Published online Sep 18, 2021. doi: 10.5312/wjo.v12.i9.629
Peer-review started: January 20, 2021
First decision: May 3, 2021
Revised: May 3, 2021
Accepted: August 3, 2021
Article in press: August 3, 2021
Published online: September 18, 2021
Processing time: 236 Days and 20.1 Hours
Hip prosthetic loosening is often difficult to detect at an early stage, and there has been uncertainty for a long time as to when the loosening occurs and thus to the basic causes. By comparing different diagnostic methods, we found that loosening is best defined as prosthetic migration and measured by radiostereometric analysis. Convincing evidence indicates that poor interlock, poor bone quality, and resorption of a necrotic bone bed may initiate loosening during or shortly after surgery; this forms the basis of the theory of early loosening. Biomechanical factors do affect the subsequent progression of loosening, which may increase subclinically during a long period of time. Eventually, the loosening may be detected on standard radiographs and may be interpreted as late loosening but should to be interpreted as late detection of loosening. The theory of early loosening explains the rapid early migration, the development of periprosthetic osteolysis and granulomas, the causality between wear and loosening, and largely the epidemiology of clinical failure of hip prostheses. Aspects discussed are definition of loosening, the pattern of early migration, the choice of migration threshold, the current understanding of loosening, a less exothermic bone cement, cemented taper-slip stems, a new exciting computed tomography-based technique for simpler implant migration studies, and research suggestions.
Core Tip: Much evidence indicates that prosthetic loosening is initiated during or shortly after surgery. The prosthetic micromovements may increase subclinically during a long period of time. Eventually, the loosening may be detected on standard radiographs and may be interpreted as late loosening but should to be interpreted as late detection of loosening. The discussion includes the definition of loosening, the pattern of early migration, the choice of migration threshold, the current understanding of loosening, a less exothermic bone cement, cemented taper-slip stems, a new exciting computed tomography-based technique for simpler implant migration studies, and research suggestions.
- Citation: Mjöberg B. Hip prosthetic loosening: A very personal review. World J Orthop 2021; 12(9): 629-639
- URL: https://www.wjgnet.com/2218-5836/full/v12/i9/629.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i9.629
Hip arthroplasty is one of the most successful of all orthopedic operations, but the results do deteriorate with time because of loosening. Radiographic changes indicating loosening are often difficult to detect at an early stage, and there has been uncertainty for a long time as to when the loosening occurs and thus to the basic causes. Confusion also arose because some hips with obviously loose prosthetic components are not painful[1-3]. To solve these issues, a few steps are required. First, the definition of loosening must be clarified. Second, the loosening must be carefully followed from its earliest detection. Third, the most important triggering factors must be identified, as well as other factors that affect the subsequent progression of loosening. Then the simplest scientific explanation that fits the evidence should be chosen.
When I, as a newly graduated orthopedic surgeon in the early 1980s, started studying hip prosthetic loosening in Lund (in Southern Sweden), the diagnosis of loosening was based on insensitive radiographic criteria (periprosthetic radiolucent lines wider than 2 mm, prosthetic migration exceeding 4 mm, cement fracture, etc.). Several poorly defined terms were used, such as allergic loosening, aseptic loosening, mechanical loosening, progressive loosening, and reactive loosening – all without clear distinctions between each other.
Radiostereometric analysis (RSA) was introduced in Lund in 1974 by Göran Selvik (1938–1990). It is a technique for obtaining reliable three-dimensional measurements from radiographs and is based on implantation of tantalum bone markers, roentgen calibration equipment, and rigid-body kinematic analysis[4,5]. RSA was mainly used for studies of various bone growth disorders but was also found feasible for the study of hip prostheses[6,7].
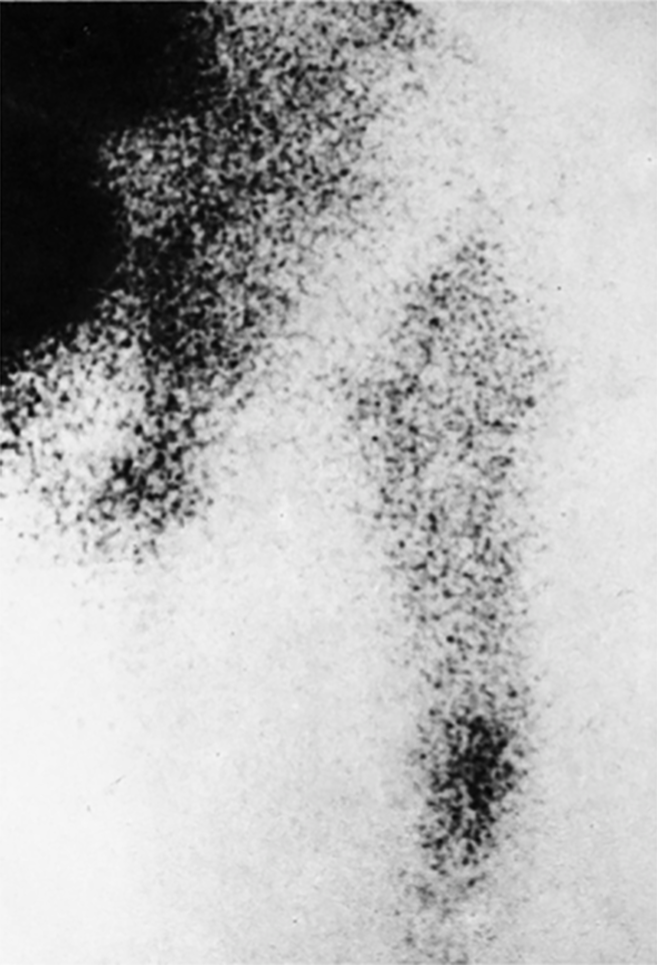
My tutor, Lars Ingvar Hansson (1937–1987), advised me to use RSA to look for any pattern in loosening. We used analog films measured with a photogrammetric instrument and assessed (by double examinations) the limit for significant migration along the longitudinal axis to be 0.2 mm. However, later RSA studies have reached a detection limit of 0.08 mm when using fully digital technology[8,9]. By comparing contrast arthrography[10,11], radionuclide arthrography[12,13], bone scintigraphy (99mTc-MDP)[14,15], and RSA (comprising both instability under load and migration with time) in 14 painful hip arthroplasties, we found that loosening is best defined as migration[16]: All prosthetic components unstable by RSA, or with abnormal arthrogram, or with increased bone scintigraphic activity, or loose at revision were migrating, but no non-migrating components demonstrated any of these signs of loosening. Interestingly, increased activity at the tip of the femoral component by bone scintigraphy (Figure 1) had high sensitivity and specificity in detecting loosening, which was also pointed out earlier[14,15].
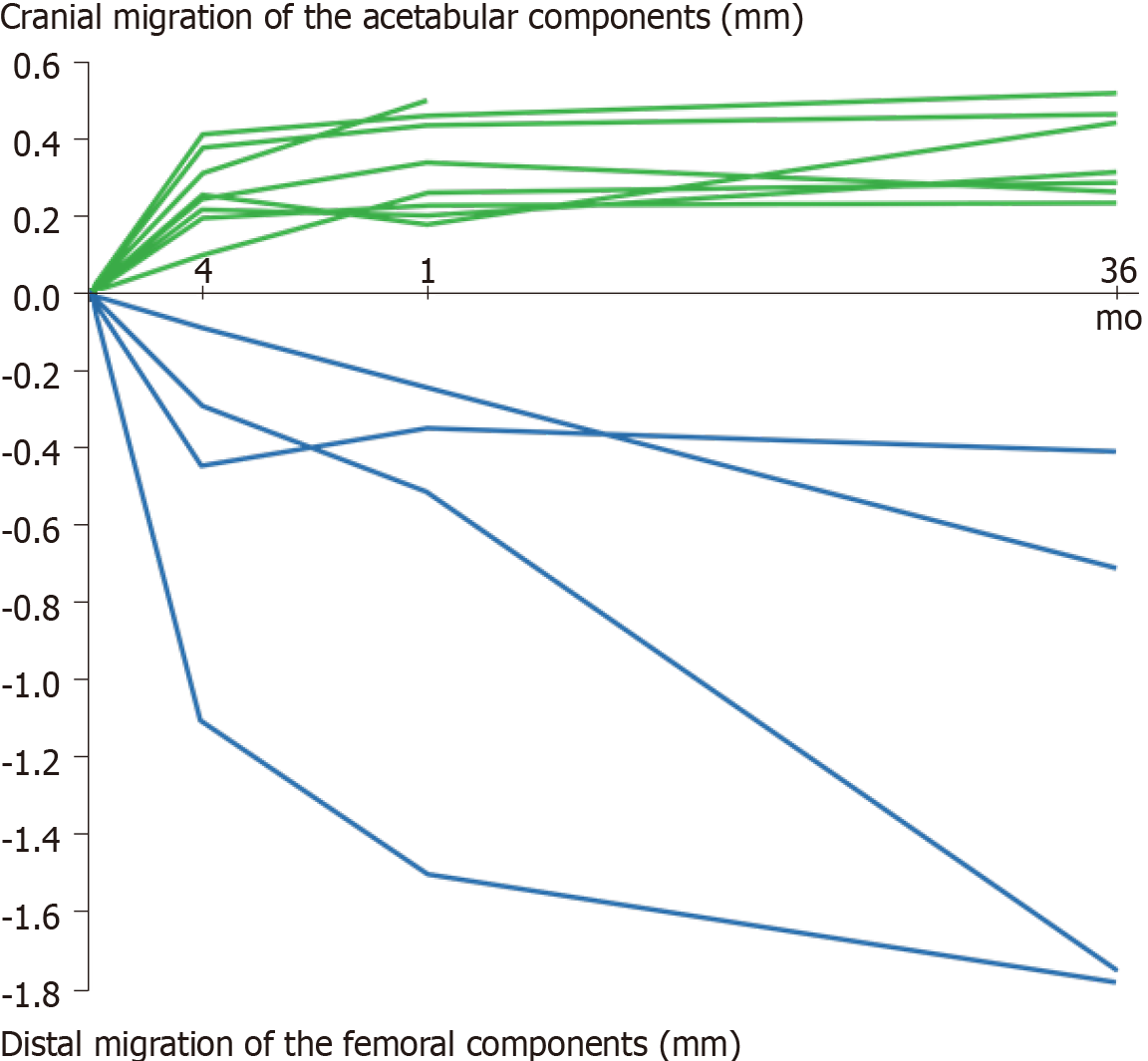
To study early prosthetic migration, RSA was performed prospectively on cemented primary hip arthroplasties in two series followed for 2–3 years after surgery[17,18]. Taking these two studies together, we found that 19 of the 36 acetabular components migrated cranially and seven of the 34 femoral components migrated distally during the observation period (two femoral components in the latter series[18] were excluded due to insufficient tantalum bone markers); and that in all the cases, but three (two acetabular and one femoral), migration was detected within 4 mo after surgery (Figure 2). We also did not find any correlation between wear and early migration of either prosthetic component[19]. We concluded: (1) That RSA may distinguish between a migrating and a non-migrating prosthetic component within 4 mo after surgery; (2) That the initial migration may be caused by insufficient initial fixation or by resorption of a necrotic bone bed formed due to the heat from curing cement but not by wear products; and (3) That “late loosening” may be the result of late detection rather than of genuine late onset of loosening.
Many RSA studies of hip prostheses have now shown that early migration poses a risk of future failure[9,20-24]. This does not mean that all early migrating components will fail in the foreseeable future. Indeed, certain early migrating uncemented femoral components appear to achieve stability during the healing period[25-27], but it does mean that the failing prosthetic components are recruited from the group of early migrating components.
Some authors have determined a high migration threshold to predict an unaccept
Others have determined a low migration threshold below which an early migration poses no or almost no risk of future failure, e.g., 0.2 mm cranial migration after 2 years for acetabular components[22] and 0.15 mm distal migration after 2 years for cemented composite-beam femoral components[23] to predict a revision rate of less than 5% within 10 years. Between these extremes (2.6 mm and 0.15 mm), of course, there is a large gray zone. The choice of migration threshold depends on the purpose. In my opinion, a high probability of permanent prosthetic fixation is a more advantageous prediction.
Inadequate preparation and cementing technique were probably the main causes of loosening during the pioneering years and greatly reduced rates of loosening were demonstrated after improved technique[3,28-30]. Convincing evidence from both clinical and experimental research indicates that the initial fixation may be insufficient due to poor interlock (inadequate cement filling, interposition of tissue debris, etc.)[31-34] or because of poor bone quality (osteoporosis, rheumatoid arthritis, etc.)[35-38]. Adequate initial fixation does, however, not eliminate the risk of loosening; resorption of a layer of a necrotic bone bed may result in early loss of otherwise optimal fixation[39,40].
The theory of early loosening[41,42] postulates (the hypothetico-deductive method) that loosening is initiated during or shortly after surgery by these factors alone: Insufficient initial fixation (poor interlock or poor bone quality) or early loss of fixation (resorption of a layer of a necrotic bone). Interestingly, the resorption of necrotic bone can be inhibited with a bisphosphonate during the healing period, which reduces early migration[43] and consequently increases the mean prosthetic survival time[44].
If initiated, the progression of loosening is affected by the degree of early instability, the bone quality, and by the magnitude of the mechanical stresses to which the prosthetic components are exposed during normal daily activity. Thus, femoral components with a high offset[24] or in a varus position[3,34] can be expected to be over-represented among prosthetic failures due to faster increase in the micro
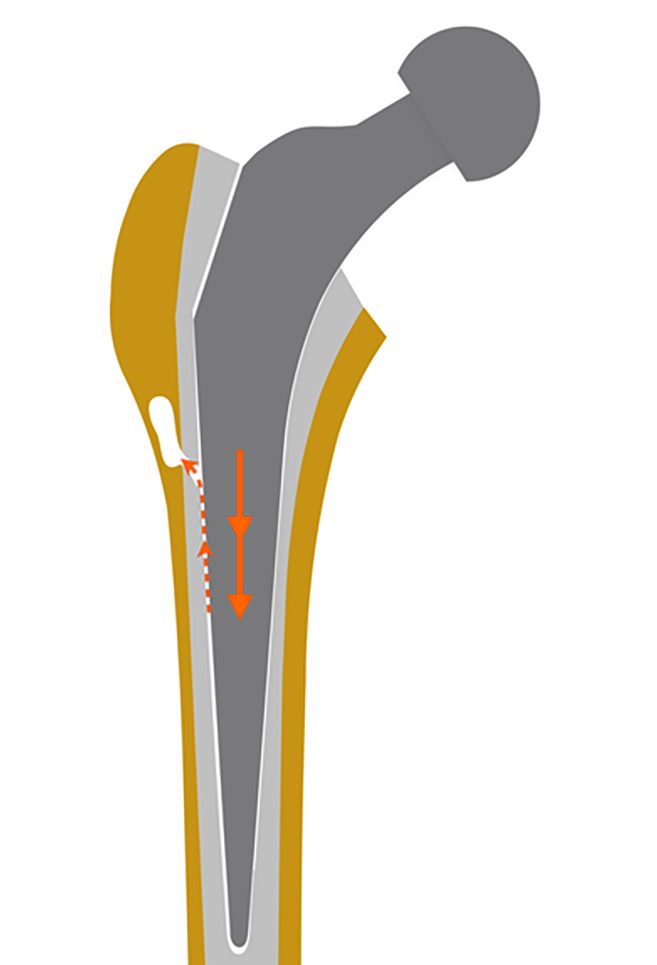
The micromovements of a loosened prosthetic component may cause devitalizing spikes of high fluid pressure in the periprosthetic interstice, which can induce osteolysis[49-51] by a complex series of inflammatory responses to the damage-associated molecular patterns of the generated necrotic cells and cell fragments[52]. The periprosthetic fluid may be forced further into the bone (Figure 3), devitalizing the bone tissue that is resorbed, and form a focal osteolysis that is invaded by granulation tissue[54]. The prosthetic micromovements and the subsequent periprosthetic osteolysis may increase subclinically during a long period of time.
The theory of early loosening explains the rapid early migration (Figure 2), the development of periprosthetic osteolysis and granulomas (Figure 3), the bone loss commonly seen in the proximal femur of distally apparently well-fixed stems, the causality between wear and loosening, and largely the epidemiology of clinical failure of hip prostheses[41,42]. But as always, if new data emerge that contradicts the predictions of the theory, the theory must be supplemented or replaced with a more complete theory.
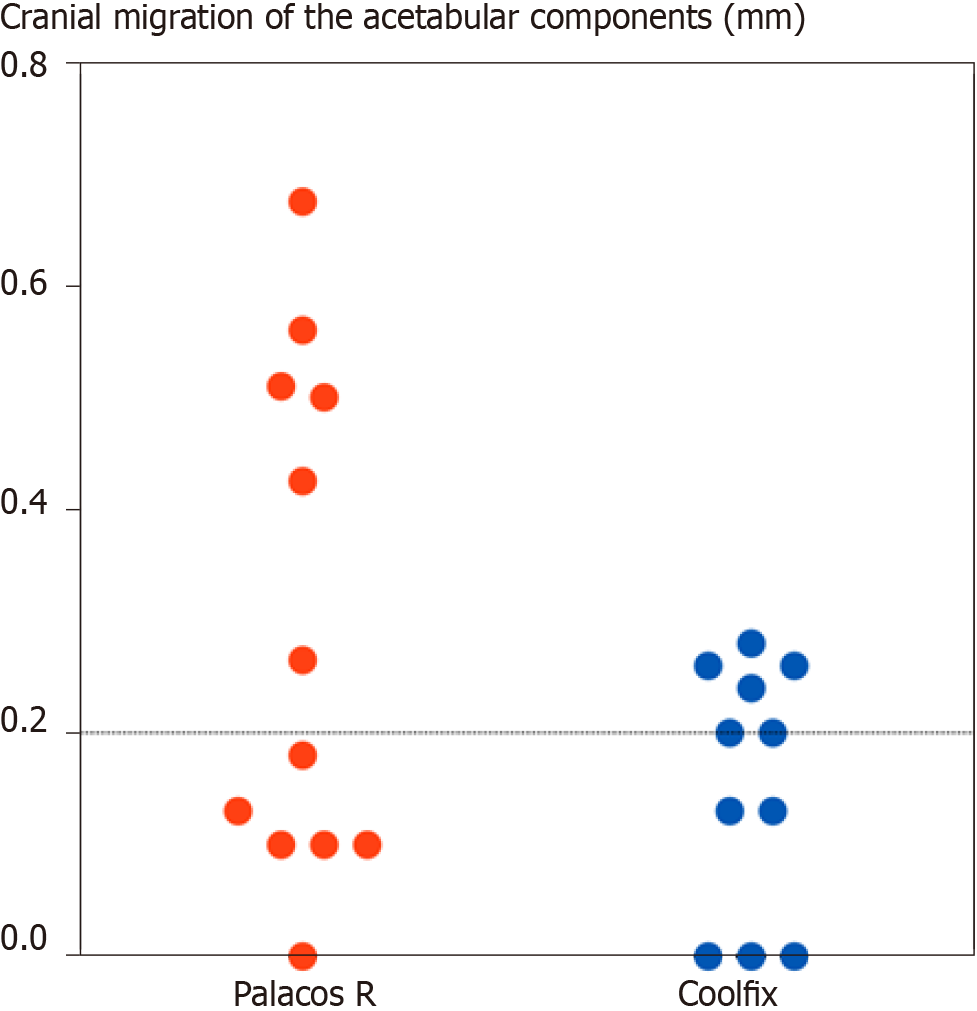
The specific heat production is directly proportional to the amount of monomer in the cement dough: 556 J/g monomer[55]. A low-monomer bone cement, Coolfix, was developed in the mid-1980s that produced less heat and less evaporating toxic monomer during the polymerization. The basic idea was to minimize the interspaces between the powder beads (which are filled with the liquid monomer) by a bimodal particle size distribution: 20 mL of liquid monomer was mixed with 70 g of Coolfix powder (instead of just about 40 g of a conventional bone cement powder). The temperature rise of Coolfix was (as expected) two-thirds of that of a conventional bone cement. The compressive strength was about 85% compared to Palacos R, probably due to the fact that the cement was made too dry (i.e. unsaturated) in the ambition to reduce the amount of liquid monomer. Therefore, this prototype Coolfix cement had a high viscosity and was more difficult to handle, especially in the acetabulum. The initial migration of the components in 24 hip prostheses was studied using RSA following randomized use of Coolfix and Palacos R[56]. After 1 year, five of the 12 acetabular components with Palacos R had migrated 0.4–0.7 mm, while all 11 acetabular components with Coolfix (one acetabular component with Coolfix was excluded due to insufficient tantalum bone markers) had migrated less than 0.3 mm (Figure 4). Only one femoral component (with Palacos R) had migrated significantly by then (0.4 mm distally).
An improved composition of Coolfix (the PMMA powders were purchased from Röhm GmbH, Darmstadt, Germany) was developed (Table 1), which had several attractive properties in addition to being less exothermic than a conventional bone cement: The improved cement was easily modeled and non-sticky, had a short mixing time, and smelled less. Unfortunately, the leadership of the Department of Orthopedics, Lund University Hospital, suddenly did not allow further clinical trials unless highly extended preclinical tests after vacuum mixing of the cement were performed; in practice, the project was stopped, and shortly afterwards I left Lund (but after my retirement and after a new department leadership had taken office in Lund, I became affiliated with the Department of Orthopedics, Lund University, once again). The improved Coolfix cement was never clinically tested.
| Plexidon M489 (300–500 µm) | 32.0 g |
| Plexidon M527 (30–60 µm) | 22.3 g |
| Plexigum M914 | 6.3 g |
| Benzoyl peroxide | 0.6 g |
| Zirconium dioxide | 8.8 g |
Later, another low-monomer cement (Cemex Rx) was marketed, where, unlike Coolfix, the smallest particles in the powder had been removed. However, compared to Palacos R, no significant difference was achieved in either curing temperature[57] or prosthetic migration[57,58].
The continuous migration of taper-slip stems has been reported to be consistent with good long-term clinical results[59-62] and has even been considered beneficial by contributing to secure fixation[59,61]. But does continuous migration really contribute to secure fixation? Or otherwise expressed: How much can a stem migrate distally without failing[63]?
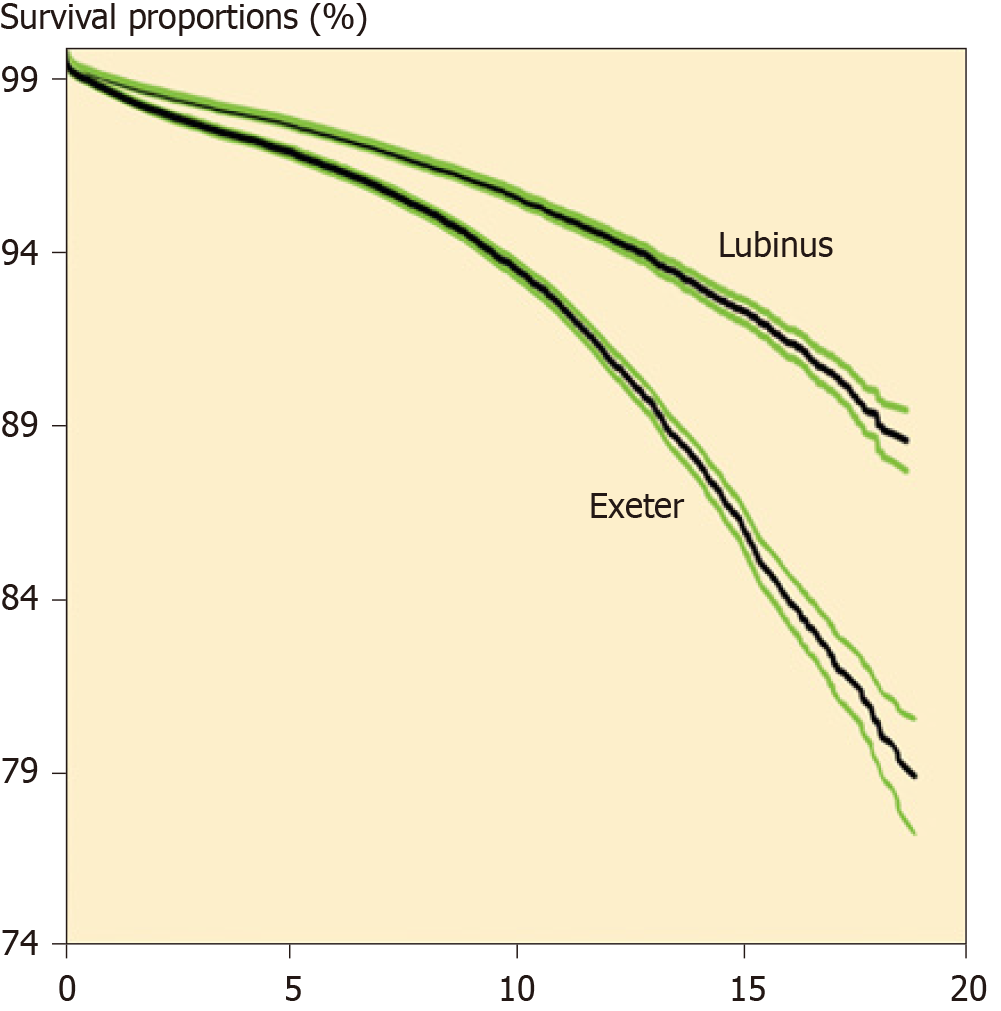
The Exeter THA has, according to the Nordic Arthroplasty Register Association database (consisting of 100000 s hip arthroplasties)[64], a survivorship fairly on par with the Lubinus THA of up to about 10 years, but afterwards, its failure rate increases faster (Figure 5).
A plausible explanation is as follows: In cases of considerable subsidence, the self-locking effect of a cemented tapered stem declines because the stem and cement mantle no longer fit well together. A play space arises around the stem, and the micromovements of the loosened stem induces periprosthetic osteolysis (due to inflammatory responses to the damage-associated molecular patterns of the necrotic cells and cell fragments generated by devitalizing spikes of high periprosthetic fluid pressure from the unstable stem). When the cement is no longer sufficiently supported by the surrounding bone, the cement mantle will crack and the stem instability will increase, resulting in a rapid subsidence[60] and ultimately a fracture of the stem[65] or a fracture of the proximal femur[66,67].
No significant prosthetic migration is safer in the long run than good 10-year clinical results!
The recently developed low-dose computed tomography-based implant motion analysis has been shown to have an accuracy and a precision on par with RSA; this measuring method (without the need for bone or implant markers and specialized RSA equipment) ”opens up the possibility for simpler implant migration studies”[68-70]. Very exciting technique! Maybe this is a future golden standard for implant motion analysis?
The less heat production and less evaporating toxic monomer during the polymerization, the more the risk of superficial bone necrosis adjacent to the Coolfix cement is reduced. A locally applied bisphosphonate inhibits the resorption of necrotic bone during the healing period[41]. The synergistic effect of this combination (the Coolfix cement and a locally applied bisphosphonate) should increase the probability of permanent prosthetic fixation. Although the improved Coolfix cement, unlike a chemically modified bone cement, after curing is chemically equivalent to conventional bone cements and should have similar mechanical properties, a preclinical characterization is required prior to a clinical trial.
Bone scintigraphy (99mTc-MDP) is extremely sensitive but generally non-specific for diagnosing loosening[71]. The scan is usually normalized within 6–9 mo after surgery[14], indicating that the healing period is over and that the prosthesis has become osseointegrated. Persistent uptake beyond 1 year represents increased bone turnover and bone perfusion – and probably continuous prosthetic migration. However, no prospective RSA study has been combined with scintigraphy, which would be interesting from both a pathophysiological and diagnostic point of view.
In contrast to the many RSA studies of hip prostheses that have shown that early migration poses a risk of future failure (the larger the early migration, the greater the risk of future failure)[9,20-24], some RSA studies indicate (as mentioned earlier) that certain uncemented femoral components appear to achieve stability during the healing period despite significant early migration[25-27]. However, do these femoral components, as suggested in these studies, really become osseointegrated or do some of them continue to migrate very slowly? Bone scintigraphy could probably tell.
Hip prosthetic loosening is often difficult to detect at an early stage. When loosening is eventually detected on standard radiographs it may be interpreted as late loosening but should be interpreted as late detection of loosening, initiated during or shortly after surgery by insufficient initial fixation or by early loss of fixation.
My thanks to the Editor for inviting me to write this very personal review and to Patricia Williamson (Skåne-Tranås, Sweden) for skillful language corrections.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country/Territory of origin: Sweden
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Isidro A, Liu P, Stogov MV S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Carlsson ÅS, Gentz CF. Mechanical loosening of the femoral head prosthesis in the Charnley total hip arthroplasty. Clin Orthop Relat Res. 1980;147:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Lindberg HO, Carlsson ÅS. Mechanical loosening of the femoral component in total hip replacement, Brunswik design. Acta Orthop Scand. 1983;54:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Paterson M, Fulford P, Denham R. Loosening of the femoral component after total hip replacement. The thin black line and the sinking hip. J Bone Joint Surg Br. 1986;68:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Selvik G. Roentgen stereophotogrammetry. A method for the study of the kinematics of the skeletal system. Acta Orthop Scand Suppl. 1989;232:1-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 398] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Kärrholm J, Gill RH, Valstar ER. The history and future of radiostereometric analysis. Clin Orthop Relat Res. 2006;448:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Baldursson H, Egund N, Hansson LI, Selvik G. Instability and wear of total hip prostheses determined with roentgen stereophotogrammetry. Arch Orthop Trauma Surg. 1979;95:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Baldursson H, Hansson LI, Olsson TH, Selvik G. Migration of the acetabular socket after total hip replacement determined by roentgen stereophotogrammetry. Acta Orthop Scand. 1980;51:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Börlin N, Thien T, Kärrholm J. The precision of radiostereometric measurements. Manual vs. digital measurements. J Biomech. 2002;35:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Klerken T, Mohaddes M, Nemes S, Kärrholm J. High early migration of the revised acetabular component is a predictor of late cup loosening: 312 cup revisions followed with radiostereometric analysis for 2-20 years. Hip Int. 2015;25:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Murray WR, Rodrigo JJ. Arthrography for the assessment of pain after total hip replacement. A comparison of arthrographic findings in patients with and without pain. J Bone Joint Surg Am. 1975;57:1060-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | O'Neill DA, Harris WH. Failed total hip replacement: assessment by plain radiographs, arthrograms, and aspiration of the hip joint. J Bone Joint Surg Am. 1984;66:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Abdel-Dayem HM, Barodawala YK, Papademetriou T. Scintigraphic arthrography. Comparison with contrast arthrography and future applications. Clin Nucl Med. 1982;7:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Uri G, Wellman H, Capello W, Robb J, Greenman G. Scintigraphic and X-ray arthrographic diagnosis of femoral prosthesis loosening: concise communication. J Nucl Med. 1984;25:661-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Häckel H, König B, Mostbeck A, Pflüger W. Zur Wertigkeit der Knochenszintigraphie bei Kunstgelenklockerungen [On the usefulness of bone scintigraphy for detecting loosening of articular prostheses (author's transl)]. Z Orthop Ihre Grenzgeb. 1978;116:727-731. [PubMed] |
| 15. | Weiss PE, Mall JC, Hoffer PB, Murray WR, Rodrigo JJ, Genant HK. 99mTc-methylene diphosphonate bone imaging in the evaluation of total hip prostheses. Radiology. 1979;133:727-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 56] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Mjöberg B, Brismar J, Hansson LI, Pettersson H, Selvik G, Önnerfält R. Definition of endoprosthetic loosening. Comparison of arthrography, scintigraphy and roentgen stereophotogrammetry in prosthetic hips. Acta Orthop Scand. 1985;56:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Mjöberg B, Selvik G, Hansson LI, Rosenqvist R, Önnerfält R. Mechanical loosening of total hip prostheses. A radiographic and roentgen stereophotogrammetric study. J Bone Joint Surg Br. 1986;68:770-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Mjöberg B, Franzén H, Selvik G. Early detection of prosthetic-hip loosening. Comparison of low- and high-viscosity bone cement. Acta Orthop Scand. 1990;61:273-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Franzén H, Mjöberg B. Wear and loosening of the hip prosthesis. A roentgen stereophotogrammetric 3-year study of 14 cases. Acta Orthop Scand. 1990;61:499-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Kärrholm J, Borssén B, Löwenhielm G, Snorrason F. Does early micromotion of femoral stem prostheses matter? J Bone Joint Surg Br. 1994;76:912-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 412] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Nieuwenhuijse MJ, Valstar ER, Kaptein BL, Nelissen RG. Good diagnostic performance of early migration as a predictor of late aseptic loosening of acetabular cups: results from ten years of follow-up with Roentgen stereophotogrammetric analysis (RSA). J Bone Joint Surg Am. 2012;94:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Pijls BG, Nieuwenhuijse MJ, Fiocco M, Plevier JW, Middeldorp S, Nelissen RG, Valstar ER. Early proximal migration of cups is associated with late revision in THA: a systematic review and meta-analysis of 26 RSA studies and 49 survivalstudies. Acta Orthop. 2012;83:583-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 23. | van der Voort P, Pijls BG, Nieuwenhuijse MJ, Jasper J, Fiocco M, Plevier JW, Middeldorp S, Valstar ER, Nelissen RG. Early subsidence of shape-closed hip arthroplasty stems is associated with late revision. A systematic review and meta-analysis of 24 RSA studies and 56 survival studies. Acta Orthop. 2015;86:575-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Johanson PE, Antonsson M, Shareghi B, Kärrholm J. Early Subsidence Predicts Failure of a Cemented Femoral Stem With Minor Design Changes. Clin Orthop Relat Res. 2016;474:2221-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Weber E, Sundberg M, Flivik G. Design modifications of the uncemented Furlong hip stem result in minor early subsidence but do not affect further stability: a randomized controlled RSA study with 5-year follow-up. Acta Orthop. 2014;85:556-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Aro E, Alm JJ, Moritz N, Mattila K, Aro HT. Good stability of a cementless, anatomically designed femoral stem in aging women: a 9-year RSA study of 32 patients. Acta Orthop. 2018;89:490-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Floerkemeier T, Budde S, Lewinski GV, Windhagen H, Hurschler C, Schwarze M. Greater early migration of a short-stem total hip arthroplasty is not associated with an increased risk of osseointegration failure: 5th-year results from a prospective RSA study with 39 patients, a follow-up study. Acta Orthop. 2020;91:266-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Mulroy RD Jr, Harris WH. The effect of improved cementing techniques on component loosening in total hip replacement. An 11-year radiographic review. J Bone Joint Surg Br. 1990;72:757-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 272] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Ballard WT, Callaghan JJ, Sullivan PM, Johnston RC. The results of improved cementing techniques for total hip arthroplasty in patients less than fifty years old. A ten-year follow-up study. J Bone Joint Surg Am. 1994;76:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 105] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Trumm BN, Callaghan JJ, George CA, Liu SS, Goetz DD, Johnston RC. Minimum 20-year follow-up results of revision total hip arthroplasty with improved cementing technique. J Arthroplasty. 2014;29:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Krause WR, Krug W, Miller J. Strength of the cement-bone interface. Clin Orthop Relat Res. 1982;290-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 60] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Kristiansen B, Jensen JS. Biomechanical factors in loosening of the Stanmore hip. Acta Orthop Scand. 1985;56:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Dohmae Y, Bechtold JE, Sherman RE, Puno RM, Gustilo RB. Reduction in cement-bone interface shear strength between primary and revision arthroplasty. Clin Orthop Relat Res. 1988;214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Franzén H, Mjöberg B, Önnerfält R. Early loosening of femoral components after cemented revision. A roentgen stereophotogrammetric study. J Bone Joint Surg Br. 1992;74:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Önsten I, Bengnér U, Besjakov J. Socket migration after Charnley arthroplasty in rheumatoid arthritis and osteoarthritis. A roentgen stereophotogrammetric study. J Bone Joint Surg Br. 1993;75:677-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Snorrason F, Kärrholm J, Holmgren C. Fixation of cemented acetabular prostheses. The influence of preoperative diagnosis. J Arthroplasty. 1993;8:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Aro HT, Alm JJ, Moritz N, Mäkinen TJ, Lankinen P. Low BMD affects initial stability and delays stem osseointegration in cementless total hip arthroplasty in women: a 2-year RSA study of 39 patients. Acta Orthop. 2012;83:107-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 38. | Finnilä S, Moritz N, Svedström E, Alm JJ, Aro HT. Increased migration of uncemented acetabular cups in female total hip arthroplasty patients with low systemic bone mineral density. A 2-year RSA and 8-year radiographic follow-up study of 34 patients. Acta Orthop. 2016;87:48-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Sih GC, Connelly GM, Berman AT. The effect of thickness and pressure on the curing of PMMA bone cement for the total hip joint replacement. J Biomech. 1980;13:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Toksvig-Larsen S, Franzén H, Ryd L. Cement interface temperature in hip arthroplasty. Acta Orthop Scand. 1991;62:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Mjöberg B. The theory of early loosening of hip prostheses. Orthopedics. 1997;20:1169-1175. [PubMed] |
| 42. | Mjöberg B. Is early migration enough to explain late clinical loosening of hip prostheses? EFORT Open Rev. 2020;5:113-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (4)] |
| 43. | Schilcher J, Palm L, Ivarsson I, Aspenberg P. Local bisphosphonate reduces migration and formation of radiolucent lines adjacent to cemented acetabular components. Bone Joint J. 2017;99-B:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Prieto-Alhambra D, Javaid MK, Judge A, Murray D, Carr A, Cooper C, Arden NK. Association between bisphosphonate use and implant survival after primary total arthroplasty of the knee or hip: population based retrospective cohort study. BMJ. 2011;343:d7222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 45. | Ramadier JO, Lelong P, Dupont JY. Rotation anormale de certaines cupules cotyloïdiennes excentrées scellées [Rotational displacement of eccentric cups cemented in the acetabulum (author's transl)]. Rev Chir Orthop Reparatrice Appar Mot. 1980;66:507-514. [PubMed] |
| 46. | Mathiesen EB, Lindgren U, Reinholt FP, Sudmann E. Wear of the acetabular socket. Comparison of polyacetal and polyethylene. Acta Orthop Scand. 1986;57:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Scholes SC, Unsworth A, Goldsmith AA. A frictional study of total hip joint replacements. Phys Med Biol. 2000;45:3721-3735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Bishop NE, Waldow F, Morlock MM. Friction moments of large metal-on-metal hip joint bearings and other modern designs. Med Eng Phys. 2008;30:1057-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Aspenberg P, van der Vis H. Fluid pressure may cause periprosthetic osteolysis. Particles are not the only thing. Acta Orthop Scand. 1998;69:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Skoglund B, Aspenberg P. PMMA particles and pressure--a study of the osteolytic properties of two agents proposed to cause prosthetic loosening. J Orthop Res. 2003;21:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Fahlgren A, Bostrom MP, Yang X, Johansson L, Edlund U, Agholme F, Aspenberg P. Fluid pressure and flow as a cause of bone resorption. Acta Orthop. 2010;81:508-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 765] [Cited by in RCA: 691] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 53. | Mjöberg B. Does particle disease really exist? Acta Orthop. 2018;89:130-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 54. | Anthony PP, Gie GA, Howie CR, Ling RS. Localised endosteal bone lysis in relation to the femoral components of cemented total hip arthroplasties. J Bone Joint Surg Br. 1990;72:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 204] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Debrunner HU, Wettstein A, Hofer P. The polymerization of self-curing acrylic cements and problems due to the cement anchorage of joint prostheses. In: Schaldach M, Hohmann D, eds. Engineering in medicine, volume 2: Advances in artificial hip and knee joint technology. Berlin, Heidelberg, New York: Springer, 1976: 294-324. |
| 56. | Mjöberg B, Selvik G. Reduced risk of loosening of hip prostheses with a new cold-curing bone cement. In: Biomaterials, Part II. Symposium organized by the Scandinavian Orthopedic Association. Ystad, Sweden, September 29-October 1, 1986. Abstracts. Acta Orthop Scand. 1988;59:343-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 57. | Nivbrant B, Kärrholm J, Röhrl S, Hassander H, Wesslén B. Bone cement with reduced proportion of monomer in total hip arthroplasty: preclinical evaluation and randomized study of 47 cases with 5 years' follow-up. Acta Orthop Scand. 2001;72:572-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Söderlund P, Dahl J, Röhrl S, Nivbrant B, Nilsson KG. 10-year results of a new low-monomer cement: follow-up of a randomized RSA study. Acta Orthop. 2012;83:604-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Yates PJ, Burston BJ, Whitley E, Bannister GC. Collarless polished tapered stem: clinical and radiological results at a minimum of ten years' follow-up. J Bone Joint Surg Br. 2008;90:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Nieuwenhuijse MJ, Valstar ER, Kaptein BL, Nelissen RG. The Exeter femoral stem continues to migrate during its first decade after implantation: 10-12 years of follow-up with radiostereometric analysis (RSA). Acta Orthop. 2012;83:129-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 61. | Murray DW, Gulati A, Gill HS. Ten-year RSA-measured migration of the Exeter femoral stem. Bone Joint J. 2013;95-B:605-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Teeter MG, McCalden RW, Yuan X, MacDonald SJ, Naudie DD. Predictive accuracy of RSA migration thresholds for cemented total hip arthroplasty stem designs. Hip Int. 2018;28:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Stefánsdóttir A, Franzén H, Johnsson R, Ornstein E, Sundberg M. Movement pattern of the Exeter femoral stem; a radiostereometric analysis of 22 primary hip arthroplasties followed for 5 years. Acta Orthop Scand. 2004;75:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Junnila M, Laaksonen I, Eskelinen A, Pulkkinen P, Havelin LI, Furnes O, Fenstad AM, Pedersen AB, Overgaard S, Kärrholm J, Garellick G, Malchau H, Mäkelä KT. Implant survival of the most common cemented total hip devices from the Nordic Arthroplasty Register Association database. Acta Orthop. 2016;87:546-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 65. | Davies BM, Branford White HA, Temple A. A series of four fractured Exeter™ stems in hip arthroplasty. Ann R Coll Surg Engl. 2013;95:e130-e132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Brodén C, Mukka S, Muren O, Eisler T, Boden H, Stark A, Sköldenberg O. High risk of early periprosthetic fractures after primary hip arthroplasty in elderly patients using a cemented, tapered, polished stem. Acta Orthop. 2015;86:169-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 67. | Chatziagorou G, Lindahl H, Kärrholm J. The design of the cemented stem influences the risk of Vancouver type B fractures, but not of type C: an analysis of 82,837 Lubinus SPII and Exeter Polished stems. Acta Orthop. 2019;90:135-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 68. | Scheerlinck T, Polfliet M, Deklerck R, Van Gompel G, Buls N, Vandemeulebroucke J. Development and validation of an automated and marker-free CT-based spatial analysis method (CTSA) for assessment of femoral hip implant migration: In vitro accuracy and precision comparable to that of radiostereometric analysis (RSA). Acta Orthop. 2016;87:139-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Brodén C, Sandberg O, Sköldenberg O, Stigbrand H, Hänni M, Giles JW, Emery R, Lazarinis S, Nyström A, Olivecrona H. Low-dose CT-based implant motion analysis is a precise tool for early migration measurements of hip cups: a clinical study of 24 patients. Acta Orthop. 2020;91:260-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 70. | Sandberg O, Tholén S, Carlsson S, Wretenberg P. The anatomical SP-CL stem demonstrates a non-progressing migration pattern in the first year: a low dose CT-based migration study in 20 patients. Acta Orthop. 2020;91:654-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 71. | Palestro CJ. Nuclear medicine and the failed joint replacement: Past, present, and future. World J Radiol. 2014;6:446-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (1)] |