Published online Jun 18, 2020. doi: 10.5312/wjo.v11.i6.278
Peer-review started: April 8, 2020
First decision: April 22, 2020
Revised: May 4, 2020
Accepted: May 12, 2020
Article in press: May 12, 2020
Published online: June 18, 2020
Processing time: 67 Days and 12.2 Hours
There is still no definitive treatment for knee osteoarthritis (OA). We are certainly far from a consensus on the best form of treatment or on an effective treatment recommendation. There are reasons for the current equivocal treatment recommendations in the face of this very serious health problem. The greatest of these reasons, undoubtedly, is the great complexity of the factors involved in the development and progression of knee OA and the complex pathophysiology including mechanical, inflammatory, metabolic, post-traumatic, molecular, genetic, and psychological changes. For several years, an attempt has been made to correlate different patient phenotypes to different patterns of response to treatment, thus creating the possibility of developing specific treatments for certain groups of patients and theoretically allowing better treatment efficacy. However, in practice we still find totally different responses and evolutions even in individuals belonging to the same phenotype. Thus, classification by phenotypes, despite being an advance, is not sufficient. The present article proposes a fragmented look at each of the many factors or targets involved in the genesis and evolution of OA. Therefore, we propose not the treatment of OA per se but the management of an individual set of targets to achieve personalized OA management. We believe that, paradoxically, by fragmenting the view of the disease we will be able to treat our patients more holistically in an individualized way.
Core tip: We are certainly far from consensus on the best form of treatment or on an effective treatment recommendation for osteoarthritis (OA). We still find totally different responses and evolutions in individuals belonging to the same OA phenotype. In this editorial, we propose a fragmented look at each of the many factors or targets involved in the genesis and evolution of OA. Therefore, we propose not the treatment of OA per se but the management of an individual set of targets to achieve personalized OA management through a target-based approach for OA treatment.
- Citation: de Campos GC, Tieppo AM, de Almeida Jr CS, Hamdan PC, Alves WM, de Rezende MU. Target-based approach for osteoarthritis treatment. World J Orthop 2020; 11(6): 278-284
- URL: https://www.wjgnet.com/2218-5836/full/v11/i6/278.htm
- DOI: https://dx.doi.org/10.5312/wjo.v11.i6.278
Knee osteoarthritis (KOA) is a heterogeneous disease at the clinical, physiologic, anatomic, and molecular levels[1] with progressive and degenerative synovial impairment that evolves from an asymptomatic phase (molecular and cellular damage level) to symptomatic phase (multitissue lesions) with joint pain, stiffness, and functional disability[2]. KOA affects not only the cartilage but the entire joint[1]. There is imbalance in joint tissues anabolism/catabolism with articular cartilage breakdown, subchondral bone remodeling, and synovial and periarticular soft tissue inflammation with joint capsule hypertrophy[3].
It is a heavy burden for society because it leads to a great loss of function and quality of life in affected individuals and generates enormous costs[4]. The latest update from the Global Burden of Disease study estimates that 242 million people worldwide live with symptoms and limitations related to OA of the knees or hips[4].
Despite the vast number of research studies, there is still no definitive treatment for OA. There are currently several guidelines with recommendations on the various treatment modalities for OA based on the medical literature[5,6]. Most of them recommend a combination of treatment modalities including non-pharmacological and pharmacological therapies, such as information/education, weight loss, exercise programs, analgesics, nonsteroidal anti-inflammatory drugs, and symptomatic slow-acting drugs[5,6].
We are, however, far from a consensus on the best form of treatment or on an effective treatment recommendation for individual patients. There are reasons for the current equivocal treatment recommendations in the face of this very serious health problem. The greatest of these, undoubtedly, is the great complexity of the factors involved in the development and progression of KOA[7] and the complex pathophysiology including mechanical, inflammatory, metabolic, post-traumatic, molecular, genetic, and psychological changes[7]. Similarly, there is a large variation among individuals in relation to disease trajectory with some patients evolving rapidly, while others remain stable for long periods of time[8]. This has led to attempts to group patients with common disease characteristics or similar treatment responses into subgroups called OA phenotypes[8].
Several studies have proposed different OA phenotypes based on clinical, laboratory, or imaging findings[8]. Correlation of different phenotypes to different patterns of response to treatment creates the possibility of developing specific treatments for certain groups of patients, theoretically allowing better treatment efficacy[8]. However, in practice we still find totally different responses and evolutions in individuals belonging to the same phenotype. This is because despite grouping patients with similar characteristics, the great variety and propensity of factors involved in the pathophysiology of each individual makes it difficult to achieve success with the same treatment for different individuals[9]. Additionally, the same individual may have characteristics common to several phenotypes, such as an obese elder who has had a meniscectomy in the past. Thus, classification by phenotypes, despite being an advance, is not sufficient. We must go further addressing specific features of these phenotypes, i.e. targets. The purpose of this publication is to introduce a novel perspective for OA treatment, which can rationalize its management and also contribute to enhance future clinical trials.
Current guidelines propose recommendations regarding several treatment modalities[5,6]. For example, the use of valgus insoles or duloxetine is a treatment of osteoarthritis. However, an OA patient with neutral alignment will not benefit from valgus insoles. Similarly, a patient without chronic and neuropathic pain will not benefit from duloxetine. Therefore, a valgus insole is not a treatment for osteoarthritis but for varus malalignment; and duloxetine is not a treatment for osteoarthritis but for chronic pain. In this context, we need to reverse the rational paradigm. Rather than treating OA as a single disease, we propose a fragmented look at each of the many different factors involved in the genesis and evolution of OA to which we will name the targets. Therefore, we propose not the treatment of OA per se but the management of an individual set of targets to achieve personalized OA management. We believe that, paradoxically, by fragmenting the view of the disease we will be able to treat our patients more holistically in an individualized way.
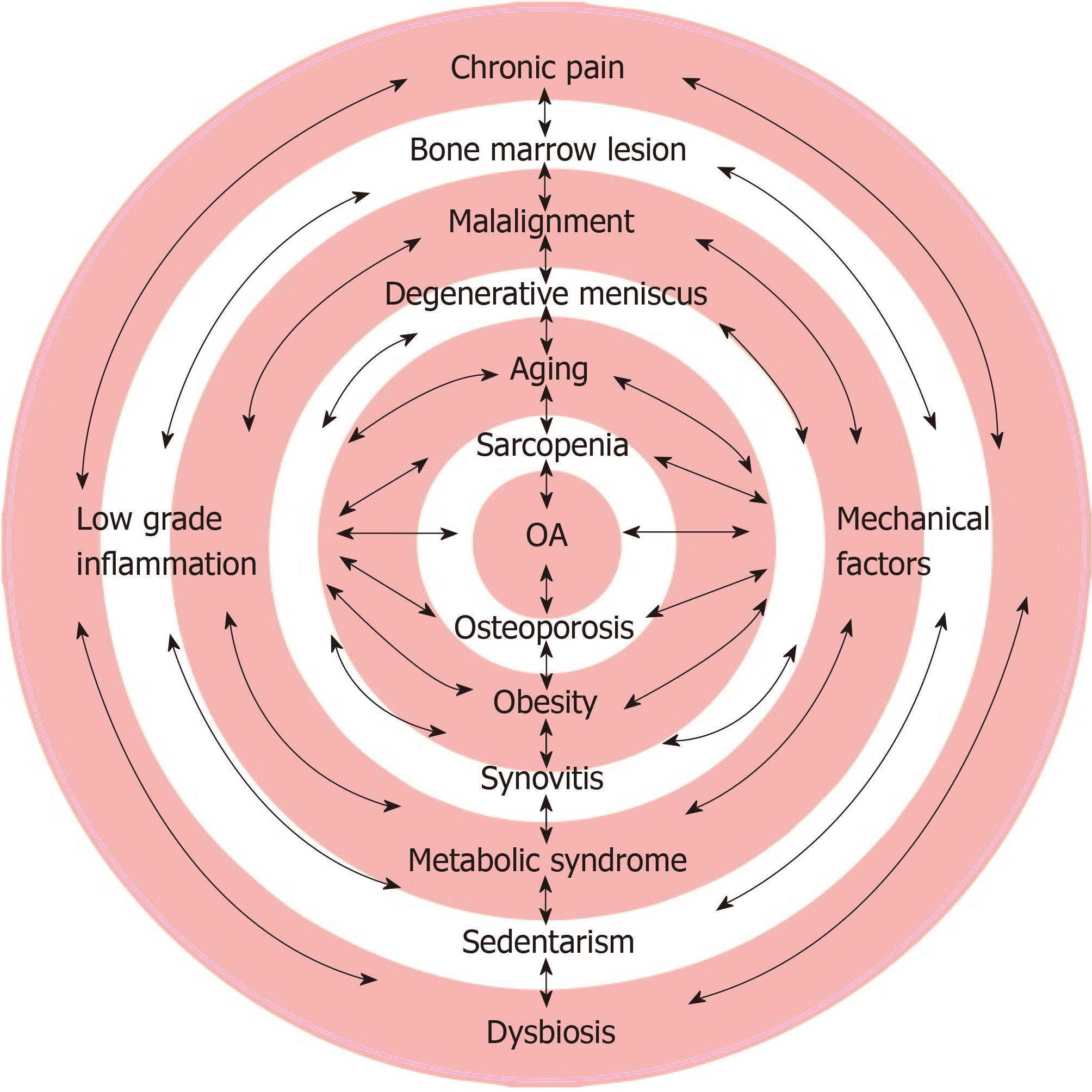
The complexity of mechanical, inflammatory, metabolic, cellular, molecular, genetic, and psychological factors is precisely what makes it difficult to classify patients into isolated groups/phenotypes given the infinite possibilities of combinations and interactions among these factors (Figure 1). By directly and exclusively considering each factor, the variability of responses narrows. The following are the most commonly encountered targets in individuals with OA. The management possibilities of these targets are also briefly discussed.
Age is undoubtedly the most important factor because OA is rare under 40 years of age and extremely frequent over 65 years of age[4]. The age factor, although it can be considered nonmodifiable, can be “treated” through a healthy lifestyle with regular practice of moderate exercise[10], adequate diet[11], and healthy attitudes such as adequate sleep and respect for the circadian cycle. These recommendations have been shown to reduce the senescence (cellular aging) of chondrocytes[7].
Obesity is another key factor in the development of OA, both due to the mechanical disturbance that being overweight causes on load-bearing joints and to the inflammatory disorder caused by the excessive production of inflammatory cytokines (adipokines)[12]. The obesity target should be addressed with diet, weight loss, and in more extreme cases bariatric surgery[13].
Metabolic syndrome, of which obesity is a part, also exerts a negative influence on OA[14]. Systemic arterial hypertension via subchondral ischemia can compromise the articular cartilage and cause bone remodeling[15]. Dyslipidemia can lead to the deposition of lipids in chondrocytes, myocytes, and hepatocytes, which deregulates their metabolism[15]. Concomitantly, increased production of free fatty acids signals greater expression of nuclear factor kappa beta and inflammasome activation, which will lead to intense production of proinflammatory cytokines, such as interleukin 1 beta, interleukin 18, and tumor necrosis factor alpha. This will increase inflammatory status with predominance of catabolism over anabolism on cartilage, synovial tissue, and subchondral bone[15]. Hyperglycemia leads to increased production of glycation end products and oxidative stress leveraging persistent low-grade systemic inflammation contributing to a toxic environment that will exacerbate OA[15]. Therefore, managing these targets is fundamental.
The relationship between the regular practice of moderate exercise and improvement in OA symptoms is well established[10]. The main treatment guidelines place exercise as a central treatment for all OA patients[5,6]. Physiological and moderate intensity movements are fundamental for normal cartilage metabolism and consequently for its maintenance[10,16].
Degenerative meniscal lesions are frequent in OA and even in knees with no radiographic signs of the disease can be considered an initial finding of OA rather than an entity of their own[17]. Degenerative meniscal lesions bring symptoms and disability and require treatment. Treatment should not be surgical even in specific cases of mechanical symptoms[17]. Currently there is evidence of successful control of this target through intra-articular corticosteroid injections or hyaluronic acid injections[18].
Poorly aligned knees have an increased risk for OA and therefore a greater risk for disease progression than knees with neutral alignment[19]. Knees with varus alignment have a four-fold increase in the risk of progression to OA in the medial compartment, and those with valgus alignment have approximately five times the risk of progression to lateral OA[20]. The association of malalignment with the size and progression of regions with bone edema as well as with accelerated cartilage loss have been demonstrated[21]. This target can be nonsurgically treated through the use of orthotics or insoles[22].
The presence of bone marrow lesions usually means a focal overload on the joint with microdamage of the trabecular bone. Subsequently, this leads to a vicious cycle of subchondral bone attrition, attempts at repair, pain, and progressive deformity[23]. It is a predictor of unfavorable disease evolution in that compartment[24]. Bone marrow lesions should also be considered a therapeutic target because they are often symptomatic and can be treated by overload reduction with the use of a cane, orthoses, body weight control, muscle rebalancing, bisphosphonates, and even surgery[25].
In patients with OA, a low inflammatory state is present and is detectable both locally and systemically[26]. Endogenous molecules called alarmins may be released into joint space after local cell stress or damage, (e.g., cartilage matrix fragments), activating inflammasome platform and/or nuclear factor kappa beta, thus leading to joint sterile inflammation and triggering cell joint senescence[27]. Chondrocytes and synovial cells (macrophages and fibroblasts) produce increased levels of inflammatory cytokines, chemokines, and lipid mediators into the synovial fluid, which in turn decreases the synthesis of collagen, aggrecans, and proteoglycans and increases catabolic mediators such as collagenases, metalloproteinases and alarmins[27].
Environmental factors, lifestyle[28], epigenomic, genomic[29], and microbiome[11] studies have been focused on clarifying molecular mechanisms of low-grade inflammation to support regulatory actions so that the expression, transcription, and translation of enzymes and structural proteins of the joints can remain at levels of homeostasis. Metabolomics is a promising field for chronic inflammation investigation[1].
Chronic low-grade inflammation plays a central role in OA pathogenesis. Apart from some promising disease modifying drugs[30], we can manage it with improvements in nutrition, rebalance of intestinal microbiome, gut health[11], restful sleep[31], and adequate physical activities[16].
Synovitis occurs even in the early stages of OA and may be subclinical[32]. Unlike rheumatoid arthritis, synovial inflammation in OA is usually found near areas with pathologically damaged bone and cartilage[32]. This hyperactive synovium can release proteinases and cytokines capable of accelerating joint destruction, thus being an important therapeutic target[32]. Special attention should be given to episodes of acute synovitis, or “flares,” which are associated with the acute worsening of pain and accompanied by joint effusion usually after joint overload. In these cases, the best treatment is certainly effusion drainage followed by intra-articular corticosteroid injection[33].
Pain is a common therapeutic target for many individuals with OA. However, acute pain, which can be treated with analgesics and anti-inflammatories, must be distinguished from chronic pain, which can be treated with drugs with different mechanisms of action[34]. Another aspect of this therapeutic target is pain perception. Psychological characteristics such as pain catastrophizing and depressive disorders can be addressed and treated with psychotherapy[35]. Conduction disorders or hypersensitization (such as regional complex pain) also require specific treatment[36].
Habits such as smoking and drinking can also be considered targets to be addressed[37]. Other situations such as hyperuricemia, hypovitaminosis, dysbiosis, and nutritional deficiencies can also be valued and treated[11]. There are large numbers of targets already identified in the medical literature and certainly many more will still be discovered. The ways to address each of the targets will also be developed and improved.
There is a great deal of difficulty in obtaining convincing conclusions from clinical trials as they are currently conducted. The focus on therapeutic targets and not on OA disease could provide more homogenous groups to be compared and studied, which would boost findings. For example, McAlindon et al[38] analyzed 140 patients with KOA comparing the use of triamcinolone infiltration versus a saline injection for a period of 2 years. They reported no difference in symptoms between groups but a greater cartilage volume loss in the group that received the triamcinolone injection. The authors concluded that the findings did not support the use of triamcinolone infiltration in patients with KOA[38]. However, a large number of studies in the literature show corticosteroid injection is an effective tool for the treatment of flares[39] (a specific target) but unsuitable for long-term treatment of general KOA.
The same reasoning holds for the conclusions (or lack thereof) of the current guidelines[5,6]. The focus of OA pathology as a whole is the current method of analysis. But the population in which treatments are being studied is usually too heterogeneous. Instead we suggest that the efficacy of a given drug, the use of orthoses, or a given infiltration should be analyzed in the context of specific targets as discussed in this paper.
There is still no ideal treatment for OA. Much difficulty arises from the treatment of it as a single disease or as groups of patients (phenotypes), which despite some common characteristics are still very different from each other. Grouping individuals according to therapeutic targets and treating them based on these targets may bring progress in outcomes. We suggest that future studies should focus on specific targets rather than focus on OA general treatment.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Sociedade Brasileira de Ortopedia e Traumatologia, No. 10607.
Specialty type: Orthopedics
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Paredes-Vieyra JP, Xiong J S-Editor: Ma RY L-Editor: Filipodia E-Editor: Liu MY
| 1. | Appleton CT. Osteoarthritis year in review 2017: biology. Osteoarthritis Cartilage. 2018;26:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Kraus VB, Blanco FJ, Englund M, Karsdal MA, Lohmander LS. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartilage. 2015;23:1233-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 429] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 3. | Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 1013] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 4. | Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4675] [Cited by in RCA: 4517] [Article Influence: 451.7] [Reference Citation Analysis (0)] |
| 5. | Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, Blanco FJ, Espinosa R, Haugen IK, Lin J, Mandl LA, Moilanen E, Nakamura N, Snyder-Mackler L, Trojian T, Underwood M, McAlindon TE. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1191] [Cited by in RCA: 1964] [Article Influence: 327.3] [Reference Citation Analysis (0)] |
| 6. | Bruyère O, Honvo G, Veronese N, Arden NK, Branco J, Curtis EM, Al-Daghri NM, Herrero-Beaumont G, Martel-Pelletier J, Pelletier JP, Rannou F, Rizzoli R, Roth R, Uebelhart D, Cooper C, Reginster JY. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arthritis Rheum. 2019;49:337-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 345] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 7. | Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med. 2016;59:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 261] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 8. | Deveza LA, Melo L, Yamato TP, Mills K, Ravi V, Hunter DJ. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthritis Cartilage. 2017;25:1926-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 220] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 9. | van Spil WE, Bierma-Zeinstra SMA, Deveza LA, Arden NK, Bay-Jensen AC, Kraus VB, Carlesso L, Christensen R, Van Der Esch M, Kent P, Knoop J, Ladel C, Little CB, Loeser RF, Losina E, Mills K, Mobasheri A, Nelson AE, Neogi T, Peat GM, Rat AC, Steultjens M, Thomas MJ, Valdes AM, Hunter DJ. A consensus-based framework for conducting and reporting osteoarthritis phenotype research. Arthritis Res Ther. 2020;22:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Ravalli S, Castrogiovanni P, Musumeci G. Exercise as medicine to be prescribed in osteoarthritis. World J Orthop. 2019;10:262-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Biver E, Berenbaum F, Valdes AM, Araujo de Carvalho I, Bindels LB, Brandi ML, Calder PC, Castronovo V, Cavalier E, Cherubini A, Cooper C, Dennison E, Franceschi C, Fuggle N, Laslop A, Miossec P, Thomas T, Tuzun S, Veronese N, Vlaskovska M, Reginster JY, Rizzoli R. Gut microbiota and osteoarthritis management: An expert consensus of the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO). Ageing Res Rev. 2019;55:100946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 12. | Francisco V, Pérez T, Pino J, López V, Franco E, Alonso A, Gonzalez-Gay MA, Mera A, Lago F, Gómez R, Gualillo O. Biomechanics, obesity, and osteoarthritis. The role of adipokines: When the levee breaks. J Orthop Res. 2018;36:594-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Groen VA, van de Graaf VA, Scholtes VA, Sprague S, van Wagensveld BA, Poolman RW. Effects of bariatric surgery for knee complaints in (morbidly) obese adult patients: a systematic review. Obes Rev. 2015;16:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Courties A, Berenbaum F, Sellam J. The Phenotypic Approach to Osteoarthritis: A Look at Metabolic Syndrome-Associated Osteoarthritis. Joint Bone Spine. 2019;86:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 15. | Gao YH, Zhao CW, Liu B, Dong N, Ding L, Li YR, Liu JG, Feng W, Qi X, Jin XH. An update on the association between metabolic syndrome and osteoarthritis and on the potential role of leptin in osteoarthritis. Cytokine. 2020;129:155043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Zampogna B, Papalia R, Papalia GF, Campi S, Vasta S, Vorini F, Fossati C, Torre G, Denaro V. The Role of Physical Activity as Conservative Treatment for Hip and Knee Osteoarthritis in Older People: A Systematic Review and Meta-Analysis. J Clin Med. 2020;9:E1167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Sihvonen R, Englund M, Turkiewicz A, Järvinen TL; Finnish Degenerative Meniscal Lesion Study Group. Mechanical Symptoms and Arthroscopic Partial Meniscectomy in Patients With Degenerative Meniscus Tear: A Secondary Analysis of a Randomized Trial. Ann Intern Med. 2016;164:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Dernek B, Kesiktas FN, Duymus TM, Diracoglu D, Aksoy C. Therapeutic efficacy of three hyaluronic acid formulations in young and middle-aged patients with early-stage meniscal injuries. J Phys Ther Sci. 2017;29:1148-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Faschingbauer M, Kasparek M, Waldstein W, Schadler P, Reichel H, Boettner F. Cartilage survival of the knee strongly depends on malalignment: a survival analysis from the Osteoarthritis Initiative (OAI). Knee Surg Sports Traumatol Arthrosc. 2020;28:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Sharma L, Chmiel JS, Almagor O, Felson D, Guermazi A, Roemer F, Lewis CE, Segal N, Torner J, Cooke TD, Hietpas J, Lynch J, Nevitt M. The role of varus and valgus alignment in the initial development of knee cartilage damage by MRI: the MOST study. Ann Rheum Dis. 2013;72:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Hayashi D, Englund M, Roemer FW, Niu J, Sharma L, Felson DT, Crema MD, Marra MD, Segal NA, Lewis CE, Nevitt MC, Guermazi A. Knee malalignment is associated with an increased risk for incident and enlarging bone marrow lesions in the more loaded compartments: the MOST study. Osteoarthritis Cartilage. 2012;20:1227-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Campos GC, Rezende MU, Pasqualin T, Frucchi R, Bolliger Neto R. Lateral wedge insole for knee osteoarthritis: randomized clinical trial. Sao Paulo Med J. 2015;133:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Klement MR, Sharkey PF. The Significance of Osteoarthritis-associated Bone Marrow Lesions in the Knee. J Am Acad Orthop Surg. 2019;27:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Nielsen FK, Egund N, Jørgensen A, Jurik AG. Risk factors for joint replacement in knee osteoarthritis; a 15-year follow-up study. BMC Musculoskelet Disord. 2017;18:510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Ververidis AN, Paraskevopoulos K, Tilkeridis K, Riziotis G, Tottas S, Drosos GI. Surgical modalities for the management of bone marrow edema of the knee joint. J Orthop. 2020;17:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Scanzello CR, Loeser RF. Editorial: inflammatory activity in symptomatic knee osteoarthritis: not all inflammation is local. Arthritis Rheumatol. 2015;67:2797-2800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Millerand M, Berenbaum F, Jacques C. Danger signals and inflammaging in osteoarthritis. Clin Exp Rheumatol. 2019;37 Suppl 120:48-56. [PubMed] |
| 28. | Lewis R, Gómez Álvarez CB, Rayman M, Lanham-New S, Woolf A, Mobasheri A. Strategies for optimising musculoskeletal health in the 21st century. BMC Musculoskelet Disord. 2019;20:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 29. | van Meurs JB. Osteoarthritis year in review 2016: genetics, genomics and epigenetics. Osteoarthritis Cartilage. 2017;25:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Herrero-Beaumont G, Pérez-Baos S, Sánchez-Pernaute O, Roman-Blas JA, Lamuedra A, Largo R. Targeting chronic innate inflammatory pathways, the main road to prevention of osteoarthritis progression. Biochem Pharmacol. 2019;165:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Park HM, Kwon YJ, Kim HS, Lee YJ. Relationship between Sleep Duration and Osteoarthritis in Middle-Aged and Older Women: A Nationwide Population-Based Study. J Clin Med. 2019;8:356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 682] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 33. | van Middelkoop M, Arden NK, Atchia I, Birrell F, Chao J, Rezende MU, Lambert RG, Ravaud P, Bijlsma JW, Doherty M, Dziedzic KS, Lohmander LS, McAlindon TE, Zhang W, Bierma-Zeinstra SM. The OA Trial Bank: meta-analysis of individual patient data from knee and hip osteoarthritis trials show that patients with severe pain exhibit greater benefit from intra-articular glucocorticoids. Osteoarthritis Cartilage. 2016;24:1143-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 34. | Qaseem A, Wilt TJ, McLean RM, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166:514-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1544] [Cited by in RCA: 1885] [Article Influence: 235.6] [Reference Citation Analysis (0)] |
| 35. | Lerman SF, Finan PH, Smith MT, Haythornthwaite JA. Psychological interventions that target sleep reduce pain catastrophizing in knee osteoarthritis. Pain. 2017;158:2189-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Shim H, Rose J, Halle S, Shekane P. Complex regional pain syndrome: a narrative review for the practising clinician. Br J Anaesth. 2019;123:e424-e433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 37. | Haugen IK, Magnusson K, Turkiewicz A, Englund M. The Prevalence, Incidence, and Progression of Hand Osteoarthritis in Relation to Body Mass Index, Smoking, and Alcohol Consumption. J Rheumatol. 2017;44:1402-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, Ward RJ. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA. 2017;317:1967-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 540] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 39. | Yaftali NA, Weber K. Corticosteroids and Hyaluronic Acid Injections. Clin Sports Med. 2019;38:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (1)] |