Published online Nov 18, 2021. doi: 10.5312/wjo.v12.i11.877
Peer-review started: May 4, 2021
First decision: June 7, 2021
Revised: June 27, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: November 18, 2021
Processing time: 195 Days and 9.9 Hours
The increased prevalence of obesity has resulted in orthopedic surgeons being likely to face many patients with a high body mass index (BMI) who warrant total hip arthroplasties (THAs) over the coming years. Studies' findings considered the postoperative clinical, and functional outcomes in these patients are controversial, and selecting the most appropriate surgical approach remains debatable.
To compare pain-levels, functionality, and quality-of-life in obese and nonobese osteoarthritic patients who have undergone primary total hip arthroplasty through either direct-anterior-approach (DAA) or Hardinge-approach.
One hundred and twenty participants (> 50 years) were divided into four groups according to the surgical approach (DAA or Hardinge) and patients' BMI (nonobese < 30 kg/m2 vs obese ≥ 30 kg/m2). Outcomes were measured preoperatively and postoperatively (6th and 12th week). Pain was measured with Face Pain Scale-Revised (FPS-R). Functionality was measured with Timed Up & Go (TUG) test and Modified Harris Hip Score-Greek version (MHHS-Gr). Quality-of-life was evaluated with the 12-item-International Hip Outcome Tool-Greek version (iHOT12-Gr) (Clinical Trial Identifier: ISRCTN15066737).
DAA vs Hardinge: (week 6) DAA-patients showed 12.2% less pain, more functionality (14.8% shorter TUG-performance time, 21.5% higher MHHS-Gr), and 38.16% better quality-of-life (iHOT12-Gr) compared to Hardinge-patients (all P values < 0.001). These differences were further increased on week 12 (all P values ≤ 0.05)]. DAA-obese vs Hardinge–obese: (week 6) DAA-obese patients had less pain, shorter TUG-performance time, better MHHS-Gr and iHOT12-Gr scores than Hardinge-obese (all P values < 0.01). (Week 12) Only the TUG-performance time of DAA-obese was significantly shortened (22.57%, P < 0.001). DAA-nonobese vs DAA-obese: no statistically significant differences were observed comparing the 6th and 12th weeks' outcomes.
DAA-groups reported less pain, more functionality and better quality-of-life, compared to the Hardinge-groups. The DAA benefited obese and nonobese patients, similarly yet faster, suggesting that it should be the more preferred choice for obese patients, instead of Hardinge. However, more comparative studies with more extended follow-up periods are needed to confirm our results and better evaluate all patients' long-term outcomes.
Core Tip: The selection of the best total hip arthroplasties (THA)-surgical approach for obese hip osteoarthritis patients is a matter of debate. In the present study, both obese and nonobese patients of direct anterior approach (DAA) groups reached equivalent pain-levels, functionality, and quality-of-life. Moreover, the comparison between obese patients showed that DAA leads faster to better functional ability and quality-of-life than the Hardinge approach. These findings suggested that DAA is a better suited THA surgical approach than Hardinge for patients with increased body mass index.
- Citation: Macheras G, Stasi S, Sarantis M, Triantafyllou A, Tzefronis D, Papadakis SA. Direct anterior approach vs Hardinge in obese and nonobese osteoarthritic patients: A randomized controlled trial. World J Orthop 2021; 12(11): 877-890
- URL: https://www.wjgnet.com/2218-5836/full/v12/i11/877.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i11.877
In recent years, there has been a growing interest in minimally invasive surgical techniques that are used for the performance of total hip arthroplasty (THA). The advantages of these techniques include lesser soft tissue trauma, lesser amount of blood loss, minor postoperative pain, shorter hospital stay, better aesthetic appearance of the incision and a faster recovery time[1-3]. Over the past decade, the direct anterior approach (DAA) has sparked scientific interest due to its soft-tissue-preserving nature (intermuscular and internerval technique), coupled with the relatively lower risk of dislocation[4]. The DAA approach can be performed through a vertical or a horizontal (bikini) incision[5].
On the other hand, THA is an effective treatment for most patients who suffer from pain and decreased functionality due to end-stage symptomatic hip osteoarthritis (OA)[6]. Epidemiological studies report that hip OA occurs in 88 out of 100000 people, and the reported prevalence was 0.9 and 1.6 per 1000 yearly, in both men and women, respectively[7]. Within the Greek population, 0.9 per 1000 people develop osteoar
Several studies have indicated that obesity is associated with both a higher complication rate after THA and poorer clinical functional outcomes[11-13]. Other studies have shown that obese patients do not differ from nonobese in terms of postoperative outcomes[14-16]. The data are considered controversial and further studies need to be performed on obese patients, especially comparative evaluations that compare minimally invasive techniques such as DAA with classical surgical techniques, such as the Hardinge approach (HA). The Hardinge was chosen because, compared to other classical surgical approaches used in obese patients, it offers better access to the hip joint and achieves a lower rate of dislocation by preserving the joint's posterior stabilizer muscles[17].
This trial aims to compare DAA and Hardinge in hip OA patients who have undergone primary THA, with regards to postoperative pain levels, functional status, and quality-of-life. In addition, it was investigated whether these parameters differ between obese and nonobese patients.
The present study was a prospective, four-group randomised controlled trial (Clinical Trial Identifier: ISRCTN15066737) conducted according to the ethical principles stated in the Declaration of Helsinki and its later amendments[18]. The Scientific Research Council of the "KAT" General Hospital of Attica, Athens, Greece approved the study's protocol (ref: No8/19-03-2019). The study conformed to the "Consolidated Standards of Reporting Trials" (CONSORT) 2010 Statement checklist of information to include when reporting a randomised trial[19].
It was estimated that a sample size of 120 patients was required in order to have a 90% probability of demonstrating a –between surgical approaches- difference of 10% change from baseline to 6th week (DAA: -50% ± 12% vs Harginge: 40% ± 12%) in TUG test performance time, with a significance of < 5% (Two-tailed test).
Participants were selected from patients who have chosen to be operated by either of the two chief orthopedic surgeons/co-researchers of the present trial. One of the head-orthopedic surgeons (GM) performs primary THA using DAA technique -through a single vertical incision-[20], whilst the other (SP) prefers the Hardinge[21]. Surgical approach and BMI were used as factors in the randomisation process, while the randomisation list was formed on the basis of these factors. An independent clinician was responsible for the random allocation sequence and assigned participants to groups. Specifically, participants were divided into four groups (30 patients per group) according to both the surgical approach used and their body mass index (BMI), as follow: (1) DAA-nonobese group: patients with BMI ≥ 20 kg/m2 and < 30 kg/m2, who underwent THA through DAA; (2) DAA-obese group: Patients with BMI ≥ 30 kg/m2, who underwent THA through DAA; (3) Harginge-nonobese group: patients with BMI ≥ 20 kg/m2 and < 30 kg/m2, who underwent THA through Harginge; and (4) Harginge-obese group: patients with BMI ≥ 30 kg/m2, who underwent THA through Harginge.
To be eligible for randomization, patients had to meet the following inclusion criteria: Symptomatic hip OA, age > 50 years, and to be ambulatory before surgery. Patients were excluded if they had dementia, chronic respiratory disease, chronic renal failure, heart failure or neurological disorder. In addition, after discharge, patients would also be excluded if they discontinued the postoperative physiotherapy before the 6th week's measurement. No patient or clinician was blinded to the group allocation.
Αn uncemented prosthesis was used in all patients. Physiotherapy intervention was started on the first postoperative day and lasted for 6 wk, firstly on an in-patient basis, and following hospital discharge, home-based. One physiotherapist was responsible for carrying out the physiotherapy during hospitalization and at home, evaluating the progress according to the protocol and ensuring the patient's compliance adherence to it, on all groups.
Outcome measures were obtained at three different time points: prior to surgery (baseline), at the end of the 6th week, and at the end of the 12th week, postoperatively. Pain-levels were measured with the Face Pain Scale – Revised (FPS-R)[22,23]. Functional ability was evaluated with the objective physical performance measure Timed Up & Go (TUG) test[24], and with the reliable and validated Greek version of the Modified Harris Hip Score (MHHS-Gr)[25]. Quality-of-life was measured with the reliable and validated Greek version of the International Hip Outcome Tool -12 items (iHOT12-Gr)[26]. One examiner carried out the measurements of all outcomes, and he did not involve in any other part of the study.
FPS-R: The FPS-R is a patient-reported instrument frequently used to measure pain intensity. It includes six facial expressions covering the entire range of pain levels in an ascending -regarding discomfort levels- order[22]. Patients describe their pain according to one of the six facial expressions that correspond to their pain and enabling them to translate their subjective experience of pain into a quantitative, numeric measure[23].
TUG test: The TUG test was used to assess participants' functionality. This test was introduced in 1991 as a modification of the "Get-Up and Go" test[24]. It is a simple, easy, and thus widespread clinical tool for measuring the lower limbs' functionality and mobility[27]. The TUG test measures the time (in seconds) taken by a participant to stand up from an armed chair with a seat height of 46 cm, walk for 3 m, turn around a cone and return to sit on that very same chair. A shorter performance time represents better functionality[24,27]. Participants were asked to perform the test as quickly as they could while still feeling safe, and were allowed to use the walking aid on which they depended on at the time of measurement. The participants performed the test twice, with a 5-minute resting interval in between. The shorter of the two performance times was then recorded.
MHHS: The MHHS is a patient-reported questionnaire that includes assessments based on pain and on function[25]. One item evaluates the pain (0-44 points), while 7 items evaluate the patient's functionality (0-47 points). The total points form a scale from 0 to 91. A multiplier of 1.1 provides a total score of 100 (best possible outcome)[28].
iHOT12: The iHOT12 questionnaire includes 12 questions on the patient's symptoms, functional and sports limitations as well as social, emotional, and occupational limitations[26]. The patient is asked to consider the problems arising from their hip disorders and quantify their quality-of-life level on a 100 mm horizontal line (visual analogue scale) by marking it with a slash. Each question has equal value, giving a mean score from 0-100. A score of 100 indicates excellent quality-of-life (full function and no symptoms), whereas zero signifies the worst quality-of-life (maximum limitations and extreme symptoms)[29].
Data was expressed as mean ± SD or mean ± SE (for two way ANOVA analysis results) for continuous variables and as percentages for categorical data. The Kolmogorov—Smirnov test was utilized for normality analysis of the continuous variables.
The two-way ANOVA model was used to examine the interaction between the "Surgical Approach" factor (DAA & Hardinge) and "BMI" factor (< 30 kg/m2 and ≥ 30 kg/m2). In the case that there was no statistically significant interaction, we compared the factor "Surgical Approach" regardless of "BMI" factor and the factor "BMI" regardless of "Surgical Approach" factor.
Due to we have found significant interaction, we created a new factor categorising the combination of the existing categories of "Surgical Approach" with "BMI" factors (DAA-nonobese, DAA-obese, Hardinge-nonobese, Hardinge-obese). The analysis of variables was performed using the "One way ANOVA model". Pairwise comparisons were performed using the Bonferroni test.
All tests were two-sided and statistical significance was set at P < 0.05. All analyses were carried out using the statistical package SPSS v21.00 (IBM Corporation, Somers, NY, United States).
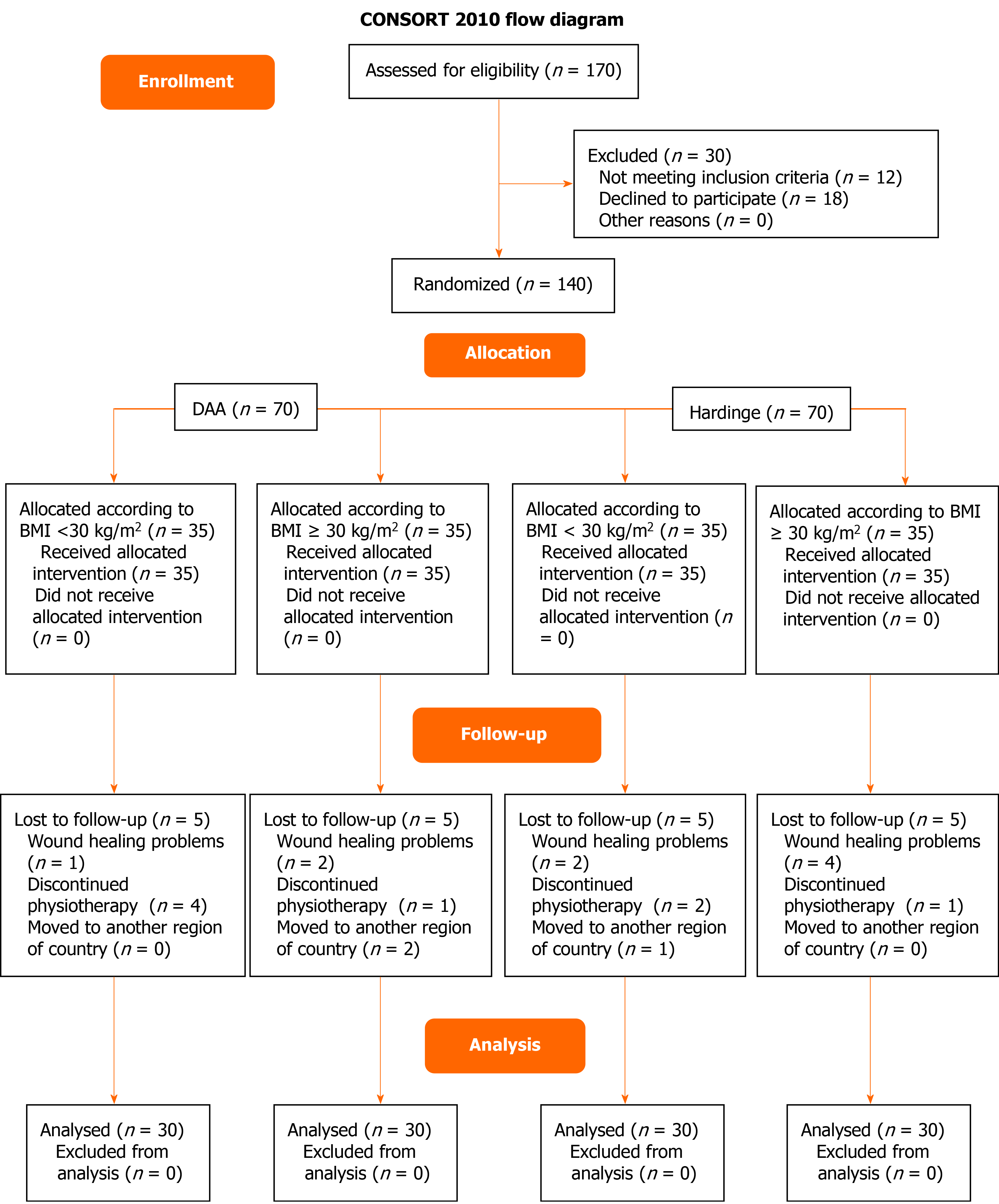
Patient recruitment lasted from January 2018 to March 2020, by which time the required number of participants had been reached. The recruitment procedure is depicted in the flow diagram in Figure 1. Eight patients (one from DAA-nonobese group, two from DAA-obese group, two from Hardinge–nonobese group and four from Hardinge-obese group) were lost to follow up due to wound healing problems. Finally, data from 120 patients (30 patients per group) were analysed (Figure 1). There is homogeneity between compared groups for all demographic and clinical characteristics apart from weight (P < 0.001) and BMI (P < 0.001), as expected (Table 1). At baseline, there is a statistically significant difference between compared groups in relation of variables, FPS-R (P = 0.045), TUG (P = 0.033), MHSS-Gr (P < 0.001); except iHOT12-Gr (P = 0.100) (Table 1); hence, the percentage (%) of changes of all variables/outcome scores in between groups - from baseline to 6th and 12th week- were presented.
| Characteristics and Clinical Measurements | DAA–nonobese (n = 30) | DAA–obese (n = 30) | Hardinge–nonobese (n = 30) | Hardinge–obese (n = 30) | P value |
| Age (yr)1 | 66.40 ± 7.31 | 63.73 ± 6.96 | 69.00 ± 9.00 | 63.73 ± 6.96 | 0.102 |
| Height (m)1 | 1.65 ± 0.09 | 1.64 ± 0.10 | 1.62 ± 0.08 | 1.64 ± 0.09 | 0.442 |
| Weight (kg)1 | 73.10 ± 10.95 | 92.63 ± 14.14a,b | 66.30 ± 8.10 | 93.93 ± 16.09a,b | < 0.001 |
| Body mass index (kg/m2)1 | 26.61 ± 2.16 | 34.27 ± 4.12a,b | 25.30 ± 2.03 | 34.73 ± 4.57a,b | < 0.001 |
| Dominant lower limb, n (%) | |||||
| Right | 24 (80.0) | 25 (83.3) | 27 (90.0) | 25 (83.3) | 0.756 |
| Left | 6 (20.0) | 5 (16.7) | 3 (10.0) | 5 (16.7) | |
| Affected hip, n (%) | |||||
| Right | 15 (50.0) | 18 (60.0) | 14 (46.7) | 18 (60.0) | 0.442 |
| Left | 15 (50.0) | 12 (40.0) | 16 (53.3) | 12 (40.0) | |
| Walking aid, n (%) | |||||
| No | 25 (83.3) | 22 (73.3) | 24 (80) | 19 (63.3) | 0.292 |
| Yes | 5 (16.7) | 8 (26.7) | 6 (20.0) | 11 (36.7) | |
| Kellgren & Lawrence classification, n (%) | |||||
| Grade 3 | 21 (70.0) | 19 (63.3) | 20 (66.7) | 19 (63.3) | 0.939 |
| Grade 4 | 9 (30.0) | 11 (36.7) | 10 (33.3) | 11 (36.7) | |
| Face Pain Scale-Revised1 (10 = worst pain) | 6.47 ± 1.55a | 6.87 ± 1.80 | 7.13 ± 1.36 | 7.60 ± 1.52 | 0.045 |
| Timed Up and Go test (s)1 | 16.09 ± 3.07a | 16.07 ± 5.56a | 17.12 ± 5.22 | 19.32 ± 4.99 | 0.033 |
| Modified Harris Hip Score – Greek version1 (100 = max best score) | 41.00 ± 6.55 | 33.94 ± 9.62b | 38.53 ± 8.15 | 31.66 ± 11.14b | < 0.001 |
| International Hip Outcome Tool (12 items) – Greek version1 (100 = max best score) | 31.43 ± 9.04 | 31.52 ± 8.26 | 28.28 ± 9.24 | 26.39 ± 10.00 | 0.100 |
The comparison of all variables/outcomes' scores during the observation period per group, showed that its absolute values were statistically significant different (P < 0.001) (Table 2). Patients of all groups benefited from the THA regarding the pain's level, functionality and quality-of-life.
| Groups | Preoperative measurement | 6th postoperative week | 12th postoperative week | P value |
| Face Pain Scale-Revised (min 0 - max 10) | ||||
| DAA-nonobese | 6.46 ± 1.55 | 1.97 ± 0.72 | 0.13 ± 0.35 | F(2.58) = 563.8, P < 0.001 |
| DAA-obese | 6.87 ± 1.79 | 1.93 ± 0.69 | 0.30 ± 0.53 | F(2.58) = 293.1, P < 0.001 |
| Hardinge-nonobese | 7.13 ± 1.36 | 3.20 ± 0.80 | 1.17 ± 0.70 | F(2.58) = 830.9, P < 0.001 |
| Hardinge-obese | 7.60 ± 1.52 | 3.00 ± 0.69 | 1.00 ± 0.69 | F(2.58) = 673.7, P < 0.001 |
| Timed Up and Go Test (second) | ||||
| DAA-nonobese | 16.09 ± 3.07 | 11.22 ± 2.29 | 7.81 ± 1.74 | F(2.58) = 399.81, P < 0.001 |
| DAA-obese | 16.07 ± 5.56 | 10.95 ± 2.52 | 8.02 ± 2.32 | F(2.58) = 89.55, P < 0.001 |
| Hardinge-nonobese | 17.12 ± 5.21 | 14.17 ± 3.69 | 11.62 ± 3.00 | F(2.58) = 122.04, P < 0.001 |
| Hardinge-obese | 19.32 ± 5.00 | 16.60 ± 4.14 | 14.33 ± 3.84 | F(2.58) = 289.56, P < 0.001 |
| Modified Harris Hip Score – Greek version (min 0 – max 100) | ||||
| DAA-nonobese | 41.00 ± 6.54 | 70.75 ± 8.20 | 92.41 ± 3.83 | F(2.58) = 1148.1, P < 0.001 |
| DAA-obese | 33.94 ± 9.62 | 63.98 ± 10.86 | 87.83 ± 6.29 | F(2.58) = 893.7, p<0.001 |
| Hardinge-nonobese | 38.53 ± 8.15 | 60.68 ± 10.68 | 82.17 ± 9.00 | F(2.58) = 1291.8, P < 0.001 |
| Hardinge-obese | 32.07 ± 10.41 | 53.82 ± 15.00 | 74.38 ± 11.78 | F(2.58) = 1066.6, P < 0.001 |
| International Hip Outcome Tool (12 items) – Greek Version (min 0 – max 100) | ||||
| DAA-nonobese | 31.43 ± 9.04 | 67.00 ± 10.05 | 86.18 ± 10.00 | F(2.58) = 703.53, P < 0.001 |
| DAA-obese | 31.52 ± 8.25 | 66.06 ± 12.95 | 88.43 ± 7.77 | F(2.58) = 740.71, P < 0.001 |
| Hardinge-nonobese | 28.28 ± 9.24 | 50.39 ± 15.72 | 67.96 ± 14.77 | F(2.58) = 411.82, P < 0.001 |
| Hardinge-obese | 26.39 ± 10.00 | 47.21 ± 16.88 | 63.46 ± 16.21 | F(2.58) = 351.81, P < 0.001 |
The exploration of interaction between "BMI" and "Surgical Approach" factors revealed statistically significant difference in pain-levels (FPS-R) at 12th week [F(1.116) = 4.11, P = 0.045] (Table 3). Exploring the data to illustrate the source of that interaction, we also compared the percentage of change of the other variables/ outcome measures (TUG, MHHS-Gr, iHOT112-Gr) from baseline to 6th and 12th week: a) across the two surgical approaches by BMI-level, and b) across BMI-level per surgical approach, adjusting significance cut-off points for multiple comparison.
| Outcomes/Variables | 6th postoperative week | 12th postoperative week |
| Face Pain Scale-Revised | F(1.116) = 1.96, P = 0.164 | F(1.116) = 4.11, P = 0.045 |
| Timed Up and Go Test | F(1.116) = 0.97, P = 0.328 | F(1.116) = 0.79, P = 0.376 |
| Modified Harris Hip Score – Greek version | F(1.116) = 1.01, P = 0.316 | F(1.116) = 0.93, P = 0.337 |
| International Hip Outcome Tool (12 items) – Greek Version | F(1.116) = 0.80, P = 0.373 | F(1.116) = 0.11, P = 0.917 |
DAA vs Hardinge regardless of BMI: At the 6th postoperative week's measurements, DAA patients reported 12.2 % lesser pain (FPS-R), more functional ability (14.8% faster TUG test performance time and 21.5% higher MHHS-Gr score), and 38.2% better quality-of-life (iHOT12-Gr) compared to Hardinge patients, with statistically significant differences (all P values <0.001) (Table 4). At 12th postoperative week's measurements these differences of both DAA groups' outcomes were further increased [FPS-R: 9.9% (P < 0.001), TUG test: 21.2 % (P < 0.001), MHHS-Gr: 22.5% (P = 0.05), and iHOT12-Gr: 40.5% (P < 0.001)] (Table 4). The DAA resulted in less postoperative pain, and offered faster and increased functional ability and better quality-of-life compared to the Hardinge.
| Nonobese (ΒΜΙ < 30 kg/m2) | Obese (ΒΜΙ ≥ 30 kg/m2) | Comparison between surgical approaches regardless of BMI | ||||||||
| Outcomes/ | Postope | DAA1 | Hard | P value3 | DAA1 | Hard | P value3 | DAA2 | Hardinge2 | P value3 |
| Face Pain Scale - Revised (%) | 6th week | -70.1 ± 6.7 | -55.3 ± 7.3 | < 0.001 | -70.0 ± 15.8 | -60.3 ± 6.9 | 0.002 | -70.0 ± 1.3 | -57.8 ± 1.3 | < 0.001 |
| 12th week | -98.4 ± 4.1 | -84.4 ± 8.2 | < 0.001 | -93.3 ± 18.7 | -87.5 ± 8.3 | 0.429 | -95.8 ± 1.4 | -86.0 ± 1.4 | < 0.001 | |
| Timed Up and Go Test (%) | 6th week | -30.1 ± 6.5 | -16.5 ± 4.8 | < 0.001 | -29.9 ± 10.9 | -13.9 ± 2.9 | < 0.001 | -30.0 ± 0.9 | -15.2 ± 0.9 | < 0.001 |
| 12th week | -51.2 ± 6.4 | -31.4 ± 5.9 | < 0.001 | -48.5 ± 14.4 | -25.9 ± 3.9 | < 0.001 | -49.9 ± 1.1 | -28.7 ± 1.1 | < 0.001 | |
| Modified Harris Hip Score – Greek version (%) | 6th week | 76.2 ± 27.4 | 59.6 ± 13.9 | 0.026 | 97.9 ± 38.5 | 71.4 ± 23.6 | 0.012 | 87.0 ± 3.5 | 65.5 ± 3.5 | < 0.001 |
| 12th week | 132.9 ± 50.4 | 121.4 ± 43.2 | 0.777 | 181.4 ± 89.6 | 147.8 ± 56.4 | 0.317 | 157.1 ± 8.1 | 134.6 ± 8.1 | 0.050 | |
| International Hip Outcome Tool (12 items) – Greek version (%) | 6th week | 124.4 ± 51.6 | 79.5 ± 22.9 | < 0.001 | 115.7 ± 39.4 | 84.3 ± 46.0 | 0.031 | 120.1 ± 5.3 | 81.9 ± 5.3 | < 0.001 |
| 12th week | 192.0 ± 76.1 | 150.3 ± 43.7 | 0.048 | 195.4 ± 64.2 | 156.1 ± 67.5 | 0.077 | 193.7 ± 8.3 | 153.2 ± 8.3 | < 0.001 | |
DAA-nonobese vs Hardinge-nonobese: At the measurement of the 6th postoperative week, the DAA-nonobese group had better % percentage of change in all outcomes: [FPS-R: 14.8% (P < 0.001), TUG test: 13.5 % (P < 0.001), MHHS-Gr: 16.5% (P = 0.026), and iHOT12-Gr: 44.9% (P < 0.001)] in comparison to the Hardinge –nonobese (Table 4). At the 12th postoperative week's measurements the DAA-nonobese FPS-R was lowered by 14.0% (P < 0.001), the TUG test performance time was shortened by 19.8 % (P < 0.001), and iHOT12-Gr got higher by 41.7% (P = 0.048), than Hardinge –nonobese. Although the DAA-nonobese group MHHS-Gr was higher by 11.5%, no statistically significant difference was revealed (P = 0.777) (Table 4). The DAA leads faster to better functional ability and quality-of-life compared to the Hardinge, in nonobese patients.
DAA-obese vs Hardinge-obese: At the 6th postoperative week's measurements DAA-obese patients had a greater % percentage of change of all outcomes [FPS-R: 9.7% (P = 0.002), TUG test performance time: 16.0% (P < 0.001), MHHS-Gr: 26.6% (P = 0.012), and iHOT12-Gr: 31.4% (P = 0.031) compared to Hardinge-obese patients (Table 4). At the 12th postoperative week's measurements, the only statistically significant difference was revealed at TUG test performance time of DAA-obese: 22.6% (P < 0.001), while the differences of the FPS-R, MHHS-Gr and iHOT-Gr were not statistically significant (Table 4). Regarding obese patients, DAA leads faster to better functional ability and quality-of-life compared to the Hardinge; at 12 wk the statistically significant differences between groups were narrowed.
Nonobese vs obese regardless of surgical approach: At the 6th postoperative week's measurements obese patients reported higher MHHS-Gr (16.8%) with statistically significant difference (P = 0.001) than nonobese. No statistically significant differences were revealed regarding the other outcomes (Table 5). At the 12th postoperative week's measurements obese patients' MHHS-Gr score was further increased (37.5%, P = 0.001), but still no statistically significant differences were revealed regarding the other outcomes (Table 5). Overall, regardless of the surgical approach, the only statistically significant difference between obese and nonobese patients was revealed in the self-reported functional ability as expressed by the MHHS-Gr questionnaire.
| Outcomes/ | Postope | DAA | Hardinge approach | Comparison between ΒΜΙ groups regardless of surgical approach | ||||||
| Nonobese1 | Obese1 | P value3 | Nono | Obese1 | P value | Nono | Obese1 | P value3 | ||
| Face Pain Scale - Revised (%) | 6th week | -70.1 ± 6.7 | -70.0 ± 15.8 | 1.000 | -55.3 ± 7.3 | -60.3 ± 6.9 | 0.304 | -62.7 ± 01.3 | -65.1 ± 1.3 | 0.178 |
| 12th week | -98.4 ± 4.1 | -93.3 ± 18.7 | 0.468 | -84.4 ± 8.2 | -87.5 ± 8.3 | 0.451 | -91.4 ± 1.4 | -90.4 ± 1.4 | 0.631 | |
| Timed Up and Go Test (%) | 6th week | -30.1 ± 6.5 | -29.9 ± 10.9 | 1.000 | -16.5 ± 4.8 | -13.9 ± 2.9 | 0.102 | -23.3 ± 0.9 | -21.9 ± 0.9 | 0.278 |
| 12th week | -51.2 ± 6.4 | -48.5 ± 14.4 | 0.783 | -31.4 ± 5.9 | -25.9 ± 3.9 | 0.001 | -41.3 ± 1.1 | -37.2 ± 1.1 | 0.010 | |
| Modified Harris Hip Score – Greek version (%) | 6th week | 76.1 ± 27.4 | 97.9 ± 38.5 | 0.068 | 59.6 ± 13.9 | 71.4 ± 23.6 | 0.102 | 67.9 ± 3.5 | 84.6 ± 3.5 | 0.001 |
| 12th week | 132.9 ± 50.4 | 181.4 ± 89.6 | 0.061 | 121.4 ± 43.2 | 147.8 ± 56.4 | 0.186 | 127.1 ± 8.1 | 164.6 ± 8.1 | 0.001 | |
| International Hip Outcome Tool (12 items) – Greek version (%) | 6th week | 124.4 ± 51.6 | 115.7 ± 39.4 | 0.841 | 79.5 ± 22.9 | 84.3 ± 46.0 | 0.957 | 102.0 ± 5.3 | 100.0 ± 5.3 | 0.793 |
| 12th week | 192.0 ± 76.1 | 195.4 ± 64.2 | 1.000 | 150.3 ± 43.7 | 156.1 ± 67.5 | 1.000 | 171.1 ± 8.3 | 175.7 ± 8.3 | 0.697 | |
DAA-nonobese vs DAA-obese: At the 6th postoperative weeks' measure
Hardinge-nonobese vs Hardinge-obese: At the 6th postoperative weeks' measure
In the present study, the effect of two different THA surgical approaches (DAA vs Hardinge) on postoperative pain levels, functionality and quality-of-life in both obese and nonobese hip OA patients was explored. Our results showed that the DAA resulted in less postoperative pain, and offered faster achieved and increased functional ability and better quality-of-life compared to the Hardinge. In addition, the measured outcomes of the aforementioned parameters did not differ between obese and nonobese DAA patients; DAA similarly benefited both obese and nonobese patients.
The selection of the best surgical approach for THA remains a matter of debate. While Hardinge is widely used, mainly due to its reduced dislocation rate[17], DAA is gaining popularity as its intermuscular pathway preserves soft tissues, ensuring an excellent functional outcome and reduced postoperative pain[4]. Our results are in line with other studies which suggest that DAA is more beneficial to the patients in terms of postoperative pain relief and faster recovery, than Hardinge[30]. A recent meta-analysis has concluded that, in comparison to the lateral approach, the anterior approach is correlated with reduced pain at 6 wk postoperatively, increased walking velocity, stride length and step length, while no difference was found in the Harris Hip Score and the rate of complications[31]. In the present study, we found that DAA was associated with higher pain relief, enhanced functional outcomes and quality-of-life at the 6th week, while these outcomes increased even more at 12th week.
Several studies have shown that obese patients do not differ from the nonobese ones in terms of postoperative outcomes[14-16]. Similarly, in the present study, both obese and nonobese patients of DAA groups reached equivalent pain-levels, functionality and quality-of-life, suggesting that BMI is indeed not a factor that will influence the postoperative outcomes of DAA. However, this was not observed in Hardinge-groups. Although at 6th weeks' measurements, no statistically significant differences were observed between Hardinge-nonobese and Hardinge-obese groups, at 12th week, Hardinge-nonobese presented significantly improved TUG test performance time in comparison to Hardinge-obese patients. This could be explained by the fact that, preoperatively, Hardinge-nonobese had no-significant shorter TUG test performance time than Hardinge-obese (Table 1), which became significant after the THA.
The comparison between obese patients showed that, DAA leads faster to better functional ability and quality-of-life compared to the Hardinge. At 12th postoperative week's measurements, the only statistically significant difference was revealed at TUG test performance time of DAA-obese group. This is not surprising since it is well known that the hip abductor strength improved at 3 mo following THA performed by a conventional approach, such as Hardinge[32], while the hip abductor strength was linear associated with TUG test[33].
In the present study, most cases with wound healing problems were reported in Hardinge-obese patients (four out of 35 patients, 11.4%), as shown in our flow diagram. This rate was lower than the rates reported in the literature, for obese patients, on other classical surgical approaches, ranging from 14.5% to 22%[34,35]. Regarding DAA, one incidence in nonobese and two incidences in obese participants were found (3.5 % and 5.7%, respectively). The study's results are within the rates reported in the literature: for DAA-nonobese ranging between 0.8% and 3%, while for DAA-obese patients ranging from 4.46% to 10.0%[36-38]. It is worth to be noted that the horizontal (bikini) incision was shown to facilitate even more wound healing in obese patients. In the retrospective comparative study by Manrique et al[39], involving obese patients (BMI > 30 kg/m2) it was reported that patients with horizontal incision had significant lower rates of wound healing problems compared to patients with vertical incision (0.00% vs 16.6%, P = 0.04)[39]. Nevertheless, the current evidence is limited, and further trials are warranted to identify differences between the two DAA skin incisions regarding wound healing in obese patients.
While the risks associated with THA in obese patients are well documented [11,34], the present study results show that DAA would be a preferable THA approach for obese patients. Since it is a minimally invasive surgical technique that provides the most direct access to the hip joint, DAA can be safely performed, by an experienced surgeon and under certain precautions, without an increased and adverse risk for obese patients[4,40].
The present study was a four-group randomised controlled trial. All patients underwent uncemented THA, and the same physiotherapist was responsible for the physiotherapy intervention in all four groups. After discharge, the supervision and guidance from the physiotherapist, during in-home sessions, helped ensure patient adherence to protocol and thus, the study's dropout rate was minimal. Moreover, all measurements were made by the same examiner, who was not involved in any other part of the study. These factors added strength and statistical power to the results of this study.
On the other hand, there are important limitations that ought to be mentioned. Patients were followed up until the 12th postoperative week; it is, therefore, unclear whether the observed postoperative differences between DAA and Hardinge will be maintained over time, even though several studies consistently showed better outcomes with DAA during functional rehabilitation within one year of surgery[41-44]. However, the fact that DAA allowed our patients to achieve a more rapid recovery than Hardinge concluded to their postoperative rehabilitation being less costly, since their independency was faster obtained, regardless of BMI. Another limitation is that DAA and Hardinge were performed by two different orthopaedic surgeons, a fact that may predispose biased conclusions. Even though our results are indicative, further research is needed to produce safer results regarding the impact of obesity in pain, functional outcomes, and quality-of-life after THA in between different approaches, which is ultimately more appropriate for obese hip OA patients.
In conclusion, the patients of our study reported less pain, more functionality and quality-of-life improvements, more so after THA with DAA, compared to the Hardinge. Moreover, DAA exhibits equivalent postoperative outcomes in obese and nonobese patients, suggesting a better-suited THA surgical approach for patients with increased BMI. Understanding the postoperative changes in pain's level, functional outcomes and quality-of-life in both obese and nonobese patients, as reported in the current study, will be helpful for both the patients and the surgeons regarding the decision-making process for the more appropriate THA surgical technique.
Total hip arthroplasty (THA) is an effective treatment for most patients who suffer from pain and decreased functional ability due to hip osteoarthritis (OA). The main risk factors for developing hip OA are advanced age, family history of OA, previous hip injury, hip dysplasia, and obesity. The increased prevalence of obesity has resulted in orthopedic surgeons being likely to face many patients with a high body mass index (BMI) who warrant THAs over the coming years. On the other hand, there has been growing interest in the direct anterior approach (DAA) in recent years because of its soft-tissue–preserving nature. Total hip arthroplasty (THA) is an effective treatment for most patients who suffer from pain and decreased functional ability due to hip osteoarthritis (OA). The main risk factors for developing hip OA are advanced age, family history of OA, previous hip injury, hip dysplasia, and obesity. The increased prevalence of obesity has resulted in orthopedic surgeons being likely to face many patients with a high body mass index (BMI) who warrant THAs over the coming years. On the other hand, there has been growing interest in the direct anterior approach (DAA) in recent years because of its soft-tissue–preserving nature.
In the literature, it has been reported that obesity is significantly associated with a greater need for joint replacement and that compared to patients with normal body mass index (BMI), obese patients may require a THA up to ten years earlier. Some studies indicate that obesity is associated with poorer clinical, functional outcomes, while others have shown that obese patients do not differ from the nonobese in this respect. The data are considered controversial, and further studies need to be performed on obese patients, especially comparative evaluations that compare minimally invasive techniques such as DAA with classical surgical techniques, such as the Hardinge approach. Compared to other classical surgical approaches used in obese patients, the Hardinge was chosen because it offers better access to the hip joint and achieves a lower dislocation rate by preserving its posterior stabilizer muscles.
We aimed to compare DAA and Hardinge in hip OA patients who have undergone primary THA regarding postoperative pain levels, functional status, and quality-of-life. In addition, it was investigated whether these parameters differ between obese and nonobese patients.
The present study was a prospective, four-group randomized controlled trial (Clinical Trial Identifier: ISRCTN15066737). One hundred twenty participants were divided into four groups (30 patients per group) according to both the surgical approach used and their body mass index (BMI) as follow: DAA-nonobese group (BMI < 30 kg/m2), DAA-obese group (BMI ≥ 30 kg/m2), Harginge-nonobese group (BMI < 30 kg/m2) and Harginge-obese group (BMI ≥ 30 kg/m2). Measurements were carried out prior to surgery (baseline) and postoperatively (at the end of the 6th week and 12th week). Pain levels were measured with the Face Pain Scale – Revised (FPS-R). Functional ability was evaluated with the Timed Up & Go (TUG) test and the Greek version of the Modified Harris Hip Score (MHHS-Gr). Quality-of-life was measured with the Greek version of the International Hip Outcome Tool -12 items (iHOT12-Gr).
DAA vs Hardinge regardless of BMI: The DAA resulted in less postoperative pain and offered faster and increased functional ability and better quality-of-life than the Hardinge. DAA-nonobese vs Hardinge-nonobese: The DAA leads faster to better functional ability and quality-of-life compared to the Hardinge in nonobese patients. DAA-obese vs Hardinge-obese: DAA leads faster to better functional ability and quality-of-life of obese patients than the Hardinge; at 12 wk, statistically significant differences between groups were narrowed. Nonobese vs obese regardless of surgical approach: the only statistically significant difference between obese and nonobese patients was revealed in the self-reported functional ability. DAA-nonobese vs DAA-obese: no statistically significant differences were observed in comparing postoperative outcomes. The DAA similarly benefited both obese and nonobese patients. Hardinge-nonobese vs Hardinge-obese: Hardinge-nonobese reached higher functionality than Hardinge-obese patients.
DAA patients reported less pain, more functionality, and quality-of-life improvements compared to the Hardinge. Moreover, DAA exhibits equivalent postoperative outcomes in obese and nonobese patients, suggesting a better-suited THA surgical approach for patients with increased BMI.
Further research based on well-designed studies with longer follow-up and larger samples need to be performed to elucidate the efficacy of DAA on functionality and quality of life of hip OA obese patients.
The authors would like to thank Dr. Antonios Galanos, Biostatistician of Laboratory of Research of the Musculoskeletal System (LRMS), Faculty of Medicine, National and Kapodistrian University of Athens, Greece, for the statistical analysis of the data.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Orthopedics
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Widmer KH S-Editor: Gong ZM L-Editor: A P-Editor: Xing YX
| 1. | Howell JR, Garbuz DS, Duncan CP. Minimally invasive hip replacement: rationale, applied anatomy, and instrumentation. Orthop Clin North Am. 2004;35:107-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Siddiqui NA, Mohandas P, Muirhead-Allwood S, Nuthall T. A review of minimally invasive hip replacement surgery – current practice and the way forward. Curr Orthop 2005; 19: 247-254. |
| 3. | Bergin PF, Doppelt JD, Kephart CJ, Benke MT, Graeter JH, Holmes AS, Haleem-Smith H, Tuan RS, Unger AS. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93:1392-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Connolly KP, Kamath AF. Direct anterior total hip arthroplasty: Comparative outcomes and contemporary results. World J Orthop. 2016;7:94-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Leunig M, Faas M, von Knoch F, Naal FD. Skin crease 'bikini' incision for anterior approach total hip arthroplasty: surgical technique and preliminary results. Clin Orthop Relat Res. 2013;471:2245-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Nho SJ, Kymes SM, Callaghan JJ, Felson DT. The burden of hip osteoarthritis in the United States: epidemiologic and economic considerations. J Am Acad Orthop Surg. 2013;21 Suppl 1:S1-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38:1134-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 554] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 8. | Andrianakos AA, Kontelis LK, Karamitsos DG, Aslanidis SI, Georgountzos AI, Kaziolas GO, Pantelidou KV, Vafiadou EV, Dantis PC; ESORDIG Study Group. Prevalence of symptomatic knee, hand, and hip osteoarthritis in Greece. The ESORDIG study. J Rheumatol. 2006;33:2507-2513. [PubMed] |
| 9. | Barrett M, Prasad A, Boyce L, Dawson-Bowling S, Achan P, Millington S, Hanna SA. Total hip arthroplasty outcomes in morbidly obese patients: A systematic review. EFORT Open Rev. 2018;3:507-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 407] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 11. | Sadr Azodi O, Adami J, Lindström D, Eriksson KO, Wladis A, Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. 2008;79:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Dowsey MM, Choong PF. Obesity is a major risk factor for prosthetic infection after primary hip arthroplasty. Clin Orthop Relat Res. 2008;466:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 345] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 14. | Chan CL, Villar RN. Obesity and quality of life after primary hip arthroplasty. J Bone Joint Surg Br. 1996;78:78-81. [PubMed] |
| 15. | Moran M, Walmsley P, Gray A, Brenkel IJ. Does body mass index affect the early outcome of primary total hip arthroplasty? J Arthroplasty. 2005;20:866-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | McLaughlin JR, Lee KR. The outcome of total hip replacement in obese and non-obese patients at 10- to 18-years. J Bone Joint Surg Br. 2006;88:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Skutek M, Wirries N, von Lewinski G. Hip Arthroplasty in Obese Patients: Rising Prevalence-Standard Procedures? Orthop Rev (Pavia). 2016;8:6379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | World Medical Association (WMA). Declaration of Helsinki – Ethical Princi-ples for Medical Research Involving Human Subjects. 19th October 2013. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. |
| 19. | Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7:e1000251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 919] [Cited by in RCA: 1151] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 20. | Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;441:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 444] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 21. | Hardinge K. The direct lateral approach to the hip. J Bone Joint Surg Br. 1982;64:17-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 603] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | von Baeyer C, Wood C, Jaaniste T. Instructions for administering the Faces Pain Scale-Revised (FPS-R) in languages other than English. Edition 6. 2009. Available from: http://www.sfap.org/pdf/VIII-I9e-pdf.pdf. |
| 23. | Stuppy DJ. The Faces Pain Scale: reliability and validity with mature adults. Appl Nurs Res. 1998;11:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8741] [Cited by in RCA: 9361] [Article Influence: 275.3] [Reference Citation Analysis (0)] |
| 25. | Stasi S, Papathanasiou G, Diochnou A, Polikreti B, Chalimourdas A, Macheras GA. Modified Harris Hip Score as patient-reported outcome measure in osteoarthritic patients: psychometric properties of the Greek version. Hip Int. 2021;31:516-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Stasi S, Stamou M, Papathanasiou G, Frantzeskaki P, Kanavas E, Evaggelou-Sossidis G, Gouskos A, Palantzas A, Poursanidis K, Macheras GA. International Hip Outcome Tool (12-items) as health-related quality-of-life measure in osteoarthritis: validation of Greek version. J Patient Rep Outcomes. 2020;4:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Herman T, Giladi N, Hausdorff JM. Properties of the 'timed up and go' test: more than meets the eye. Gerontology. 2011;57:203-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 28. | Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 2-year follow-up. Arthroscopy. 2000;16:578-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 457] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 29. | Griffin DR, Parsons N, Mohtadi NG, Safran MR; Multicenter Arthroscopy of the Hip Outcomes Research Network. A short version of the International Hip Outcome Tool (iHOT-12) for use in routine clinical practice. Arthroscopy. 2012;28:611-616; quiz 616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 336] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 30. | Yue C, Kang P, Pei F. Comparison of Direct Anterior and Lateral Approaches in Total Hip Arthroplasty: A Systematic Review and Meta-Analysis (PRISMA). Medicine (Baltimore). 2015;94:e2126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Wang Z, Bao HW, Hou JZ. Direct anterior versus lateral approaches for clinical outcomes after total hip arthroplasty: a meta-analysis. J Orthop Surg Res. 2019;14:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Downing ND, Clark DI, Hutchinson JW, Colclough K, Howard PW. Hip abductor strength following total hip arthroplasty: a prospective comparison of the posterior and lateral approach in 100 patients. Acta Orthop Scand. 2001;72:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Cinnamon CC, Longworth JA, Brunner JH, Chau VK, Ryan CA, Dapiton KR, Chmell SJ, Foucher KC. Static and dynamic abductor function are both associated with physical function 1 to 5 years after total hip arthroplasty. Clin Biomech (Bristol, Avon). 2019;67:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Chee YH, Teoh KH, Sabnis BM, Ballantyne JA, Brenkel IJ. Total hip replacement in morbidly obese patients with osteoarthritis: results of a prospectively matched study. J Bone Joint Surg Br. 2010;92:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | Jameson SS, Mason JM, Baker PN, Elson DW, Deehan DJ, Reed MR. The impact of body mass index on patient reported outcome measures (PROMs) and complications following primary hip arthroplasty. J Arthroplasty. 2014;29:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Antoniadis A, Dimitriou D, Flury A, Wiedmer G, Hasler J, Helmy N. Is Direct Anterior Approach a Credible Option for Severely Obese Patients Undergoing Total Hip Arthroplasty? J Arthroplasty. 2018;33:2535-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Purcell RL, Parks NL, Gargiulo JM, Hamilton WG. Severely Obese Patients Have a Higher Risk of Infection After Direct Anterior Approach Total Hip Arthroplasty. J Arthroplasty. 2016;31:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Russo MW, Macdonell JR, Paulus MC, Keller JM, Zawadsky MW. Increased Complications in Obese Patients Undergoing Direct Anterior Total Hip Arthroplasty. J Arthroplasty. 2015;30:1384-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | Manrique J, Paskey T, Tarabichi M, Restrepo C, Foltz C, Hozack WJ. Total Hip Arthroplasty Through the Direct Anterior Approach Using a Bikini Incision Can Be Safely Performed in Obese Patients. J Arthroplasty. 2019;34:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Moskal JT, Capps SG, Scanelli JA. Anterior muscle sparing approach for total hip arthroplasty. World J Orthop. 2013;4:12-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 41. | Restrepo C, Parvizi J, Pour AE, Hozack WJ. Prospective randomized study of two surgical approaches for total hip arthroplasty. J Arthroplasty. 2010;25:671-679.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 42. | Goebel S, Steinert AF, Schillinger J, Eulert J, Broscheit J, Rudert M, Nöth U. Reduced postoperative pain in total hip arthroplasty after minimal-invasive anterior approach. Int Orthop. 2012;36:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 43. | Ilchmann T, Gersbach S, Zwicky L, Clauss M. Standard Transgluteal versus Minimal Invasive Anterior Approach in hip Arthroplasty: A Prospective, Consecutive Cohort Study. Orthop Rev (Pavia). 2013;5:e31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Mirza AJ, Lombardi AV Jr, Morris MJ, Berend KR. A mini-anterior approach to the hip for total joint replacement: optimising results: improving hip joint replacement outcomes. Bone Joint J. 2014;96-B:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |