Published online Aug 18, 2020. doi: 10.5312/wjo.v11.i8.345
Peer-review started: April 3, 2020
First decision: April 22, 2020
Revised: May 23, 2020
Accepted: July 19, 2020
Article in press: July 19, 2020
Published online: August 18, 2020
Processing time: 132 Days and 11.2 Hours
The ideal lumbar and cervical discs should provide six degrees of freedom and tri-planar (three-dimensional) motion. Although all artificial discs are intended to achieve the same goals, there is considerable heterogeneity in the design of lumbar and cervical implants. The “second generation total disc replacements” are non-articulating viscoelastic implants aiming at the reconstruction of physiologic levels of shock absorption and flexural stiffness. This review aims to give an overview of the available implants detailing the concepts and the functional results experimentally and clinically. These monobloc prostheses raise new challenges concerning the choice of materials for the constitution of the viscoelastic cushion, the connection between the components of the internal structure and the metal endplates and even the bone anchoring mode. New objectives concerning the quality of movement and mobility control must be defined.
Core tip: Although all artificial discs are intended to achieve the same aims, there is considerable heterogeneity in the design of lumbar and cervical implants. The “second generation total disc replacements” are non-articulating viscoelastic implants aiming at the reconstruction of physiologic levels of shock absorption and flexural stiffness. This review aims to give an overview of the available implants. We herein discuss the new challenges concerning the choice of materials, the connection between the components of the internal structure and the metal endplates and even the bone anchoring mode. We discuss the consequences of the different technological choices and we emphasize the special features to watch out for when monitoring these implants in the future.
- Citation: Lazennec JY. Lumbar and cervical viscoelastic disc replacement: Concepts and current experience. World J Orthop 2020; 11(8): 345-356
- URL: https://www.wjgnet.com/2218-5836/full/v11/i8/345.htm
- DOI: https://dx.doi.org/10.5312/wjo.v11.i8.345
Total disc replacement (TDR) has been advocated as an alternative to spinal fusion to preserve or restore segmental motion and to prevent or delay adjacent level disc degeneration after fusion. A large amount of data in the literature report favorable clinical and radiological outcomes of TDR surgery but there are still many possible improvements on the mechanical and functional performance of the implants[1-5]. Although all artificial discs are intended to achieve the same aims, there is considerable heterogeneity in the design of lumbar and cervical implants. The natural disc is viscoelastic as it allows for variation in the degree of stiffness with the amount of load, and is compliant under loading (shock absorber).
The ideal lumbar and cervical discs should provide six degrees of freedom (DOF) and tri-planar (three-dimensional) motion: Flexion and extension in sagittal plane, lateral bending in frontal plane, rotation and compression in axial plane.
Artificial discs can be classified according to their structure (uni- and bi-articulating or non-articulating) or to their motion characteristics (constrained or unconstrained). The “first generation” TDR were “mechanical implants” based on the experience of hip and knee replacement. These articulating designs involve a mechanical interface with a specific friction couple but do not replicate the elasticity and the shock absorbance of the native disc.
The “second generation TDR” are non-articulating disc implants (elastomeric, deformable core). Their structure is more complex with the objective of better reconstructing the physiologic levels of shock absorption and flexural stiffness.
These monobloc implants raise new challenges concerning the choice of materials for the constitution of the viscoelastic cushion, the connection between the components of the internal structure and the metal endplates and even the bone anchoring mode. New objectives concerning the quality of movement and mobility control must be defined.
At the lumbar level 3 “viscoelastic” TDR available for implantations have a significant follow up.
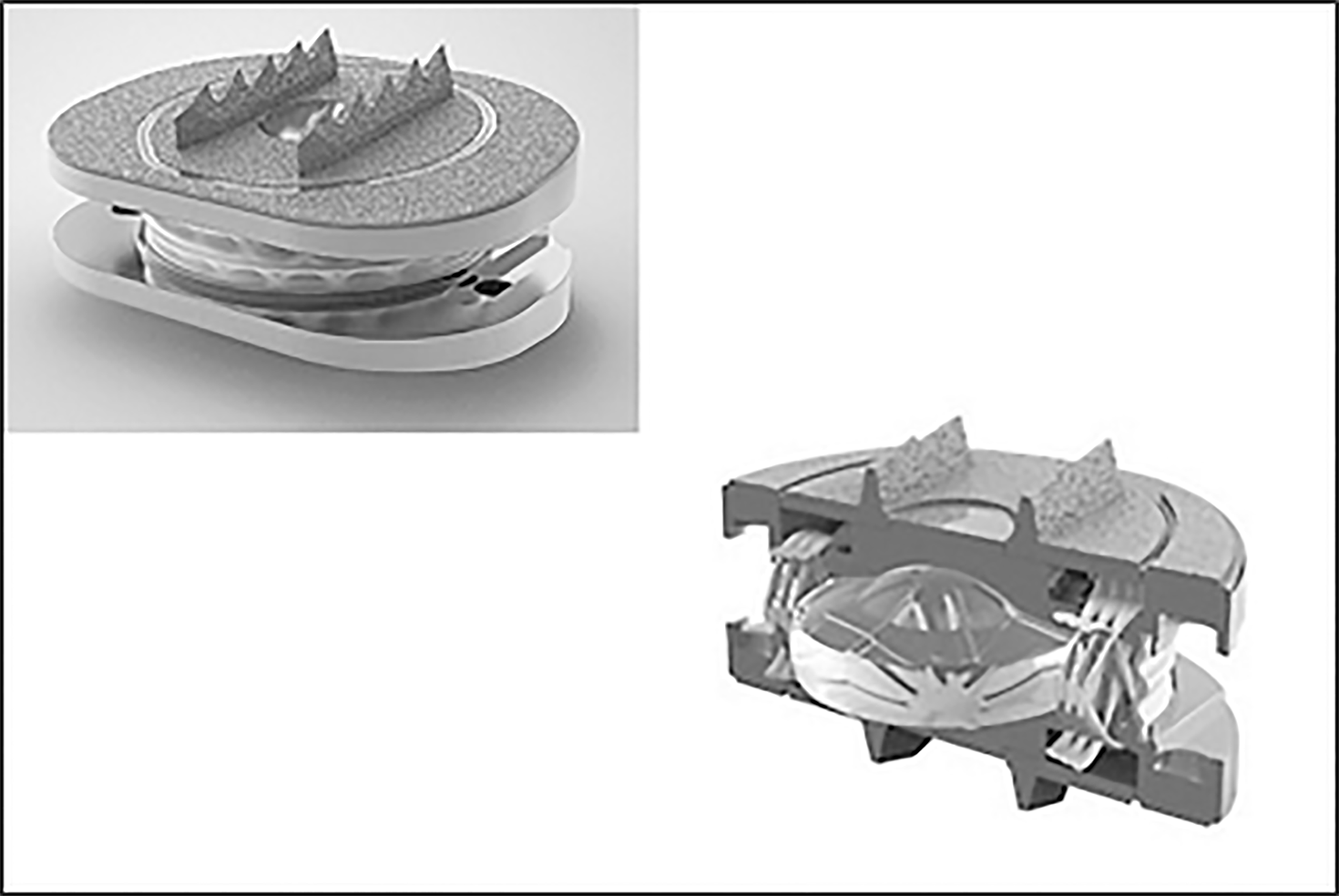
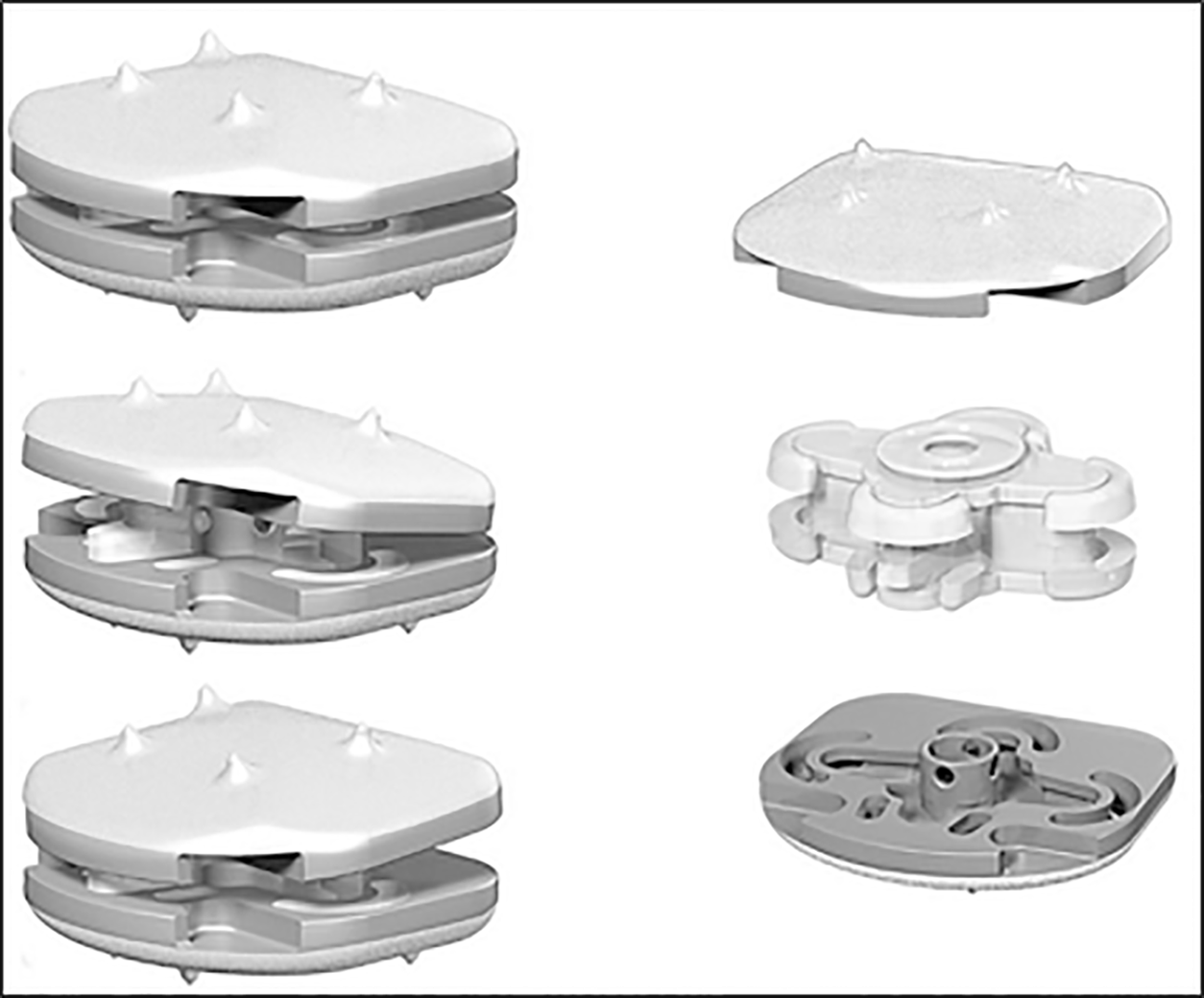
Freedom lumbar disc (FLD) is a one-piece implant consisting of an elastomeric core bonded to titanium alloy Ti-6Al-4V endplates. The core material is CarboSil™ TSPU, a silicone polycarbonate urethane (PCU) thermoplastic elastomer. A urethane adhesive is used at the interface. The implant is available in 8° and 12° lordotic configurations with 3 footprints (Figure 1).
Wear testing[6,7] reported that implants lost an average of 0.07 g weight over 30 million device cycles of wear testing. The parts lost approximately 1/4 of a millimeter in height over 30 million cycles, while the periphery dimension increased by about 3/4 of a millimeter. FLD was first implanted in 2009[8].
The device combines a mobile polymer core between 2 metal endplates to simulate the structure of the nucleus and peripheral fibers designed to simulate the annulus. The titanium alloy endplates are coated in titanium plasma spray. Keels are used to secure the implant. The disc also has a PCU polymer sheath surrounding the core and fiber construct to avoid tissue in growth and the migration of wear debris. The M6-L is available in lordotic configurations of 3°, 6°, 10°, 16° with two implant heights, 10 mm and 12 mm and 3 footprints (Figure 2).
Wear testing report[9] can be found for 20 million cycles. Mass loss was 16.9 ± 12.0 mg. An average of an additional 25.9 mg of debris were generated and trapped inside the inside the device. Total device debris were therefore less than 43 mg. M6-L was first implanted in 2009[10].
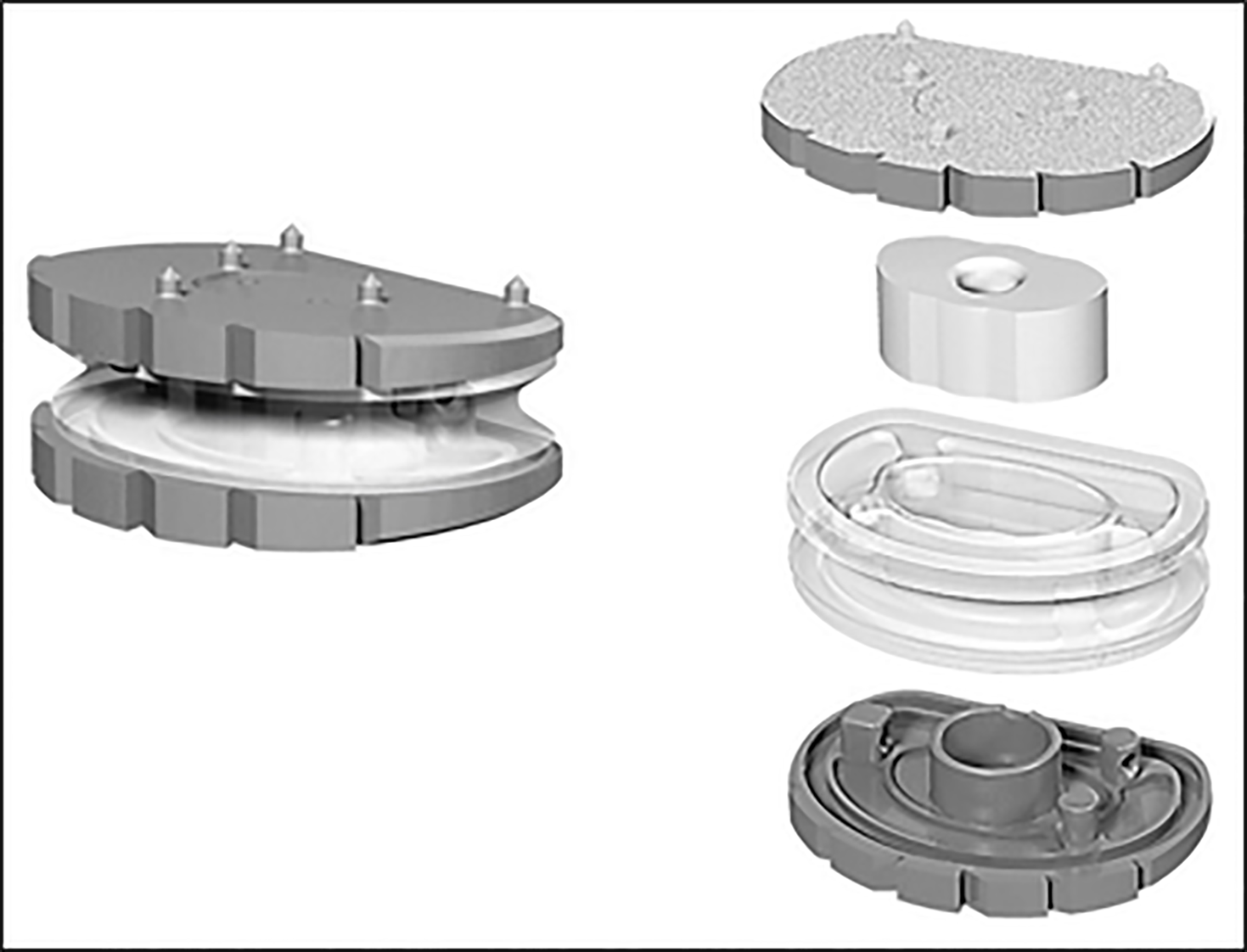
LP ESP is a one-piece deformable implant with a composite cushion combining an inner core made with a silicone nucleus, and an outer core made with a PCU annulus. The peripheral core (figuring the annulus) is securely fixed to the titanium alloy endplates by a patented adhesion-molding technology reinforced by a peripheral groove. This fixation process avoids fluid infiltration and the risk of fatigue lesions of the interface despite the very different mechanical properties of polymer and metal endplates. The PCU annulus is stabilized for rotational mobility by additional pegs located on the internal surface of the titanium endplates between the peripheral groove and the central area of the endplates. The PCU cushion is stabilized by ‘‘male’’ and ‘‘female’’ contactless inner pegs to control compression and translation.
Endplates have anchoring pegs for primary fixation and are covered by a textured T40 titanium layer and hydroxyapatite. Depending on the size, the titanium endplates differ in thickness and angulation. LP-ESP is available in 2 heights (10 and 12 mm), each with 3 lordosis angles (7°, 9°, and 11°). The differences in thickness and lordotic angle do not affect the implant mobility or its cushioning (Figure 3).
Wear tests were performed to reproduce the most unfavorable scenario for TDR implantation. Inclination of the prosthesis was 45° to reproduce the sagittal orientation of the disk in functional situations and extended to 40 million cycles without any sign of mechanical failure (no loss of cohesion and stiffness remained stable). Residual gap between metal endplates was 0.55 mm after 20 million cycles and 0.78 mm after 40 million cycles. Reported loss of mass after 20 million cycles was less than 0.5% (slight degradation of the endplates coating). LP ESP was first implanted in 2005 in its actual configuration[11].
Physio L prosthesis is a one-piece device with over molded elastomeric polycarbonate-urethane core attached (titanium lock wire) to 2 titanium alloy endplates coated with titanium beads. The prosthesis was implanted for the first time in 2005[12] but to our best knowledge is no longer made available by the company.
At the cervical level 4 prostheses claim viscoelastic properties.
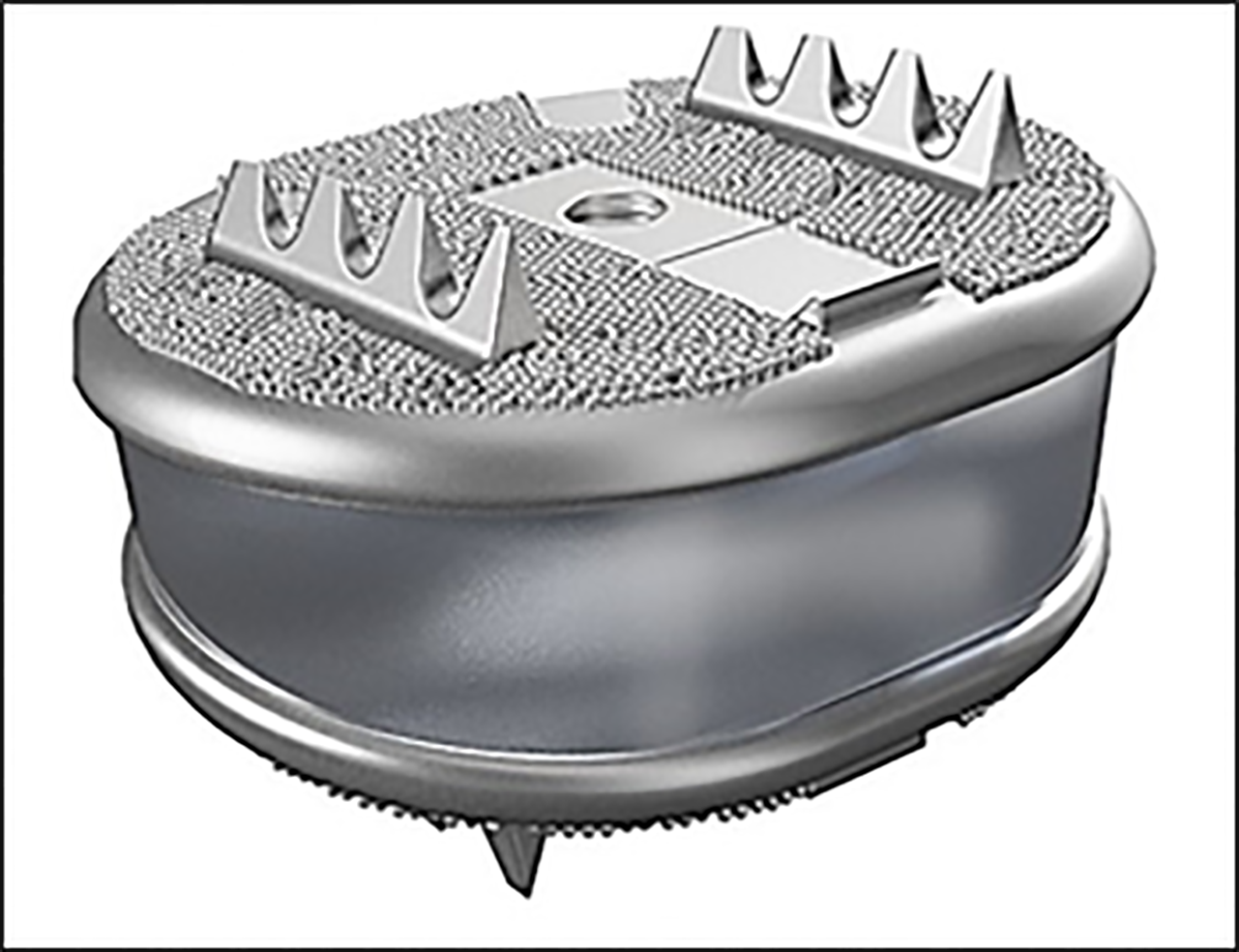
Freedom cervical disc (FCD) is a one-piece viscoelastic implant. The elastomeric core (CarboSil polymer) is bonded to titanium alloy endplate plates with the same design as the FLD. FCD is available with 8° lordosis in 3 footprints with different corresponding posterior heights (5.7/6.7 mm; 5.8/6.8 mm; 5.9/6.9 mm). (Figure 4) Mechanical tests were done with 45º compressive shear loading to provide maximum AP translation of the cushion and therefore a severe testing of the polymer and bond. Two implants were tested and survived 50 million cycles at twice the average daily living load (200 N) with no mechanical or functional failures. One device completed 10 million cycles with no functional failures at 800 N, and another at 1250 N. The dynamic stiffness of each test specimen remained constant at the supra-physiologic loads of 800 and 2150 N.
Particulate analysis showed that the wear rate was 0.028 mg/million cycles, with 0.016 mg being CarboSil polymer and 0.012 g being titanium[13]. FCD was first implanted in 2012[14].
The mobile central PCU polymeric core figuring the nucleus is surrounded by a polyethylene woven-fiber construct designed to simulate the annulus. The concept is the same as for the M6-L implant. M6-C is available in two implant heights, 6 mm and 7 mm and 4 footprints (Figure 5).
Mechanical tests[15] reported 20 million cycles (10M cycles lateral bending, 10M cycles flexion/extension) without implants lesions. Average height loss under a physiologic 100N axial compressive load was 0.47 ± 0.05 mm. Axial compressive stiffness was 873 ± 168 N/mm. After 10 million cycles for compression shear load at 45°, all implants were intact including physiologic height (< 0.25 mm height loss) and stiffness (761 ± 200 N/mm). To our best knowledge wear rate data are not available.
The CP ESP is a one-piece deformable implant including a central core made of PCU fixed to titanium endplates covered by a textured T 40 titanium layer and hydroxyapatite. The design is an adaptation of the concept of the LP ESP prosthesis to the specifics of the cervical spine. The cushion fixation to the endplates is similar using adhesion molding and a peripheral groove to prevent fluid infiltration and fatigue fractures of the interface. The specific shape of the cushion is designed to optimize the mobility as well as the control of translation and shear movements. The PCU cushion is stabilized by ‘‘male’’ and ‘‘female’’ contactless inner pegs to control compression and translation.
CP-ESP is available in three thicknesses (5, 6 and 7 mm), each with three sizes in AP and lateral dimensions (13 mm × 15 mm, 14 mm × 17 mm and 15 mm × 20 mm). The size of the cushion and the mechanical properties of the prosthesis are the same regardless of the model (Figure 6). CP-ESP was first implanted in 2012[18].
Wear tests were conducted according to the ISO 18192 Norm with different configurations for the location of the rotation centers to simulate the worst-case scenarios. According to the different protocols, the loss of height ranged from 0.02 to 0.12 mm after 10 million cycles. The mean variation of weight was 0.8 mg for 10 million cycles. The alignment of the two endplates has been studied to assess the cohesion of the cushion and metal parts. After 10 million cycles, the variation was insignificant (0.2 mm for AP and lateral measurements).
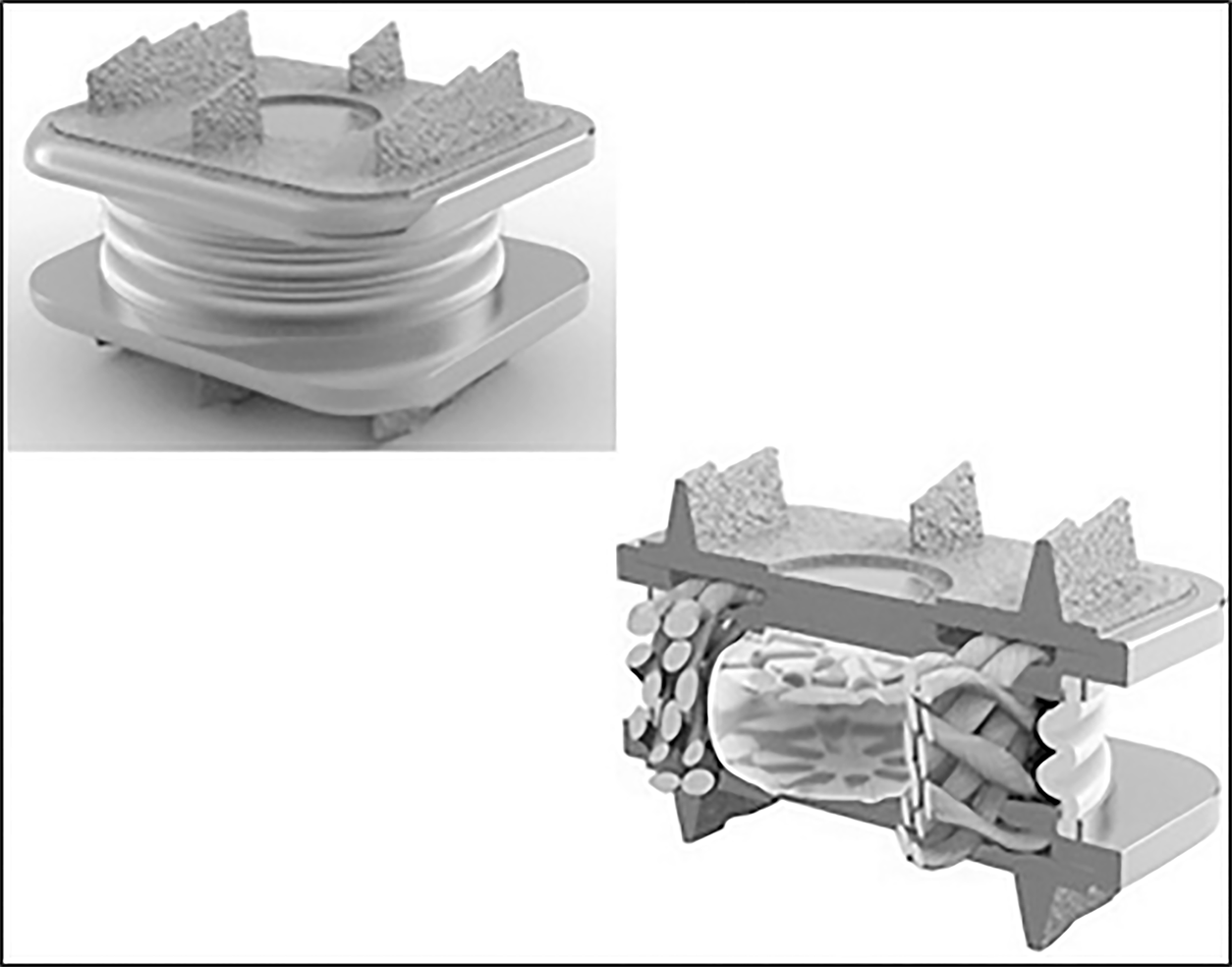
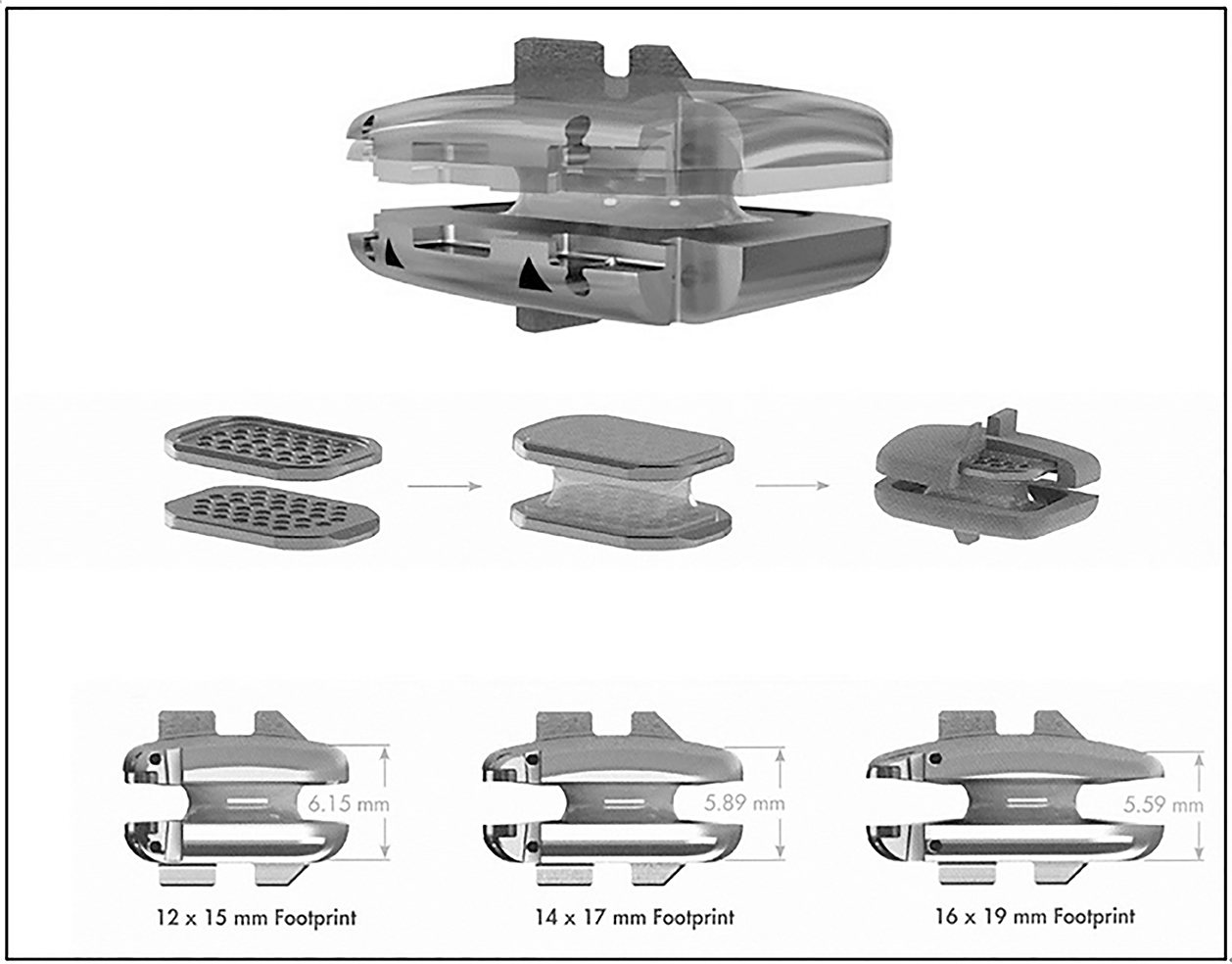
The implant is designed as a one-piece compressible polymer core with dome-shaped, plasma-coated endplates and a central-split keel (Figure 7).
The core is bonded to 2 perforated metal plates. This component is secured to the prosthesis endplates by locking wires. The molding technology claims for a “reduction in wear between the polymer core and metal endplates." The design is an adaptation of the concept of the Physio L prosthesis[19].
The Rhine cervical disc is available in three sizes 12 mm x 15 mm, 14 mm x 17 mm, 16 mm x 19 mm footprint. The thickness of the implants depends on the size of the implants respectively. For the “6 mm implants group” the anterior leading-edge height (without the superior dome) is respectively 6.15, 5.89 and 5.59 mm. For the “7 mm implants” all values are increased by 1 mm.
Wear tests according to ISO 18191 and 18192 Norms reported 11 Million cycles with no function failures: The height loss was 0.11 mm, representing only 1.89% of the original height. The weight loss was 0.056 mg/million cycles representing only 0.08% of original weight after correction for fluid absorption. Additional characterization testing completed 25 Million cycles without functional failure. Dynamic tests were performed for compression load (150 N) shear load (106 N) on 10MC cycles without wear or failure after inspection. The prosthesis was first implanted in 2016.
Literature of first-generation lumbar prostheses reports early anterior migration following hyperextension motion with or without sub-optimally positioned prosthesis. Some authors suggested the role of an inadequate release of the posterior longitudinal ligament potentiated by an anterior positioning of the implant, explaining that the compressive loads make the prostheses more sensitive to anterior migration[20,21].
The second-generation viscoelastic prostheses are likely to present the same risk. Metal endplates are a key component for the segmental stabilization. Titanium alloy is used for all 3 designs as its osteoconductive property is advocated to enhance adhesion with the adjacent vertebra. In one TDR system (LP ESP) additional hydroxy apatite (HA) coating is used. This technology required additional evaluations to demonstrate that temperature increase at the time of industrial coating did not negatively affect the PCU cushion. To date no adverse effects from this coating have been reported which is consistent with previous long-term experience for HA coating in cementless total hip arthroplasty (THA). Additional spikes or keels on the endplate are used to improve initial stabilization. As an analogy with the experience for cementless THA these features are involved in the “primary fixation” stage. Secondary fixation is devoted to the surface characteristics of the endplates for the final bone anchoring. Implant fixation can be considered in two ways “biomechanical” and “histological”.
Primary stability is a biomechanical phenomenon related to bone quality at the implant site, to the implant properties and to the surgical technique for vertebral endplate preparation and implant size selection. Primary stability is a necessary condition to obtain implant osseointegration. The endplates must be carefully prepared to remove all soft tissue and give a surface of congruent contact with the implant to favor bone regrowth. The implantation principles are the same as for cementless knee or hip prostheses[22]. The initial bone healing after surgery involves the production of new bone to fill the gap between mature bone tissue and the implant surface. Low level micromotion between the implant and the bone tissue is considered to be responsible for biomechanical stimulation of bone turnover. When primary stability is insufficient or in the event of spinal overuse immediately after surgery, micro-movements can lead to the formation of fibrous tissue instead of an osteointegrated interface. The literature reports that the relative micromotions between the implant and the bone tissue should not exceed approximately 150 µm[23]. During weeks or months after the implant surgery, mature bone tissue replaces the newly formed bone at the implant surface, leading to the final bone–implant contact ratio[24]. This global process may fail if the gaps are too large or impossible to fill due to soft tissue interpositions. This can lead to early migration of fluid accumulation with cysts formation under the metal endplates[23]. The "biological" stage of fixation requires special attention for 6 to 8 weeks as with any cementless implantation.
The biomechanical conditions of viscoelastic implants are significantly different from those of articulated TDR. Stresses on the vertebral endplates are greater at the primary stage of fixation due to flexural stiffness with these one-piece implants and expose to two potential concerns: Subsidence and translation.
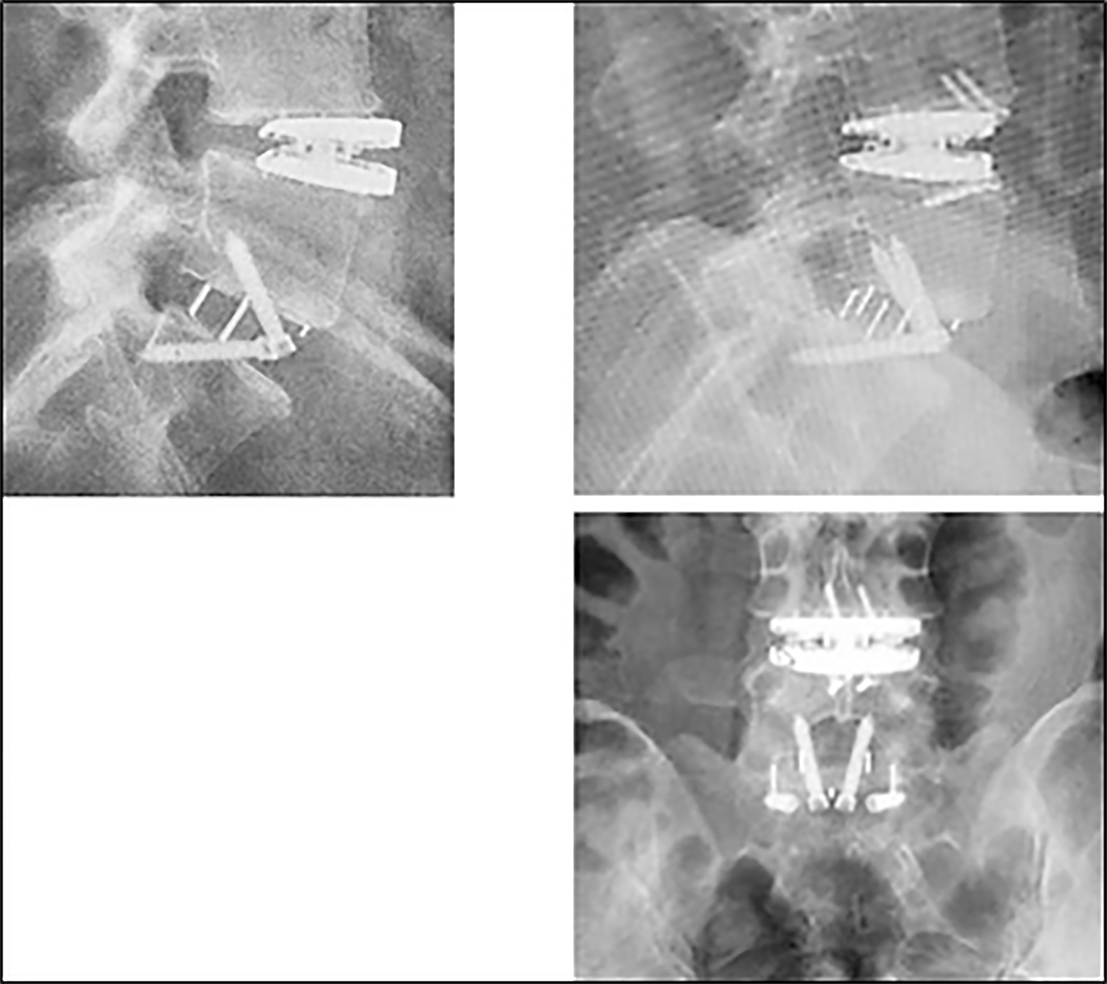
Flexural rigidity is a characteristic of each viscoelastic implants. It evolves after implantation (local temperature and hydratation). It also depends on the implants design and the integrated restrictions for shear and translation as for the LP ESP prosthesis. Thus for this last implant, additional compression of the bone interface has been suggested for optimizing this primary phase of integration of the metal plates (“kyphosing” the implanted segment at the end of the intervention by “delordosis” on the operating table or elevation of the legs). In all cases, the posterior part of the disc space must be thoroughly cleaned to place the implant in an adequate posterior position so as to avoid the risk of anterior slippage (Figure 8). In a way, the implantation method is close to that of a classic interbody cage since the implant is monobloc. The sagittal introduction of the implant must be done strictly in the axis of the intervertebral space to avoid an impaction in one of the vertebral endplates which can cause an unexpected locking or secondary sagittal translation. The two metal plates must be introduced in a perfectly coherent way to avoid any anteroposterior shift of one relative to the other which can damage the viscoelastic component.
The first difficulty is solving the difficulty of attaching the elastomeric part of the implant to the metallic endplates. The interface must be fatigue resistant for an optimal adaptation to the damping spring system claimed by the viscoelastic disc concepts. Two types of implants must be individualized according to the mode of connection with or without local mobility at the metal endplates interface.
The M6-L attachment of the biomechanically active component is based on an “over molding process” called PPA (Perforated Plate Attachment). The M6 compressible PCU polymeric core is surrounded by a high–tensile strength, ultrahigh-molecular weight polyethylene fibers construct. The fibers are designed to simulate the annulus and to provide a progressive resistance to the motion of the polymer nucleus. Movement between the endplates and the biomechanically active component is not suppressed as friction occurs between the fibers wound and the endplates. The mobility of the nucleus provides the adaptability and the self-centering property claimed by this design. The M6-L has a polymer sheath encasing the core and fiber construct to capture potential wear debris. Favorable biomechanical tests have been reported regarding motion quality assessed by load–displacement curves in flexion–extension. Encouraging clinical results have been reported on 83 patients at 24 months follow-up[10].
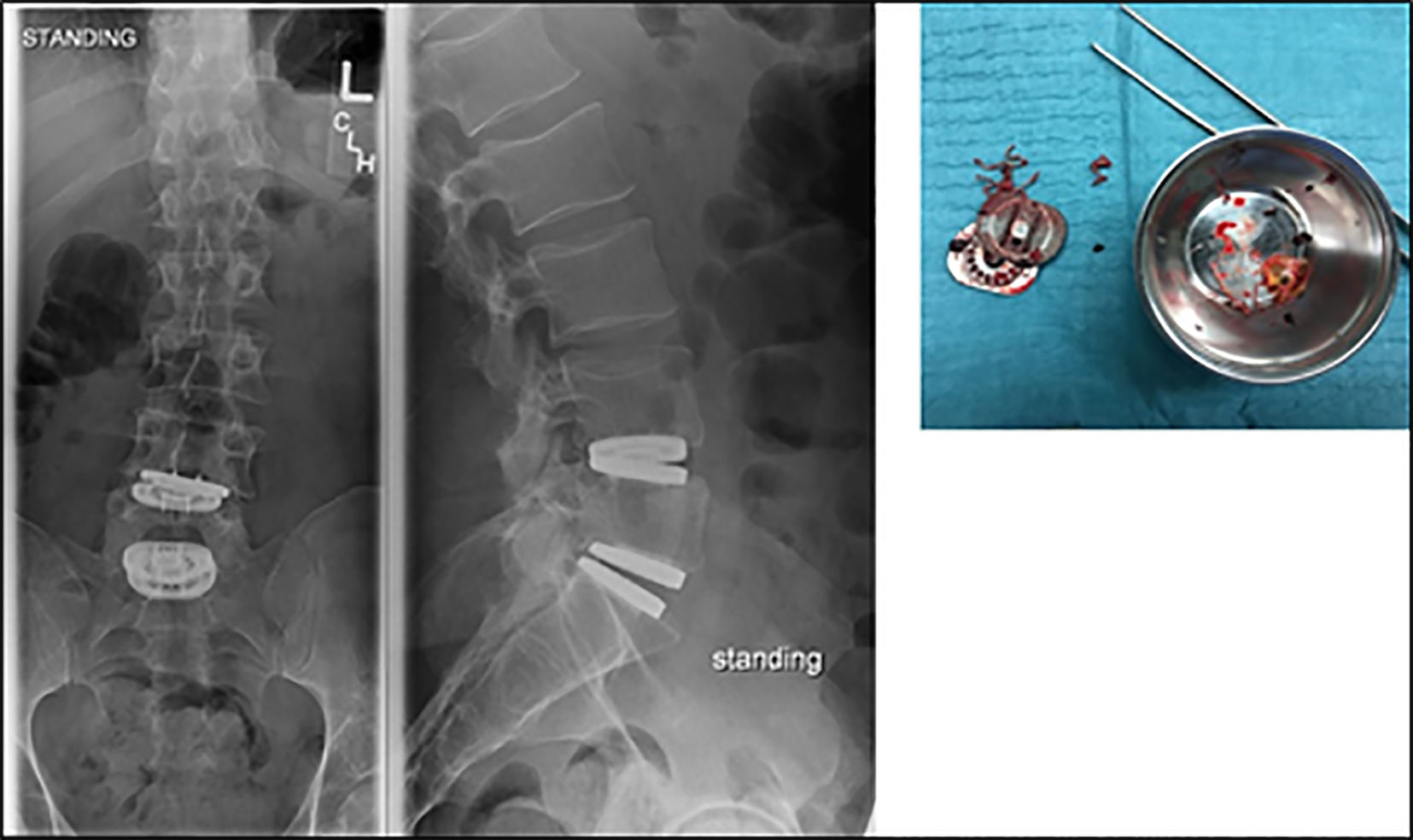
The potential weakness of this design is represented by the friction of the fibers on the metal plates, a potential source of breakage or wear products. The risk is greater if the implant is facing atypical conditions of rotation or inclination in the frontal or sagittal plane. Another mode of failure can be the blocking of the core in flexion or in extension causing a limitation of mobility or abnormal stresses of the peripheral structure with lesions of the polyethylene fibers construct, risk of nucleus expulsion and direct endplates contact with metallosis (Figure 9).
The 2 other implants claim a direct and stable fixation of the viscoelastic component on the metal endplates. But the concepts of fixation and the structures of the viscoelastic cushion are very different.
The FLD monoblock elastomeric core is “bonded” to titanium retaining plates. But few informations are available about the precise adhesion process of this “bonding” technology, the influence of the cushion thickness on mobility properties and fatigue characteristics of the viscoelastic core structure and its endplate fixation. Encouraging clinical results have been reported on 48 patients when compared to ALIF procedure at 1.3 years follow-up[7]. To our best knowledge no publication provides results with longer follow-up.
LP ESP disc prosthesis design is fundamentally different. The cushion is a composite structure with a PCU annulus and a silicone nucleus. To overcome the problems posed by the risk of hypermobility and fatigue damage in the event of a pure elastomeric component[25], movement limiters are included in the structure to control translation and axial compression. The location of the 3 peripheral plots in the annulus is intended for shear control. Significant inclination of the endplates can be observed in the event of high pelvic incidence or frontal obliquity of the sacrum. 45º compressive shear has been studied as an extreme loading scenario which translates the top of the device over the bottom and places the anterior and posterior sides of the cushion into tension. In addition, male and female central metal limiters contribute to shear and compression control. The combination of central and peripheral limiters claims better control of the coupling of the 6 functional 6 DOFs[26]. Whatever the size of the implants used, the cushion is exactly the same with the same mechanical characteristics which avoids heterogeneity in possible mobility. The fixation to the endplate is based on a patented adhesion molding technology without any chemical adhesion process.
Current experience with 15 years follow-up has not shown any particular weakness of the design in terms of detachment of the viscoelastic cushion, fatigue rupture or particles release. On the other hand, this type of implant exposes to more problems for primary stability and initial bone ingrowth due to the greater flexural stiffness chosen to protect the facet joints. Above all, excellent preparation of implantation sites is essential and more demanding than for other implants. Post-operative follow-up for the first weeks include caution on authorized activity to avoid the risks of migration and encourage rehabitation of the endplates. The determination of the centers of rotation reported a different location depending on the implanted levels. This is a promising result which seems to show the adaptability of a viscoelastic cushion according to the level and the anatomical and postural particularities[27].
The same observations can be made on the preparation of the bony implantation site as flexural stiffness of these monobloc implants differentiates them from the first-generation articulated implants.
The size of the implant must be selected carefully to avoid subsidence. Over-distraction of disc space increases the load on facet joints and can cause postoperative neck pain or limited motion within the artificial disc. In case with too loose disc space or too low-height implant, suboptimal device function or migration may occur. Adaptation of the implantation site may be challenging in some cases as the vertebral endplates can be flat or concave. Aggressive bone preparation can be unfavorable with a risk of heterotopic ossifications (HO). The motion characteristics of the different designs, the extent and quality of end-plate coverage, an inadequate position of the center of rotation or a kyphotic adaptation may be significant factors.
These are also questions raised for the evaluation of HO occurrence[28]. To date HO risk is difficult to precise as the length of follow-up is a key point[29]. The use of plain radiographs in the literature may underestimate the rate and grade of HO. In addition, some surgeons use post operatively non-steroidal anti-inflammatory medications to prevent HO formation despite the lack of evidence for this strategy. The published rate ranges from 0 at 29 months follow-up (25 patients)[30], or at 34 months (43 patients)[16] to 15.1% McAfee-Suchomel grade I and 10.7% grade II in (112 patients, 36 months follow-up)[31], or 29% no HO 13% grade I and 58% grade II or more[32]. As for lumbar levels, the implantation must avoid any anteroposterior shift of one metal endplate relative to the other which can damage the viscoelastic component. Due to its miniaturization the elastomeric component is more sensitive to parasitic translation of the endplates linked to the operating technique.
As for lumbar implants, 2 types of implants must be individualized according to the mode of connection with or without local mobility at the interface.
The design of M6-C Cervical Disc Prosthesis is similar to the one of M6-L with a mobile central core and peripheral ultrahigh-molecular weight polyethylene fibers for stabilization. The same observations and comments can be done especially for the potential over solicitation of the implant in case of increased sagittal inclination of vertebral endplates and high rotational mobility. As at the lumbar level the weak point may be linked to the friction of the polyethylene fibers on the metal endplates with granulomatous reaction or the unexpected locking of the mobile nucleus[33,34].
The other 3 implants claim a direct and stable fixation of the viscoelastic component on the metal endplates. The FCD and the Rhine cervical disc (RCD) have an homogeneous elastic core. The influence of the height of the core on mobility must be carefully monitored on the long term as the available range of motion could be affected. This point should be followed carefully on multi-level implantations. In addition, the original endplates design of FCD implies an 8° lordosis in the metal endplates, the impact of which is unknown in the event of cervical kyphosis posture. Surprisingly, the impact of cervical posture is not analyzed for cervical implants despite normal cervical spinal alignment may vary from lordosis to neutral or to kyphosis[35,36]. Due to variations in sagittal posture, the "neutral position" of the disc prostheses when the gaze is horizontal may involve more kyphosis or lordosis inside the mechanically active cushion. This phenomenon may have different consequences according to the endplate’s designs.
The CP ESP design is based on the experience with the LP ESP implant. The viscoelastic core is one piece but not homogeneous: a mobility limiter is positioned in the center of the cushion to control translation and compression. In addition, the shape of the cushion is asymmetrical to control rotation and translation. These two particularities are intended to avoid the implant kyphosis described for the Bryan type viscoelastic prostheses and potential asymmetry of sagittal ROM in case of kyphotic spine. Like for the LP ESP implant, the combination of central limiter and the asymmetric shape of the cushion claims better control of the coupling of the 6 functional 6 DOFs[26]. Due to the small size of the cushion and its axial limiter, the introduction of this implant must be very rigorous to avoid the relative sliding of the plates and the lesion of the central stabilizer. Like RCD implant CP ESP does not provide intrinsic lordosis in the endplates.
The long-term adaptability of the specific viscoelastic cushion according to the level and the anatomical and postural particularities must be confirmed.
The restitution of the amplitude of movement (ROM) for the flexion- extension is similar to that of the first-generation implants. But it is not the only relevant parameter to characterize the functional quality of these implants. The location of the axis of rotation is another clinically relevant measure of quality of motion for flexion–extension, lateral bending, and axial rotation. These implants claim adaptability of the sagittal axis of rotation according to the disc levels. This could be a relevant argument to advocate for the protection of adjacent levels. One experimental study on the RCD prosthesis[19] showed a fair restitution for centers of rotation on 2 specimens for C5-C6 and C6-C7 levels.
Another experimental study on M6-C[37] reported the location of C5-C6 COR before and after implantation on 12 specimens for flexion–extension mode. The location of the COR for the implanted segments was posterior to the midpoint of the C6 superior endplate (similar to vivo data reported in the literature) but more cranial than intact controls. Currently, there is insufficient evidence to assess the long-term effects of a more cranial COR location. In addition, no information was available for the other segments
A recent study on the evolution of centers of rotation in vivo for CP ESP implants at 2 years of follow-up[38] reports an adaptability of the mean center of rotation at the prosthesis level and adjacent disks as a function of time and posture of the cervical spine.
The restoration of lateral bending motion and motion coupling is also a relevant parameter for the functional quality of cervical disc prostheses. This can also be a determining factor in the longevity of viscoelastic implants depending on the shape and characteristics of the mechanically active cushion. As for the first-generation disc protheses, longer follow-up is needed to understand and interpret the effects of coupled motions on the PCU component and its interface with metal endplates.
Aseptic loosening related to debris deposit in the area surrounding the prosthesis could also be a problem affecting the implant survival curve. These prostheses are supposed to avoid any friction with the exception of M6 implants (mobility of the nucleus and contact of the peripheral fibers of the annulus with the metal plates). A longer follow-up is necessary to accurately assess the rate of aseptic loosening and the possibility of osteolysis due to wear or deterioration of the mechanically active cushion.
Anterior bone loss (ABL) in cervical disc arthoplasties (CDA) has been reported for first-generation implants[39,40]. According to the literature, osteolysis evolution is not consistent with wear debris or low-grade infection. For Kieser et al[41] ABL is common (57.1% of CDA), occurs within 3 months and remains stable after the first year. Osteolysis only affects the not loaded bone. It could be linked to the resection of the anterior longitudinal ligament and likely caused by avascular necrosis of the anterior subchondral bone. ABL could be expected in viscoelastic CDA as this bone remodeling does not seem related to the implant.
The kinematics of the lumbar and cervical discs is defined by six mechanically DOFs. The multi-axial mechanics of the discs and the coupling between the DOFs is a relevant argument in favor of viscoelastic disc prostheses. The current viscoelastic designs using PCU passed extensive fatigue and endurance bending tests with a special focus on creep deformations and hysteresis properties. They are resistant to tearing, corrosion and wear. The follow-up is not the same for all implants but the longest clinical and radiological evaluations show that they are biomechanically compatible with natural movements without significant problems of loss of adhesion or osteolytic response to particles of debris. The use of first-generation mechanical prostheses accustomed us to consider the quality of disc reconstruction in terms of the range of motion at the operated and adjacent levels.
The development of “second-generation” viscoelastic implants offers new possibilities to better restore the disc function by providing damping capacity, flexural stiffness and coupled movements at the implanted level.
These new concepts must make us go beyond the description of the amplitude of segmental mobility in degrees. We now need to think in terms of stiffness (Nm/deg) for flexion-extension or inclination. The tolerance and/or the reconstitution capacity for the coupled movements may make the difference between the implants currently available and for the protection of the adjacent levels.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang X S-Editor: Ma YJ L-Editor: A E-Editor: Xing YX
| 1. | Formica M, Divano S, Cavagnaro L, Basso M, Zanirato A, Formica C, Felli L. Lumbar total disc arthroplasty: outdated surgery or here to stay procedure? A systematic review of current literature. J Orthop Traumatol. 2017;18:197-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 2. | Othman YA, Verma R, Qureshi SA. Artificial disc replacement in spine surgery. Ann Transl Med. 2019;7:S170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (2)] |
| 3. | Abi-Hanna D, Kerferd J, Phan K, Rao P, Mobbs R. Lumbar Disk Arthroplasty for Degenerative Disk Disease: Literature Review. World Neurosurg. 2018;109:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Uschold TD, Fusco D, Germain R, Tumialan LM, Chang SW. Cervical and lumbar spinal arthroplasty: clinical review. AJNR Am J Neuroradiol. 2012;33:1631-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 5. | Frost BA, Camarero-Espinosa S, Foster EJ. Materials for the Spine: Anatomy, Problems, and Solutions. Materials (Basel). 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | AxioMed. White Paper: Fatigue Characterization of the Freedom®Lumbar Disc. Available from: http://www.axiomed.com/pdf/WPaperFatigueCharF.pdf. |
| 7. | AxioMed. White Paper: Freedom® Lumbar Disc Wear Testing and Evaluation of Wear Debris in an Animal Model. Available from: http://www.axiomed.com/pdf/WPaperWearTestingF.pdf. |
| 8. | Rischke B, Zimmers KB, Smith E. Viscoelastic Disc Arthroplasty Provides Superior Back and Leg Pain Relief in Patients with Lumbar Disc Degeneration Compared to Anterior Lumbar Interbody Fusion. Int J Spine Surg. 2015;9:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Spinal Kinetics, Inc. White Paper: Wear debris analysis of the m6-l artificial lumbar disc. Available from: http://www.spinalkinetics.com/wp-content/themes/sk/resources/M034-Rev-1-M6-L-Wear-Debris.pdf. |
| 10. | Schätz C, Ritter-Lang K, Gössel L, Dreßler N. Comparison of Single-Level and Multiple-Level Outcomes of Total Disc Arthroplasty: 24-Month Results. Int J Spine Surg. 2015;9:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Lazennec JY, Aaron A, Brusson A, Rakover JP, Rousseau MA. The LP-ESP(®) lumbar disc prosthesis with 6 degrees of freedom: development and 7 years of clinical experience. Eur J Orthop Surg Traumatol. 2013;23:131-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Pimenta L, Marchi L, Oliveira L, Nogueira-Neto J, Coutinho E, Amaral R. Elastomeric Lumbar Total Disc Replacement: Clinical and Radiological Results With Minimum 84 Months Follow-Up. Int J Spine Surg. 2018;12:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | AxioMed. White Paper: Durability Characterization of the Freedom® Cervical Disc. Available from: https://www.axiomed.com/pdf/WPaperDurabilityFCD.pdf. |
| 14. | Chin KR, Lubinski JR, Zimmers KB, Sands BE, Pencle F. Clinical experience and two-year follow-up with a one-piece viscoelastic cervical total disc replacement. J Spine Surg. 2017;3:630-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Spinal Kinetics, Inc. White Paper: Available from: http://mst.ru/publications/eng/m6/M008%20M6-C%20Mechanical Characterization%20Compendium%20Piece.pdf. |
| 16. | Oltulu İ, Korkmaz Ö, Sarıoğlu E, Aydoğan M. A Retrospective Review of Radiographic and Clinical Findings from the M6 Cervical Prosthesis. Asian Spine J. 2019;13:913-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Lauryssen C, Coric D, Dimmig T, Musante D, Ohnmeiss DD, Stubbs HA. Cervical total disc replacement using a novel compressible prosthesis: Results from a prospective Food and Drug Administration-regulated feasibility study with 24-month follow-up. Int J Spine Surg. 2012;6:71-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Lazennec JY, Aaron A, Ricart O, Rakover JP. The innovative viscoelastic CP ESP cervical disk prosthesis with six degrees of freedom: biomechanical concepts, development program and preliminary clinical experience. Eur J Orthop Surg Traumatol. 2016;26:9-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Guyer RD, Voronov LI, Havey RM, Khayatzadeh S, Carandang G, Blank KR, Werner S, Rubin J, Padovani N, Patwardhan AG. Kinematic assessment of an elastic-core cervical disc prosthesis in one and two-level constructs. JOR Spine. 2018;1:e1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Stieber JR, Donald GD. Early failure of lumbar disc replacement: case report and review of the literature. J Spinal Disord Tech. 2006;19:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Mobbs RJ, Li JXJ, Phan K. Anterior Longitudinal Ligament Reconstruction to Reduce Hypermobility of Cervical and Lumbar Disc Arthroplasty. Asian Spine J. 2017;11:943-950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Viceconti M, Monti L, Muccini R, Bernakiewicz M, Toni A. Even a thin layer of soft tissue may compromise the primary stability of cementless hip stems. Clin Biomech (Bristol, Avon). 2001;16:765-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Raffa ML, Nguyen VH, Haiat G. Micromechanical modeling of the contact stiffness of an osseointegrated bone-implant interface. Biomed Eng Online. 2019;18:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Gao X, Fraulob M, Haïat G. Biomechanical behaviours of the bone-implant interface: a review. J R Soc Interface. 2019;16:20190259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 25. | Lee CK, Langrana NA, Parsons JR, Zimmerman MC. Development of a prosthetic intervertebral disc. Spine (Phila Pa 1976). 1991;16:S253-S255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | DeLucca JF, Amin D, Peloquin JM, Vresilovic EJ, Costi JJ, Elliott DM. Off-axis response due to mechanical coupling across all six degrees of freedom in the human disc. JOR Spine. 2019;2:e1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Lazennec JY, Rakover JP, Rousseau MA. Five-year follow-up of clinical and radiological outcomes of LP-ESP elastomeric lumbar total disc replacement in active patients. Spine J. 2019;19:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Suchomel P, Jurák L, Benes V, Brabec R, Bradác O, Elgawhary S. Clinical results and development of heterotopic ossification in total cervical disc replacement during a 4-year follow-up. Eur Spine J. 2010;19:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Kong L, Ma Q, Meng F, Cao J, Yu K, Shen Y. The prevalence of heterotopic ossification among patients after cervical artificial disc replacement: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e7163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Reyes-Sanchez A, Miramontes V, Olivarez LM, Aquirre AA, Quiroz AO, Zarate-Kalfopulos B. Initial clinical experience with a next-generation artificial disc for the treatment of symptomatic degenerative cervical radiculopathy. SAS J. 2010;4:9-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Byval'tsev VA, Kalinin AA, Stepanov IA, Pestryakov YY, Shepelev VV. [Analysis of the results of total cervical disc arthroplasty using a M6-C prosthesis: a multicenter study]. Zh Vopr Neirokhir Im N N Burdenko. 2017;81:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Hui N, Phan K, Kerferd J, Lee M, Mobbs RJ. Comparison of M6-C and Mobi-C cervical total disc replacement for cervical degenerative disc disease in adults. J Spine Surg. 2019;5:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Baltus C, Costa E, Vaz G, Raftopoulos C. Granulomatous Reaction on a Double-Level Cervical Total Disc Arthroplasty. World Neurosurg. 2019;122:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Brenke C, Schmieder K, Barth M. Core herniation after implantation of a cervical artificial disc: case report. Eur Spine J. 2015;24 Suppl 4:S536-S539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Ames CP, Blondel B, Scheer JK, Schwab FJ, Le Huec JC, Massicotte EM, Patel AA, Traynelis VC, Kim HJ, Shaffrey CI, Smith JS, Lafage V. Cervical radiographical alignment: comprehensive assessment techniques and potential importance in cervical myelopathy. Spine (Phila Pa 1976). 2013;38:S149-S160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 392] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 36. | Hey HWD, Lau ET, Wong GC, Tan KA, Liu GK, Wong HK. Cervical Alignment Variations in Different Postures and Predictors of Normal Cervical Kyphosis: A New Understanding. Spine (Phila Pa 1976). 2017;42:1614-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Patwardhan AG, Tzermiadianos MN, Tsitsopoulos PP, Voronov LI, Renner SM, Reo ML, Carandang G, Ritter-Lang K, Havey RM. Primary and coupled motions after cervical total disc replacement using a compressible six-degree-of-freedom prosthesis. Eur Spine J. 2012;21 Suppl 5:S618-S629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Lazennec JY, Rischke B, Rakover JP, Ricart O, Rousseau MA. Two years' follow-up with clinical and radiological results. Evolution of segmental mobility and location of mean centres of rotation. Orthop Proceedings. 2019;101-B. [DOI] [Full Text] |
| 39. | Tumialán LM, Gluf WM. Progressive vertebral body osteolysis after cervical disc arthroplasty. Spine (Phila Pa 1976). 2011;36:E973-E978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Heo DH, Lee DC, Oh JY, Park CK. Bone loss of vertebral bodies at the operative segment after cervical arthroplasty: a potential complication? Neurosurg Focus. 2017;42:E7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Kieser DC, Cawley DT, Fujishiro T, Tavolaro C, Mazas S, Boissiere L, Obeid I, Pointillart V, Vital JM, Gille O. Anterior Bone Loss in Cervical Disc Arthroplasty. Asian Spine J. 2019;13:13-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |