Vermeulen RJ, Oostrom KJ, Huisman J, Veltman DJ, Delemarre-Van de Waal HA. Preparing children with a mock scanner training protocol results in high quality structural and functional MRI scans. Eur J Pediatr. 2010;169:1079-1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 36. | Hoang D, Perrault D, Stevanovic M, Ghiassi A. Surgical applications of three-dimensional printing: a review of the current literature & how to get started. Ann Transl Med. 2016;4:456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 37. | Ballard DH, Mills P, Duszak R Jr, Weisman JA, Rybicki FJ, Woodard PK. Medical 3D Printing Cost-Savings in Orthopedic and Maxillofacial Surgery: Cost Analysis of Operating Room Time Saved with 3D Printed Anatomic Models and Surgical Guides. Acad Radiol. 2020;27:1103-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |