Published online Feb 18, 2020. doi: 10.5312/wjo.v11.i2.90
Peer-review started: July 24, 2019
First decision: October 24, 2019
Revised: November 7, 2019
Accepted: November 28, 2019
Article in press: November 28, 2019
Published online: February 18, 2020
Processing time: 209 Days and 18.8 Hours
Postoperative delirium (POD) is one of the most common complications in older adult patients undergoing elective surgery. Few studies have compared, within the same institution, the type of surgery, risk factors and type of anesthesia and analgesia associated with the development of POD.
To investigate the following three questions: (1) What is the incidence of POD after non-ambulatory orthopedic surgery at a high-volume orthopedic specialty hospital? (2) Does surgical procedure influence incidence of POD after non-ambulatory orthopedic surgery? And (3) For POD after non-ambulatory orthopedic surgery, what are modifiable risk factors?
A retrospective cohort study was conducted of all non-ambulatory orthopedic surgeries at a single orthopedic specialty hospital between 2009 and 2014. Patients under 18 years were excluded from the cohort. Patient characteristics and medical history were obtained from electronic medical records. Patients with POD were identified using International Classification of Diseases, 9th Revision (ICD-9) codes that were not present on admission. For incidence analyses, the cohort was grouped into total hip arthroplasty (THA), bilateral THA, total knee arthroplasty (TKA), bilateral TKA, spine fusion, other spine procedures, femur/pelvic fracture, and other procedures using ICD-9 codes. For descriptive and regression analyses, the cohort was grouped, using ICD-9 codes, into THA, TKA, spinal fusions, and all procedures.
Of 78492 surgical inpatient surgeries, the incidence from 2009 to 2014 was 1.2% with 959 diagnosed with POD. The incidence of POD was higher in patients undergoing spinal fusions (3.3%) than for patients undergoing THA (0.8%); THA patients had the lowest incidence. Also, urgent and/or emergent procedures, defined by femoral and pelvic fractures, had the highest incidence of POD (7.2%) than all other procedures. General anesthesia was not seen as a significant risk factor for POD for any procedure type; however, IV patient-controlled analgesia was a significant risk factor for patients undergoing THA [Odds ratio (OR) = 1.98, 95% confidence interval (CI): 1.19 to 3.28, P = 0.008]. Significant risk factors for POD included advanced age (for THA, OR = 4.9, 95%CI: 3.0-7.9, P < 0.001; for TKA, OR = 2.16, 95%CI: 1.58-2.94, P < 0.001), American Society of Anesthesiologists score of 3 or higher (for THA, OR = 2.01, 95%CI: 1.33-3.05, P < 0.001), multiple medical comorbidities, hyponatremia (for THA, OR = 2.36, 95%CI: 1.54 to 3.64, P < 0.001), parenteral diazepam (for THA, OR = 5.05, 95%CI: 1.5-16.97, P = 0.009; for TKA, OR = 4.40, 95%CI: 1.52-12.75, P = 0.007; for spine fusion, OR = 2.17, 95%CI: 1.19-3.97, P = 0.01), chronic opioid dependence (for THA, OR = 7.11, 95%CI: 3.26-15.51, P < 0.001; for TKA, OR = 2.98, 95%CI: 1.38-6.41, P = 0.005) and alcohol dependence (for THA, OR = 5.05, 95%CI: 2.72-9.37, P < 0.001; for TKA, OR = 6.40, 95%CI: 4.00-10.26, P < 0.001; for spine fusion, OR = 6.64, 95%CI: 3.72-11.85, P < 0.001).
POD is lower (1.2%) than previously reported; likely due to the use of multi-modal regional anesthesia and early ambulation. Both fixed and modifiable factors are identified.
Core tip: This original research adds significantly to the perioperative literature. At this single orthopedic institution, the effects of different procedures, and effects of the different management practices of these procedures, on postoperative delirium were examined. The incidence of post-operative delirium was found to be lower at this institution than many other previous reports. Potentially modifiable risk factors for post-operative delirium in patients undergoing common orthopedic procedures, for whom higher vigilance is warranted were also identified.
- Citation: Urban MK, Sasaki M, Schmucker AM, Magid SK. Postoperative delirium after major orthopedic surgery. World J Orthop 2020; 11(2): 90-106
- URL: https://www.wjgnet.com/2218-5836/full/v11/i2/90.htm
- DOI: https://dx.doi.org/10.5312/wjo.v11.i2.90
Postoperative delirium (POD) is one of the most common complications in older adult patients undergoing elective surgery. The reported incidence ranges from 3%-25% after elective surgery[1,2]. Many perioperative characteristics have been associated with the development of POD including increased length of recovery and hospital stay, as well as increased morbidity and mortality[1,3]. Fixed risk factors often associated with POD include advanced age, pre-existing central nervous system deficits, psychiatric disease, alcohol abuse, emergency surgery and the presence of multiple comorbidities[3,4]. Few studies have compared, within the same institution, the type of surgery, risk factors and type of anesthesia and analgesia associated with the development of POD[5]. Our goal was to assess the incidence of POD after non-ambulatory orthopedic surgery, evaluate the influence of the surgical procedure on this incidence, and identify possible modifiable risk factors.
With the approval of the Institutional Review Board a retrospective cohort study was conducted of all non-ambulatory orthopedic surgeries at a single orthopedic specialty hospital between 2009 and 2014. Specifically, the study population was patients aged 18 years or older who underwent inpatient orthopedic surgeries from January 1, 2009 to December 31, 2014 at a single institution. Excluded populations were patients aged 17 years or younger and patients who did not undergo an inpatient orthopedic surgery. These patient populations were excluded to minimize selection bias as younger patients are less likely to develop delirium and ambulatory patients are not routinely followed to see if there is an occurrence of delirium after surgery.
The initial study design included years prior to 2009; but it was determined that differences in coding before 2009 would affect the interpretation of the data and contribute to disease misclassification bias, so data prior to 2009 was excluded. Patient characteristics and medical history were obtained from electronic medical records. Patient’s medical diagnoses, including Elixhauser comorbidity score determination[6] and procedure types were obtained from International Classification of Diseases, 9th Revision (ICD-9) codes. Age, sex, BMI, postoperative medications, laboratory values, American Society of Anesthesiologists’ (ASA) score, and whether a computerized axial tomography (CT) scan or magnetic resonance imaging (MRI) scan of the brain was ordered were obtained from the electronic health record (Allscripts, Atlanta, Georgia).
For incidence analyses, the cohort was grouped into total hip arthroplasty (THA), bilateral THA, total knee arthroplasty (TKA), bilateral TKA, spine fusion, other spine procedures, femur/pelvic fracture and other procedures using ICD-9 procedure codes. For descriptive and regression analysis, the cohort was grouped, using ICD-9 procedure codes, into THA, TKA, spinal fusions, and all procedures. Emergent procedures were excluded in the descriptive and regression analyses to minimize selection bias as the characteristics of patients undergoing emergent surgeries and the characteristics of these surgeries themselves may be related to the development of delirium. Patients in the THA and TKA group were identified by 81.51 and 81.54 ICD-9 codes, respectively. Bilateral THA and bilateral TKA patients were identified by the presence of 81.51 coded twice or 81.54 coded twice during one admission stay, respectively. Spinal fusion patients were identified using one or more of the following codes: 81.03, 81.05, 81.07, 81.08, 81.33, 81.33, 81.35, 81.37, 81.38, 81.62, 81.63, 81.64 and 84.51, representing anterior, posterior, or revision spine fusions. Other spine patients were identified by a presence of one or more of 3.0-3.99, 81.00-81.08, 81.30-81.39, 84.60-84.69 and 84.80-84.85 codes. Femur and/or pelvic fracture patients were identified by one or more of the following codes: 808.0-808.9, 820.0-820.9 and 821.0-821.3 ICD-9 codes. Patients in the other-procedure grouping were inpatients who underwent orthopedic surgery without the presence of any of the codes listed above during the duration of the study. The all-procedure grouping consisted of all adult inpatient orthopedic surgeries that occurred during the study period, which encompasses all the groups listed above. To examine non-emergent surgeries, the THA, TKA and spinal fusion groupings excluded patients if they had the following present on admission: Cardiovascular accident, deep vein thrombosis, pulmonary embolism, acute myocardial infarction, acute kidney failure, hip fracture, pneumonia, respiratory failure, sepsis and trauma. The all-procedure grouping included emergent procedures. The THA grouping only included patients with an ASA score of 2 and above. Since no THA patients with an ASA score of 1 had POD, the regression analysis was restricted to patients with ASA score of 2 and above to allow for THA patients with ASA score 2 to serve as the reference group in the regression model. This was preferred over grouping patients with ASA score 1 and 2 into one group to serve as the reference since this would bias the results as there would be no THA POD patients with an ASA score of 1 in this group.
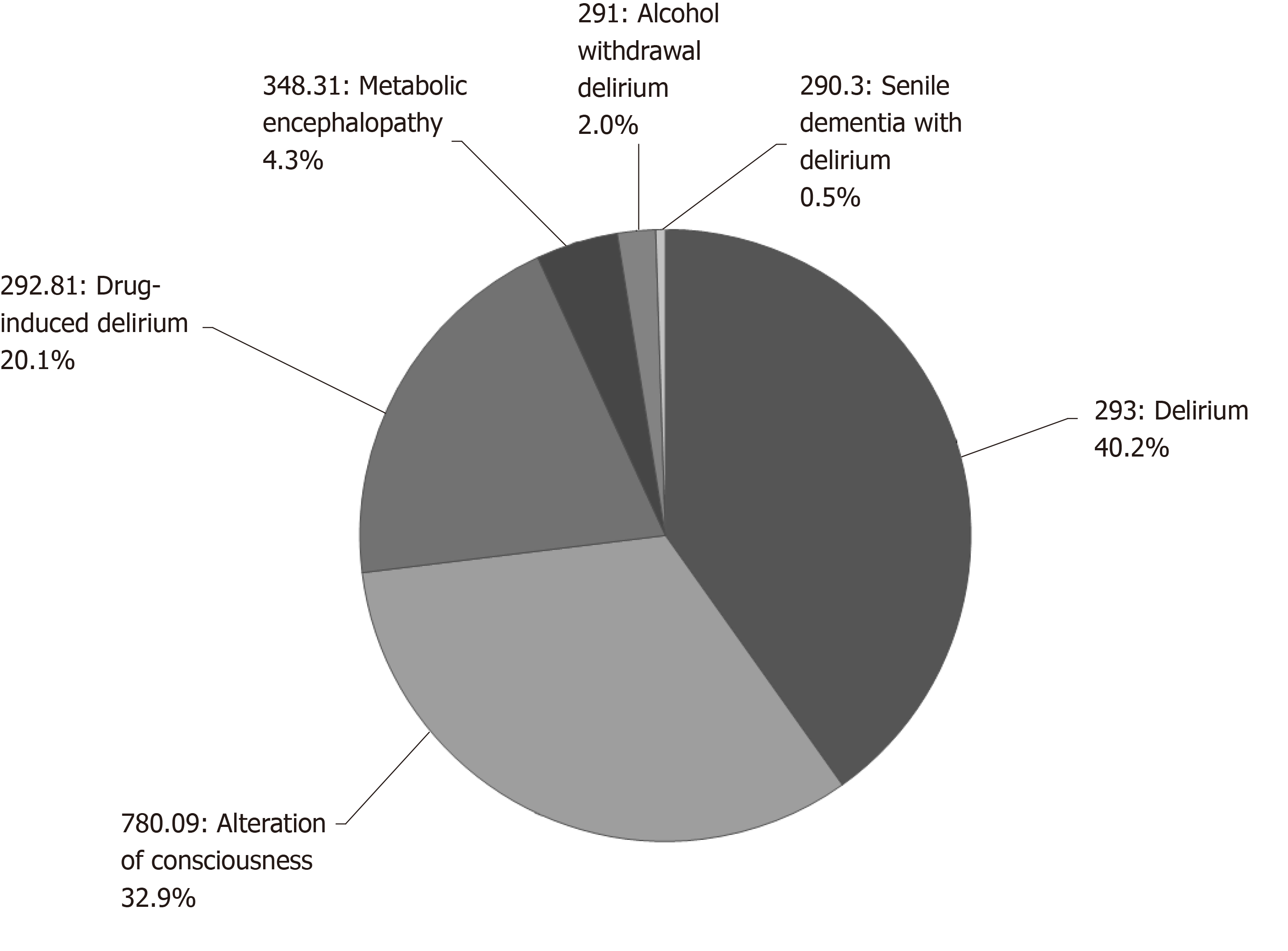
Patients with POD were identified using the following ICD-9 codes, provided that these conditions were not present on admission: 290.11; 290.3; 291.0; 292.81; 293.0; 293.1; 293.9; 300.11; 300.14; 300.15; 348.31; and 780.09 (Figure 1). There were no patients with ICD-9 codes 290.11 and 300.14 and only a total of 4 with ICD-9 codes 293.1, 300.11 and 300.15. All patients in the post-operative care unit, step down unit and intensive care unit (ICU) were assessed for delirium using the confusion assessment method for the ICU (CAM-ICU) scale. Once transferred to a non-monitored bed in the hospital, during each nursing shift, the covering nurse conducted a CAM-ICU assessment on their assigned patients. If the nurses detected a change in mental status, a physician assistant or nurse practitioner was requested to confirm the diagnosis and report the change to a covering physician. The diagnosis of delirium and/or change in mental status was only made after a practitioner entered the diagnosis in the patient’s medical record. Opioid dependence was identified using any ICD-9 diagnosis code, if present on admission, between 304.00 and 304.93, as well as code V58.69. The occurrence of a postoperative thiamine order was used as a proxy for alcohol abuse. Pressure ulcers were identified by the following ICD-9 diagnosis codes if present on admission: 707.01, 707.02, 707.03, 707.04, 707.05, 707.06, 707.07, 707.09, 707.23 and 707.24. atrial fibrillation (Afib) was identified by the 427.31 ICD-9 diagnosis code if present on admission. Preoperative hyponatremia was defined by a sodium value < 135 mmol/L within 30 days before admission.
Preliminary descriptive statistical analysis consisted of frequency counts and percentages for discrete variables and median, intra-quartile range, and minimum and maximum values for continuous variables. Crude inferential analysis consisted of Chi-square and Fisher Exact tests for discrete comparisons and independent samples t-tests for continuous variables. When continuous variables failed to meet the assumption of normality using the Kolmogorov-Smirnov test, non-parametric Mann Whitney U tests were used in place of t-tests. Multivariable logistic regression analysis was used to identify potential risk factors POD while adjusting for any potential confounding. Records with missing data for the regression variable candidates were removed from the analyses. Patients with more than one admission in the study had each admission treated separately. Separate models for spinal fusion, hip arthroplasty, and knee arthroplasty were constructed and separate models with Elixhauser comorbidities and Charlson Comorbidity scores[7,8] were constructed for each procedure type. The models with the Charlson Comorbidity scores were used for sensitivity analyses. Based on age categorizations in the literature, age was treated as a binary variable for all procedure type models; arthroplasty models had patients 70 years old or older vs patients less than 70 years old and spine models had patients 65 years old or older vs patients less than 65 years old[4,9-11]. Multicollinearity for each model was checked and no covariates had a variance inflation factor above 2.0, so they were considered not to be collinear. A model was constructed including, a priori, all patient and clinical variables that were thought to be risk factors, based on literature and physician expertise. Significance was set at 0.05 for all analyses without multiple comparison adjustment since the study was evaluating a single hypothesis: Predictors of delirium. All analyses were performed using SAS 9.3 (Cary, NC, United States). The statistical methods of this study were reviewed by Kara Fields and Joseph T Nguyen from Hospital for Special Surgery.
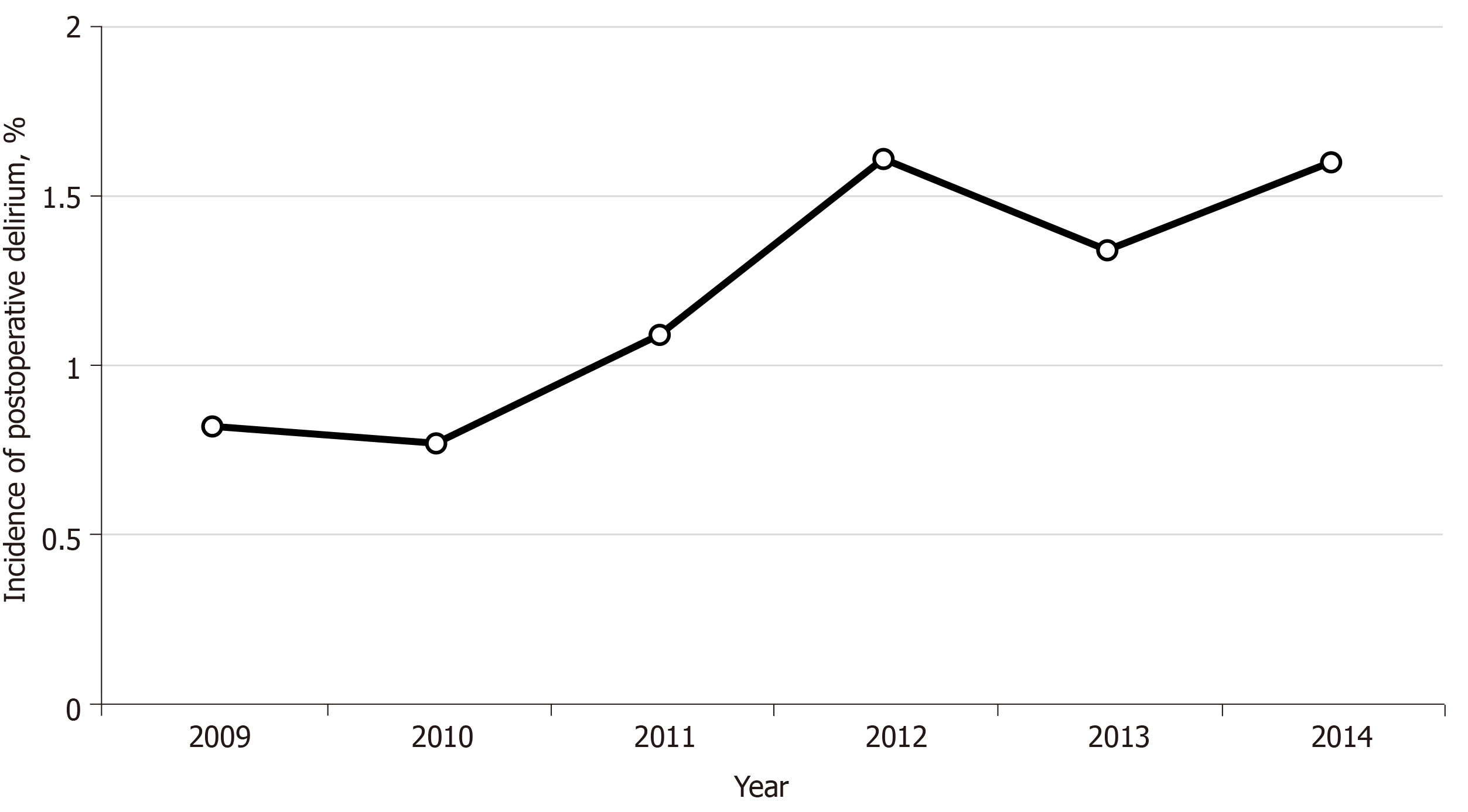
The incidence of POD was assessed in patients 18 years old or older undergoing non-ambulatory surgery at an orthopedic institution from 2009 to 2014. During this time, there were 78492 surgical inpatient surgeries. Of these patients; 959 were diagnosed with POD; an incidence of 1.2%. The most common diagnostic categories of delirium included altered consciousness, drug induced delirium, and metabolic encephalopathy. The reported incidence of POD increased from 2009 to 2012, and then plateaued from 2012 to 2014 (Figure 2); the majority of the increase occurred in the diagnostic ICD-9 code 293.0 for “delirium due to conditions classified elsewhere.”
The incidence of delirium varied between the orthopedic surgical procedures (Table 1). The incidence of POD was higher in patients undergoing spinal fusions than for patients undergoing THA, THA patients had the lowest incidence. Also, urgent and/or emergent procedures defined by femoral and pelvic fractures had the highest incidence of POD than all other procedures (Table 1). Of the 78492 inpatient surgeries, 35166 patients were males (44.8%) and 43326 were females (55.2%). The mean age was 62 years (SD ± 14). The mean BMI was 28.8 (SD ± 6.1). Table 2 contains the patient characteristics for all patients undergoing a major elective orthopedic surgery. For these patients, there was a statistically significant difference in age between POD and non-POD patients, with an incidence of 2.5% in patients 70 years old or older compared to an incidence of 0.006% in patients under 70 years old. Patients with POD had higher ASA scores, where 45.7% of POD patients had an ASA score of 3 or higher with an incidence of 3.0% compared to a POD incidence of 0.008% for patients with an ASA score of 2 or lower. Additionally, POD was significantly higher in patients receiving postoperative benzodiazepines; 10.2% of the patients with POD received diazepam vs only 4.0% of the non-POD population. Significantly more narcotics were used by patients with POD than those without. Perioperative thiamine administration which is a marker for pre-existing alcohol use was more common in POD patients. Also, Afib on admission was more common in patients who developed POD than in non-POD patients. Tables 3 and 4 show the patient characteristics of THA and TKA patients. POD was more common in patients 70 years or older in patients undergoing THA and TKA procedures; 80.0% of THA and 69.8% of TKA patients with POD were 70 years old or older vs 35.7% of THA and 41.8% of TKA patients without POD. For TKA patients, the incidence of patients who received a general anesthetic (GA) was 4.9% in POD patients; however only 2.7% of the non-POD TKA patients received a GA for surgery. This statistically significant difference was also seen with the type of postoperative analgesia. The incidence of POD among patients who received epidural patient-controlled analgesia (PCA) was 0.7% for THA patients and 1.2% for TKA patients. In comparison, the incidence of POD among THA patients who received intravenous PCA was 1.2% for THA and 2.1% for TKA patients. In our analysis, 90.7% of the THA patients with a diagnosis of POD had either a CT, MRI or both.
| Procedure | Incidence (%) |
| Primary THA | 0.8 |
| Bilateral THA | 0.4 |
| Primary TKA | 1.2 |
| Bilateral TKA | 1.2 |
| Spine fusion | 3.3 |
| Other spine procedures | 1.9 |
| Femur/pelvic fracture | 7.2 |
| Other procedures | 1.0 |
| POD (Mean +/- SD or n, %), n = 959 | Non-POD (Mean +/- SD or n, %), n = 77533 | P value | |
| Age, yr | 72.7+/-12.9 | 62.3+/-14.1 | < 0.001a |
| ≥ 70 | 610 (63.6) | 24038 (31.0) | < 0.001a |
| Male | 389 (40.6) | 34777 (44.9) | 0.008a |
| BMI | 28.2+/-6.2 | 28.0+/-6.1 | 0.003a |
| Procedure type | < 0.001a | ||
| THA | 161 (16.8) | 19753 (25.5) | |
| TKA | 249 (26.0) | 20106 (25.9) | |
| Bilateral THA | 3 (0.3) | 853 (1.1) | |
| Bilateral TKA | 29 (3.0) | 2337 (3.0) | |
| Spine fusions | 140 (14.6) | 4135 (5.3) | |
| Other spine procedures | 126 (13.1) | 6587 (8.5) | |
| Other procedures | 251 (26.2) | 23762 (30.7) | |
| Trauma | < 0.001a | ||
| Fracture of femur/pelvis | 57 (5.9) | 731 (0.9) | |
| Other trauma | 30 (3.1) | 2607 (3.4) | |
| No trauma | 872 (90.9) | 74195 (95.7) | |
| Length of stay | 6.0+/-7.5 | 3.0+/-3.2 | < 0.001a |
| Latest creatinine before surgery | < 0.001a | ||
| < 1.2 mg/dL | 825 (86.0) | 71048 (91.6) | |
| 1.2-2.0 mg/dL | 123 (12.8) | 6229 (8.0) | |
| > 2.0 mg/d | 11 (1.2) | 256 (0.3) | |
| Preoperative hyponatremia | < 0.001a | ||
| Yes | 367 (38.3) | 21290 (27.5) | |
| No | 591 (61.6) | 56190 (72.5) | |
| Missing | 1 (0.1) | 53 (0.07) | |
| Postoperative thiamine order | < 0.001a | ||
| Yes | 119 (12.4) | 1390 (1.8) | |
| No | 839 (87.5) | 76122 (98.2) | |
| Missing | 1 (0.1) | 21 (0.03) | |
| Atrial fibrillation (present on admission) | 130 (13.6) | 3815 (4.9) | < 0.001a |
| Pressure ulcers (present on admission) | 23 (2.4) | 323 (0.42) | < 0.001a |
| Opioid dependence or long-term use | 29 (6.2) | 1245 (1.6) | < 0.001a |
| Psychiatric disease (present on admission) | 356 (37.1) | 16716 (21.6) | < 0.001a |
| Anesthesia type | < 0.001a | ||
| General | 344 (35.9) | 16356 (21.9) | |
| Other | 578 (60.3) | 56067 (72.3) | |
| Missing | 37 (3.9) | 4510 (5.8) | |
| Patient-controlled analgesia | < 0.001a | ||
| Epidural | 413 (43.1) | 40991 (52.9) | |
| IV | 441 (46.0) | 22458 (29.0) | |
| Peripheral nerve infusion | 11 (1.2) | 949 (1.2) | |
| Missing | 94 (9.8) | 13135 (16.9) | |
| Received diazepam | < 0.001a | ||
| Yes | 98 (10.2) | 3135 (4.0) | |
| No | 860 (89.7) | 74377 (95.9) | |
| Missing | 1 (0.1) | 21 (0.03) | |
| CT, MRI or both performed | < 0.001a | ||
| Yes | 872 (90.9) | 64838 (83.6) | |
| No | 86 (9.0) | 12674 (16.4) | |
| Missing | 1 (0.1) | 21 (0.03) | |
| ASA score | < 0.001a | ||
| 1 | 7 (0.73) | 4933 (6.4) | |
| 2 | 477 (49.7) | 53801 (69.4) | |
| ≥ 3 | 438 (45.7) | 14274 (18.4) | |
| Missing | 37 (3.9) | 4525 (5.8) |
| POD (Mean +/- SD or n, %), n = 140 | Non-POD (Mean +/- SD or n, %), n = 18232 | P value | |
| Age, yr | 77.6+/-10.4 | 65.5+/-11.4 | < 0.001a |
| ≥ 70 | 112 (80.0) | 6499 (35.65) | < 0.001a |
| Male | 57 (40.7) | 7891 (43.28) | 0.54 |
| BMI | 26.7+/-5.2 | 28.4+/-5.8 | < 0.001a |
| Length of stay | 6.1+/-4.0 | 3.3+/-1.5 | < 0.001a |
| Latest creatinine before surgery | < 0.001a | ||
| < 1.2 mg/dL | 107 (76.4) | 16376 (89.8) | |
| 1.2-2.0 mg/dL | 29 (20.7) | 1781 (9.8) | |
| > 2.0 mg/dL | 4 (2.9) | 75 (0.4) | |
| Preoperative hyponatremia | < 0.001a | ||
| Yes | 40 (28.6) | 2525 (13.9) | |
| No | 100 (71.4) | 15700 (86.1) | |
| Missing | 0 (0) | 7 (0.04) | |
| Postoperative thiamine order | < 0.001a | ||
| Yes | 17 (12.1) | 387 (2.1) | |
| No | 123 (87.9) | 17844 (97.9) | |
| Missing | 0 (0) | 1 (0.01) | |
| Atrial fibrillation (present on admission) | 5 (3.6) | 119 (0.7) | < 0.001a |
| Pressure ulcers (present on admission) | 1 (0.7) | 47 (0.3) | 0.31 |
| Opioid dependence or long-term use | 9 (6.4) | 199 (1.1) | < 0.001a |
| Psychiatric disease (present on admission) | 47 (33.6) | 3584 (19.7) | < 0.001a |
| Anesthesia type | 0.55 | ||
| General | 4 (2.7) | 384 (2.1) | |
| Other | 136 (97.1) | 17805 (97.7) | |
| Missing | 0 (0) | 40 (0.2) | |
| Patient-controlled analgesia | < 0.001a | ||
| Epidural | 102 (72.9) | 14235 (78.1) | |
| IV | 24 (17.1) | 1306 (7.2) | |
| Missing | 14 (10) | 2691 (14.8) | |
| Received diazepam | 0.03a | ||
| Yes | 8 (5.7) | 453 (2.5) | |
| No | 132 (94.3) | 17778 (97.5) | |
| Missing | 0 (0) | 1 (0.01) | |
| CT, MRI or both performed | 0.009a | ||
| Yes | 127 (90.7) | 15616 (85.7) | |
| No | 13 (9.3) | 2615 (14.3) | |
| Missing | 0 (0) | 1 (0.01) | |
| ASA score | < 0.001a | ||
| 2 | 73 (52.1) | 14667 (80.5) | |
| ≥ 3 | 67 (47.9) | 3519 (19.3) | |
| Missing | 0 (0) | 46 (0.25) |
| POD (Mean +/- SD or n, %), n = 245 | non-POD (Mean +/- SD or n, %), n = 19864 | P value | |
| Age, yr | 75.1+/-9.3 | 67.6+/-10 | < 0.001a |
| ≥ 70 | 171 (69.8) | 8304 (41.8) | < 0.001a |
| Male | 89 (36.3) | 7364 (37.1) | 0.81 |
| BMI | 30.0+/-6.1 | 30.6+/-6.3 | 0.13 |
| Length of stay | 6.0+/-3.2 | 3.8+/-1.5 | < 0.001a |
| Latest creatinine before surgery | < 0.001a | ||
| < 1.2 mg/dL | 203 (82.9) | 17828 (89.8) | |
| 1.2-2.0 mg/dL | 37 (15.1) | 1954 (9.8) | |
| > 2.0 mg/dL | 5 (2.0) | 82 (0.4) | |
| Preoperative hyponatremia | 0.07 | ||
| Yes | 45 (18.4) | 2837 (14.3) | |
| No | 200 (81.6) | 17019 (85.7) | |
| Missing | 0 (0) | 8 (0.04) | |
| Postoperative thiamine order | < 0.001a | ||
| Yes | 28 (11.4) | 332 (1.7) | |
| No | 217 (88.6) | 19530 (98.3) | |
| Missing | 0 (0) | 2 (0.01) | |
| Atrial fibrillation (present on admission) | 46 (18.8) | 1190 (6.0) | < 0.001a |
| Pressure ulcers (present on admission) | 1 (0.4) | 29 (0.2) | 0.31 |
| Opioid dependence or long-term use | 9 (3.7) | 198 (1.0) | 0.001a |
| Psychiatric disease (present on admission) | 77 (31.4) | 3915 (19.7) | < 0.001a |
| Anesthesia type | 0.04a | ||
| General | 12 (4.9) | 545 (2.7) | |
| Other | 233 (95.1) | 19252 (96.9) | |
| Missing | 0 (0) | 67 (0.3) | |
| Patient-controlled analgesia | 0.005a | ||
| Epidural | 179 (73.1) | 15151 (76.3) | |
| IV | 38 (15.5) | 1801 (9.1) | |
| Peripheral nerve infusion | 9 (3.7) | 711 (3.6) | |
| Missing | 19 (7.8) | 2201 (11.1) | |
| Received diazepam | 0.003a | ||
| Yes | 12 (4.9) | 381 (1.9) | |
| No | 233 (95.1) | 19481 (98.1) | |
| Missing | 0 (0) | 2 (0.01) | |
| CT, MRI or both performed | 0.14 | ||
| Yes | 226 (92.2) | 17733 (89.3) | |
| No | 19 (7.8) | 2129 (10.7) | |
| Missing | 0 (0) | 2 (0.01) | |
| ASA score | < 0.001a | ||
| 1 | 2 (0.8) | 489 (2.46) | |
| 2 | 130 (53.1) | 14933 (75.2) | |
| ≥ 3 | 113 (46.1) | 4327 (22.0) | |
| Missing | 0 (0) | 70 (0.4) |
Table 5 presents the data for spine fusion patients, with similar findings that patients with POD are older with a history of psychiatric illness, opioid dependence, alcohol use and have more comorbidities.
| POD (Mean +/- SD or n, %), n = 137 | non-POD (Mean +/- SD or n, %), n = 4107 | P value | |
| Age, yr | 67.5+/-12.5 | 60.1+/-15.6 | < 0.001a |
| ≥ 65 | 91 (66.4) | 1765 (43.0) | < 0.001a |
| Male | 54 (39.4) | 1810 (44.1) | 0.28 |
| BMI | 28.5+/-6.5 | 28.1+/-5.9 | 0.40 |
| Length of stay | 9.0+/-5.1 | 5.6+/-3.6 | < 0.001a |
| Latest creatinine before surgery | 0.01a | ||
| < 1.2 mg/dL | 116 (84.7) | 3754 (91.4) | |
| 1.2-2.0 mg/dL | 20 (14.6) | 346 (8.4) | |
| > 2.0 mg/dL | 1 (0.7) | 7 (0.2) | |
| Preoperative hyponatremia | 0.16 | ||
| Yes | 43 (31.4) | 1070 (26.1) | |
| No | 94 (68.6) | 3033 (73.9) | |
| Missing | 0 (0) | 4 (0.1) | |
| Postoperative thiamine order | < 0.001a | ||
| Yes | 20 (14.6) | 93 (2.3) | |
| No | 117 (85.4) | 4013 (97.7) | |
| Missing | 0 (0) | 1 (0.02) | |
| Atrial fibrillation (present on admission) | 12 (8.8) | 135 (3.3) | 0.003a |
| Pressure ulcers (present on admission) | 3 (2.2) | 13 (0.32) | < 0.001a |
| Opioid dependence or long-term use | 11 (8.0) | 124 (3.0) | 0.004a |
| Psychiatric disease (present on admission) | 65 (47.5) | 1185 (28.9) | < 0.001a |
| Anesthesia type | 1 | ||
| General | 136 (99.3) | 4052 (98.7) | |
| Other | 0 (0) | 8 (0.2) | |
| Missing | 1 (0.7) | 47 (1.1) | |
| Patient-controlled analgesia | 1 | ||
| Epidural | 0 (0) | 18 (0.4) | |
| IV | 136 (99.3) | 3968 (96.6) | |
| Missing | 1 (0.7) | 121 (3.0) | |
| Received diazepam | < 0.001a | ||
| Yes | 34 (24.8) | 476 (11.6) | |
| No | 103 (75.2) | 3630 (88.4) | |
| Missing | 0 (0) | 1 (0.02) | |
| CT, MRI or both performed | 0.26 | ||
| Yes | 136 (99.3) | 3999 (97.4) | |
| No | 1 (0.7) | 107 (2.6) | |
| Missing | 0 (0) | 1 (0.02) | |
| ASA score | < 0.001a | ||
| 1 | 1 (0.7) | 214 (5.2) | |
| 2 | 78 (56.9) | 2974 (72.4) | |
| ≥ 3 | 57 (41.6) | 871 (21.2) | |
| Missing | 1 (0.7) | 48 (1.2) |
Using regression analysis we identified perioperative risk factors with a significant association with POD, for patients undergoing a THA, TKA or spinal fusion, while controlling for age, sex, BMI, creatinine levels, hyponatremia, thiamine order (alcohol abuse), ASA status, Afib, opioid dependence, pressure ulcers (for THA and spine fusion models only), PCA route, anesthesia type, surgery length, administration of parenteral diazepam, and Elixhauser comorbidities (Table 6). Three additional models were built with the same covariates but with Charslon Comorbidity scores in place of the Elixhauser comorbidities for sensitivity analyses. 42725 of 78492 surgical inpatient surgeries were eligible for regression analyses as only non-emergent THA, TKA and spine fusion patients were included in the regression analyses. Records with missing data for candidate variables were also removed from analyses resulting in 18276 of 18372 eligible patients in the THA model, with 140 of 140 eligible cases remaining; 19987 of 20109 eligible patients in the TKA model, with 245 of 245 eligible cases remaining; and 4183 of 4244 eligible patients in the spine fusion model, with 136 of 137 cases remaining.
| THAOR (95%CI), n = 18276 | THA P value | TKAOR (95%CI), n = 19987 | TKA P value | SFOR (95%CI), n = 4183 | SF P value | |
| Age, yr | ||||||
| ≥ 70 vs < 70 | 4.9 (3.0, 7.9) | < 0.001a | 2.16 (1.58, 2.94) | < 0.001a | - | - |
| ≥ 65 vs < 65 | - | - | - | - | 2.76 (1.79, 4.25) | < 0.001a |
| Male | 0.90 (0.60, 1.35) | 0.62 | 0.82 (0.60, 1.12) | 0.21 | 0.73 (0.48, 1.10) | 0.13 |
| BMI | 0.94 (0.90, 0.98) | 0.004a | 0.99 (0.96, 1.02) | 0.37 | 0.99 (0.95, 1.02) | 0.46 |
| Latest creatinine value before surgery | ||||||
| 1.2-2.0 mg/dL vs < 1.2 mg/dL | 1.77 (1.05, 3.00) | 0.03a | 0.79 (0.49, 1.27) | 0.32 | 1.40 (0.73, 2.68) | 0.31 |
| > 2.0 mg/dL vs < 1.2 mg/dL | 4.08 (1.06, 15.66) | 0.04a | 1.39 (0.46, 4.20) | 0.56 | 2.26 (0.20, 26.24) | 0.51 |
| Preoperative hyponatremia | 2.36 (1.54, 3.64) | < 0.001a | 1.25 (0.87, 1.79) | 0.23 | 1.24 (0.82, 1.88) | 0.31 |
| PCA route | ||||||
| IV vs Epidural | 1.98 (1.19, 3.28) | 0.008a | 1.26 (0.83, 1.92) | 0.27 | - | - |
| Peripheral nerve infusion vs Epidural | - | - | 0.93 (0.47, 1.86) | 0.85 | - | - |
| Postoperative thiamine order | 5.05 (2.72, 9.37) | < 0.001a | 6.40 (4.00, 10.26) | <0.001a | 6.64 (3.72, 11.85) | <0.001a |
| ASA Score | ||||||
| 2 vs 1 | - | - | 1.91 (0.26, 13.88) | 0.52 | 1.90 (0.25, 14.19) | 0.53 |
| > 3 vs 1 | - | - | 3.63 (0.49, 26.74) | 0.21 | 2.38 (0.31, 18.46) | 0.41 |
| ≥ 3 vs 2 | 2.01 (1.33, 3.05) | < 0.001a | - | - | - | - |
| Anesthesia type | ||||||
| Other vs general | 2.92 (0.66, 12.85) | 0.16 | 0.89 (0.43, 1.82) | 0.74 | - | - |
| Surgery length | 1.00 (1.00, 1.01) | 0.10 | 1.00 (0.99, 1.01) | 0.55 | 1.00 (1.00, 1.00) | <0.001a |
| Atrial fibrillation, present on admission | 0.97 (0.53, 1.77) | 0.91 | 1.93 (1.32, 2.81) | <0.001a | 2.19 (1.09, 4.41) | 0.03a |
| Pressure ulcers, present on admission | 0.65 (0.08, 5.05) | 0.68 | - | - | 7.56 (1.89, 30.24) | 0.004a |
| Opioid dependence or long-term use | 7.11 (3.26, 15.51) | < 0.001a | 2.98 (1.38, 6.41) | 0.005a | 1.88 (0.91, 3.90) | 0.09 |
| Parenteral diazepam | 5.05 (1.5, 16.97) | 0.009a | 4.40 (1.52, 12.75) | 0.007a | 2.17 (1.19, 3.97) | 0.01a |
| Elixhauser comorbidity | ||||||
| Deficiency anemias | 1.64 (0.74, 3.63) | 0.22 | 1.10 (0.57, 2.11) | 0.78 | 1.11 (0.42, 2.93) | 0.83 |
| Congestive heart failure | 2.35 (0.93, 5.96) | 0.07 | 1.59 (0.74, 3.38) | 0.23 | 1.54 (0.39, 6.11) | 0.54 |
| Rheumatoid arthritis/collagen vascular diseases | 1.06 (0.45, 2.51) | 0.900 | 0.35 (0.13, 0.94) | 0.04a | 1.02 (0.49, 2.13) | 0.95 |
| Chronic pulmonary disease | 1.13 (0.67, 1.92) | 0.64 | 0.84 (0.55, 1.28) | 0.41 | 1.06 (0.65, 1.73) | 0.82 |
| Coagulopathy | 1.32 (0.39, 4.53) | 0.66 | 1.60 (0.70, 3.62) | 0.26 | 2.80 (1.05, 7.50) | 0.04a |
| Depression | 1.70 (1.05, 2.75) | 0.03a | 1.47 (1.02, 2.11) | 0.04a | 2.61 (1.73, 3.92) | <0.001a |
| Diabetes w/o chronic complications | 1.54 (0.90, 2.64) | 0.12 | 1.90 (1.36, 2.66) | < 0.001a | 1.54 (0.92, 2.58) | 0.10 |
| Diabetes w chronic complications | 2.05 (0.45, 9.23) | 0.35 | 1.77 (0.68, 4.58) | 0.24 | 3.34 (1.02, 10.91) | 0.046a |
| Hypertension | 1.62 (1.06, 2.48) | 0.03a | 1.21 (0.59, 1.64) | 0.23 | 1.00 (0.66, 1.51) | 0.99 |
| Hypothyroidism | 1.01 (0.61, 1.66) | 0.98 | 1.46 (1.06, 2.02) | 0.02a | 1.07 (0.65, 1.76) | 0.80 |
| Liver disease | 0.41 (0.05, 3.22) | 0.40 | 0.70 (0.16, 3.07) | 0.63 | 0.71 (0.15, 3.30) | 0.66 |
| Lymphoma | 1.33 (0.25, 7.20) | 0.74 | - | - | - | - |
| Fluid and electrolyte disorders | 0.31 (0.04, 2.43) | 0.26 | 1.41 (0.44, 4.56) | 0.57 | 1.14 (0.36, 3.63) | 0.82 |
| Other neurological disorders | 2.04 (0.98. 4.27) | 0.06 | 2.79 (1.69, 4.60) | < 0.001a | 2.14 (1.10, 4.14) | 0.02a |
| Obesity | 1.06 (0.53, 2.12) | 0.88 | 0.79 (0.53, 1.19) | 0.27 | 1.56 (0.90, 2.69) | 0.11 |
| Peripheral vascular disorders | 1.20 (0.47, 3.10) | 0.70 | 1.40 (0.62, 3.16) | 0.42 | 1.68 (0.64, 4.42) | 0.30 |
| Psychoses | 2.52 (0.81, 7.90) | 0.11 | 6.39 (3.51, 11.62) | < 0.001a | 4.95 (2.38, 10.28) | <0.001a |
| Pulmonary circulation disorders | 1.20 (0.47, 3.10) | 0.70 | 1.69 (0.89, 3.23) | 0.11 | 1.57 (0.51, 4.88) | 0.44 |
| Renal failure | 0.94 (0.41, 2.15) | 0.88 | 2.32 (1.31, 4.11) | 0.004a | 0.97 (0.39, 2.45) | 0.95 |
| Solid tumor w/o metastasis | - | - | 1.42 (0.31, 6.45) | 0.65 | - | - |
| Valvular disease | 1.08 (0.59, 1.97) | 0.80 | 1.23 (0.79, 1.92) | 0.37 | 1.17 (0.61, 2.26) | 0.63 |
| Weight loss | 0.61 (0.05, 7.46) | 0.70 | 7.14 (0.62, 82.11) | 0.11 | - | - |
Older age remained a significant risk factor for POD for THA, TKA and spine fusion patients. GA was not seen as a significant risk factor for POD for any procedure type. However, IV PCA remained a significant risk factor for patients undergoing a THA. ASA score of 3 or higher and preoperative hyponatremia remained significant risk factors for THA patients only. Finally, parenteral diazepam, chronic opioid dependence and postoperative thiamine order were significant risk factors for POD for THA, TKA, and spine fusion patients. Models with the Charlson Comorbidity scores provided similar results as with models that included the Elixhauser comorbidities.
Over a 6-year period in a cohort of 78492 adult patients undergoing non-ambulatory orthopedic surgery, the incidence of POD was 1.2%. Many of the risk factors identified have been cited in previous reports and are not amenable to modification: Advanced age, medical comorbidities, and a history of psychiatric disease. However, some risk factors such as pre-existing narcotic dependence, alcoholism, and hyponatremia are potentially modifiable. In addition to surgical procedure, type of anesthesia and type of postoperative analgesia may affect the incidence of POD and as such be targeted in an attempt to reduce the incidence of POD.
This study had some limitations. First, this study provided insight into the incidence of POD at a single orthopedic specialty institution where the contribution of different procedures and anesthetic/analgesic approaches to the development of POD could be assessed. Since we relied on the reporting of mental status changes using the CAM-ICU methodology, patients with subtle changes in cognition or hypoactive delirium may have been omitted in our tabulation biasing the results toward the null, although the magnitude of this bias would be small. Second, older patients with unrecognized dementia and confusion on admission may have been incorrectly diagnosed with new acute POD biasing the results away from the null, the magnitude of this bias would also be small. A modifiable approach to reduce the incidence of POD has been to target and decrease the use of preoperative polypharmacy involving psychotropic medications[12,13]. This report did not track preoperative medications thus allowing for possible confounding where preoperative psychotropic medications may account for some of the POD incidence seen in this population. Third, we, as others[14], noted an association between preoperative alcohol use and POD. In this report, however we use postoperative administration of thiamine as an indicator of increased preoperative alcohol consumption. Although, it is our policy to administer thiamine to all patients believed to be at risk for alcohol withdrawal, it is possible that some patients may have been omitted biasing the results toward the null, the magnitude of this bias would be small.
The incidence of POD reported in this study is lower than what has been reported in many previous studies[1,15]. Even in patients greater than 70 years old, our reported incidence of 2.5% is considerably below the reported rates of 15%-20% after elective surgery[12] and 50% after the repair of hip fractures[16]. However, there are reports of a lower incidence of POD in at-risk patient populations[5,17]; Chung et al[18], reported a POD incidence of 3.1% after TKA. Several studies have suggested that the hospital incidence of delirium is under-reported due to the methods used to identify patients with delirium, which often miss patients with hypoactive delirium[19,20]. In the present report, the CAM-ICU algorithm was utilized by nurses to identify patients with a change in mental status. The diagnosis of delirium using CAM-ICU features has been shown to have improved sensitivity compared to observational assessment alone[21]. In addition, in this study the diagnosis was confirmed by a practitioner before the diagnosis was entered into the medical record. Furthermore, early ambulation and multi-modal analgesia which in multiple studies has been shown to reduce the incidence of POD, is a major factor in the postoperative management of the patients in this report. Thus, we believe the incidence of POD reported in this study is accurately represented.
The incidence of delirium varied between the various non-ambulatory surgical procedures. Pelvic and hip fractures demonstrated the highest rate followed by spinal fusions and then knee arthroplasty. Patients undergoing TKA are older, generally have more pain, increased comorbidities, lose more blood with subsequent increased intravenous fluid infusions, and are hospitalized longer at our institution than those undergoing THA[22]. All of these factors could have contributed to an increase in POD. Weinstein et al[5] also reported an increased incidence of POD in TKA patients compared to THA patients. Although the spinal fusion patients were younger; these patients all were subjected to GA including 97% who also received intravenous PCA narcotics for analgesia. Fineberg et al[9] also reported a higher incidence of POD among spinal fusion patients. We did not find arthroplasty patients undergoing GA had a higher risk of POD than patients who received a regional anesthetic. However, this difference was present in the type of postoperative analgesia received for THA patients; epidural PCA vs intravenous PCA. Some studies have suggested an association between GA and the development of delirium[5]. However, conflicting reports have been published questioning the role of GA in POD[23,24]. Weinstein et al[5] using a similar data base at our institution, found similarly low rates of POD for arthroplasty patients with a higher reported incidence in those patients receiving GA. However, at this institution over 97% of the primary arthroplasty patients received a neuraxial anesthetic for surgery with most cases having GA reserved for patients with contraindications to a spinal or epidural anesthetic (e.g., coagulopathy or previous spinal fusion). Hence, this finding may be confounded by uncontrolled factors. Furthermore, the degree of sedation delivered for the regional anesthetic patients was not recorded or controlled for in this study. Sieber et al[25] reported that in those patients who received spinal anesthesia with deep sedation vs “light” sedation for the repair of hip fractures, the incidence of POD was twice as high. However, this dramatic reduction in POD with reduced sedation was not confirmed in the STRIDE study, where the authors suggested that the benefits of reduced sedation may be obscured by competing baseline comorbidities[26].
We found that preoperative narcotic dependence was a major risk factor for the development of POD for THA, TKA and spine fusion patients. Opioid-tolerant patients require higher doses of postoperative opioids, and their pain is more difficult to control. The administration of postoperative opioids, particularly intravenous PCA, has been associated with sleep disturbances, cognitive impairment, and delirium[11,15]. Some studies have also suggested an association between ketamine administration and postoperative confusion[5]. However, perioperative administration of ketamine is often used to manage chronic pain in patients and reduce narcotic requirements[27]. Hence, a direct association between ketamine and delirium is inconclusive[28]. In this study and others[12,19], the postoperative administration of diazepam was associated with the development of POD. Diazepam is not utilized at this institution to treat postoperative confusion, but is instead used to treat anxiety or to prevent benzodiazepine withdrawal.
In summary, we found, in an orthopedic surgical population, an association between POD and many of the unmodifiable risk factors which have been identified in previous reports, including older age, history of psychiatric disease and multiple medical comorbidities. The incidence of POD was lower than many other previous reports, possibly due to our reliance on regional anesthesia and analgesia for many procedures, a commitment to early ambulation for all of our patients and the pursuit of narcotic-avoidance postoperative analgesia. Entering surgery as an opioid tolerant patient significantly increases the risk of POD and all efforts should be aimed at reducing the preoperative narcotic requirements of these patients and a postoperative analgesic protocol which emphasizes a non-narcotic approach should be used. For those patients at risk for POD a multifactorial intervention approach which includes multi-modal analgesia which de-emphasizes opioids, a reduction in the administration of psycho-active medications, preoperative alcohol use counselling and abstinence, early postoperative ambulation and possible early intervention with dexmedetomidate or atypical anti-psychotic medications is recommended for patients undergoing elective orthopedic inpatient surgery.
Postoperative delirium (POD) is one of the most common complications in older adult patients undergoing elective surgery. The reported incidence ranges from 3%-25% after elective surgery. Many perioperative characteristics have been associated with the development of POD including increased length of recovery and hospital stay, as well as increased morbidity and mortality. Fixed risk factors often associated with POD include advanced age, pre-existing central nervous system deficits, psychiatric disease, alcohol abuse, emergency surgery and the presence of multiple comorbidities.
Delirium is one of the most common complications in older adult patients undergoing elective surgery. Few studies have compared, within the same institution, the type of surgery, risk factors and type of anesthesia and analgesia associated with the development of delirium.
We investigated the following three questions: (1) What is the incidence of POD after non-ambulatory orthopedic surgery at a high-volume orthopedic specialty hospital?; (2) Does surgical procedure influence incidence of POD after non-ambulatory orthopedic surgery?; and (3) For POD after non-ambulatory orthopedic surgery, what are modifiable risk factors?. Exploring these questions will help us determine how to treat patients at higher risk for POD when undergoing an orthopedic procedure.
Common epidemiological research methodology and statistical analyses were used in this investigation. Electronic health records were collected and preliminary descriptive statistical analysis were conducted. Frequency counts and percentages for discrete variables and median, intra-quartile range, and minimum and maximum values for continuous variables were reported. Crude inferential analysis consisted of Chi-square and Fisher Exact tests for discrete comparisons and independent samples t-tests for continuous variables. When continuous variables failed to meet the assumption of normality using the Kolmogorov-Smirnov test, non-parametric Mann Whitney U tests were used in place of t-tests. Multivariable logistic regression analysis was used to identify potential risk factors POD while adjusting for any potential confounding.
Of 78492 surgical inpatient surgeries, the incidence from 2009 to 2014 was 1.2% with 959 diagnosed with POD. The incidence of POD was higher in patients undergoing spinal fusions (3.3%) than for patients undergoing total hip arthroplasty (THA) (0.8%); THA patients had the lowest incidence. Also, urgent and/or emergent procedures, defined by femoral and pelvic fractures, had the highest incidence of POD (7.2%) than all other procedures. General anesthesia was not seen as a significant risk factor for POD for any procedure type; however, IV patient-controlled analgesia (PCA) was a significant risk factor for patients undergoing THA [Odds ratio (OR) = 1.98, 95% confidence interval (CI): 1.19 to 3.28, P = 0.008]. Significant risk factors for POD included advanced age (for THA, OR = 4.9, 95%CI: 3.0 to 7.9, P < 0.001; for total knee arthroplasty (TKA), OR = 2.16, 95%CI: 1.58 to 2.94, P < 0.001), American Society of Anesthesiologists (ASA) score of 3 or higher (for THA, OR = 2.01, 95%CI: 1.33 to 3.05, P < 0.001), multiple medical comorbidities, hyponatremia (for THA, OR = 2.36, 95%CI: 1.54 to 3.64, P < 0.001), parenteral diazepam (for THA, OR = 5.05, 95%CI: 1.5 to 16.97, P = 0.009; for TKA, OR = 4.40, 95%CI: 1.52 to 12.75, P = 0.007; for spine fusion, OR = 2.17, 95%CI: 1.19 to 3.97, P = 0.01) , chronic opioid dependence (for THA, OR = 7.11, 95%CI: 3.26 to 15.51, P < 0.001; for TKA, OR = 2.98, 95%CI: 1.38 to 6.41, P = 0.005) and alcohol dependence (for THA, OR = 5.05, 95%CI: 2.72 to 9.37, P < 0.001; for TKA, OR = 6.40, 95%CI: 4.00 to 10.26, P < 0.001; for spine fusion, OR = 6.64, 95%CI: 3.72 to 11.85, P < 0.001). Many of the risk factors identified have been cited in previous reports and are not amenable to modification: advanced age, medical comorbidities, and a history of psychiatric disease. However, some risk factors such as pre-existing narcotic dependence, alcoholism, and hyponatremia are potentially modifiable. In addition to surgical procedure, type of anesthesia and type of postoperative analgesia may affect the incidence of POD and as such be targeted in an attempt to reduce the incidence of POD.
The incidence of POD reported in this study is lower than what has been reported in many previous studies. Even in patients greater than 70 years old, our reported incidence of 2.5% is considerably below the reported rates of 15%-20% after elective surgery and 50% after the repair of hip fractures. The incidence of delirium varied between the various non-ambulatory surgical procedures. Pelvic and hip fractures demonstrated the highest rate followed by spinal fusions and then knee arthroplasty. Patients undergoing TKA are older, generally have more pain, increased comorbidities, lose more blood with subsequent increased intravenous fluid infusions, and are hospitalized longer at our institution than those undergoing THA. All of these factors could have contributed to an increase in POD. We did not find arthroplasty patients undergoing general anesthesia had a higher risk of POD than patients who received a regional anesthetic. However, this difference was present in the type of postoperative analgesia received for THA patients; epidural PCA versus intravenous PCA. We found that preoperative narcotic dependence was a major risk factor for the development of POD for THA, TKA and spine fusion patients. Opioid-tolerant patients require higher doses of postoperative opioids, and their pain is more difficult to control. The administration of postoperative opioids, particularly intravenous PCA, has been associated with sleep disturbances, cognitive impairment, and delirium. Some studies have also suggested an association between ketamine administration and postoperative confusion. However, perioperative administration of ketamine is often used to manage chronic pain in patients and reduce narcotic requirements. Hence, a direct association between ketamine and delirium is inconclusive. In this study and others, the postoperative administration of diazepam was associated with the development of POD. Diazepam is not utilized at this institution to treat postoperative confusion, but is instead used to treat anxiety or to prevent benzodiazepine withdrawal. Entering surgery as an opioid tolerant patient significantly increases the risk of POD and all efforts should be aimed at reducing the preoperative narcotic requirements of these patients and a postoperative analgesic protocol which emphasizes a non-narcotic approach should be used. For those patients at risk for POD a multifactorial intervention approach which includes multi-modal analgesia which de-emphasizes opioids, a reduction in the administration of psycho-active medications, preoperative alcohol use counselling and abstinence, early postoperative ambulation and possible early intervention with dexmedetomidate or atypical anti-psychotic medications is recommended for patients undergoing elective orthopedic inpatient surgery.
We hypothesized that regional anesthesia, postoperative opioid sparring techniques, and early ambulation were responsible for the lower incidence of POD in our arthroplasty patients. Future research may involve a program designed for elderly patients at risk for POD undergoing total joint arthroplasty and should include: A regional anesthetic with reduced intravenous sedation; when feasible, local anesthetic blocks for postoperative analgesia; opioid sparring medications including acetaminophen and nonsteroidal anti-inflammatory drugs; time and place orienting by nursing staff; undisturbed sleep while in the hospital; and early ambulation and discharge from the hospital. The incidence of POD in this group should then be compared to controls. The spine fusion patient population, which has a higher incidence of POD, could also be used in future research studies using the postoperative protocol designed for arthroplasty patients outlined above with an alteration to the anesthetic protocol. Spine fusion patients require general anesthesia – in these procedures rather than using a regional anesthetic or local anesthetic block, the general anesthesia can be administered to reduce the patient’s exposure to medications that have the potential to produce delirium. The anesthetic can include intravenous dexmedetomidate, lidocaine and ketamine, all of which will reduce narcotic administration. Furthermore, since preoperative narcotic dependence was associated with POD, future research should also focus on preoperative opioid reduction and clear postoperative pain management expectations.
We thank Justin Do, Research Assistant at Hospital for Special Surgery (HSS), for assistance with creating Figures 1 and 2. We also thank Kara Fields, Analyst at HSS, for using her expertise in biostatistical analysis to help guide the statistical-methods approach during the course of this study. Lastly, we thank Joseph T Nguyen, Director of Biostatistics Core at HSS, for using his expertise in biostatistical analysis to review the final statistical methods used in the study.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Emara KMS-Editor: Tang JZ L-Editor: A E-Editor: Liu MY
| 1. | Gleason LJ, Schmitt EM, Kosar CM, Tabloski P, Saczynski JS, Robinson T, Cooper Z, Rogers SO, Jones RN, Marcantonio ER, Inouye SK. Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg. 2015;150:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 350] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 2. | Marcantonio ER. Delirium in Hospitalized Older Adults. N Engl J Med. 2017;377:1456-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 648] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 3. | Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 453] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 4. | Wang LH, Xu DJ, Wei XJ, Chang HT, Xu GH. Electrolyte disorders and aging: risk factors for delirium in patients undergoing orthopedic surgeries. BMC Psychiatry. 2016;16:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Weinstein SM, Poultsides L, Baaklini LR, Mörwald EE, Cozowicz C, Saleh JN, Arrington MB, Poeran J, Zubizarreta N, Memtsoudis SG. Postoperative delirium in total knee and hip arthroplasty patients: a study of perioperative modifiable risk factors. Br J Anaesth. 2018;120:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6456] [Cited by in RCA: 7774] [Article Influence: 287.9] [Reference Citation Analysis (0)] |
| 7. | Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7532] [Cited by in RCA: 8636] [Article Influence: 261.7] [Reference Citation Analysis (0)] |
| 8. | Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 152] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Fineberg SJ, Nandyala SV, Marquez-Lara A, Oglesby M, Patel AA, Singh K. Incidence and risk factors for postoperative delirium after lumbar spine surgery. Spine (Phila Pa 1976). 2013;38:1790-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Gao R, Yang ZZ, Li M, Shi ZC, Fu Q. Probable risk factors for postoperative delirium in patients undergoing spinal surgery. Eur Spine J. 2008;17:1531-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative delirium: the importance of pain and pain management. Anesth Analg. 2006;102:1267-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 279] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 12. | Kapoor G, Saigal S, Elongavan A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J Anaesthesiol Clin Pharmacol. 2017;33:300-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 470] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 13. | Watt J, Tricco AC, Talbot-Hamon C, Pham B, Rios P, Grudniewicz A, Wong C, Sinclair D, Straus SE. Identifying Older Adults at Risk of Delirium Following Elective Surgery: A Systematic Review and Meta-Analysis. J Gen Intern Med. 2018;33:500-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 14. | Davoudi A, Ebadi A, Rashidi P, Ozrazgat-Baslanti T, Bihorac A, Bursian AC. Delirium Prediction using Machine Learning Models on Preoperative Electronic Health Records Data. Proc IEEE Int Symp Bioinformatics Bioeng. 2017;2017:568-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Krenk L, Rasmussen LS, Kehlet H. New insights into the pathophysiology of postoperative cognitive dysfunction. Acta Anaesthesiol Scand. 2010;54:951-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 16. | Juliebø V, Bjøro K, Krogseth M, Skovlund E, Ranhoff AH, Wyller TB. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 17. | Edelstein DM, Aharonoff GB, Karp A, Capla EL, Zuckerman JD, Koval KJ. Effect of postoperative delirium on outcome after hip fracture. Clin Orthop Relat Res. 2004;422:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Chung KS, Lee JK, Park JS, Choi CH. Risk factors of delirium in patients undergoing total knee arthroplasty. Arch Gerontol Geriatr. 2015;60:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Marcantonio ER, Juarez G, Goldman L, Mangione CM, Ludwig LE, Lind L, Katz N, Cook EF, Orav EJ, Lee TH. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272:1518-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 291] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 522] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 21. | Wong CL, Holroyd-Leduc J, Simel DL, Straus SE. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 283] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 22. | Urban MK, Mangini-Vendel M, Lyman S, Pan TJ, Magid SK. The Need for a Step-up in Postoperative Medical Care is Predictable in Orthopedic Patients Undergoing Elective Surgery. HSS J. 2016;12:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Davis N, Lee M, Lin AY, Lynch L, Monteleone M, Falzon L, Ispahany N, Lei S. Postoperative cognitive function following general versus regional anesthesia: a systematic review. J Neurosurg Anesthesiol. 2014;26:369-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Ellard L, Katznelson R, Wasowicz M, Ashworth A, Carroll J, Lindsay T, Djaiani G. Type of anesthesia and postoperative delirium after vascular surgery. J Cardiothorac Vasc Anesth. 2014;28:458-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Sieber FE, Zakriya KJ, Gottschalk A, Blute MR, Lee HB, Rosenberg PB, Mears SC. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 26. | Sieber FE, Neufeld KJ, Gottschalk A, Bigelow GE, Oh ES, Rosenberg PB, Mears SC, Stewart KJ, Ouanes JP, Jaberi M, Hasenboehler EA, Li T, Wang NY. Effect of Depth of Sedation in Older Patients Undergoing Hip Fracture Repair on Postoperative Delirium: The STRIDE Randomized Clinical Trial. JAMA Surg. 2018;153:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 27. | Urban MK, Ya Deau JT, Wukovits B, Lipnitsky JY. Ketamine as an adjunct to postoperative pain management in opioid tolerant patients after spinal fusions: a prospective randomized trial. HSS J. 2008;4:62-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Avidan MS, Maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO, Veselis RA, Grocott HP, Emmert DA, Rogers EM, Downey RJ, Yulico H, Noh GJ, Lee YH, Waszynski CM, Arya VK, Pagel PS, Hudetz JA, Muench MR, Fritz BA, Waberski W, Inouye SK, Mashour GA; PODCAST Research Group. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet. 2017;390:267-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 328] [Article Influence: 41.0] [Reference Citation Analysis (0)] |