Published online Oct 18, 2020. doi: 10.5312/wjo.v11.i10.442
Peer-review started: May 19, 2020
First decision: May 26, 2020
Revised: June 3, 2020
Accepted: August 25, 2020
Article in press: August 25, 2020
Published online: October 18, 2020
Processing time: 151 Days and 17.4 Hours
Polyethylene (PE) particles produced by wear of the acetabular insert are thought to cause osteolysis and thereby aseptic loosening of the implant in total hip arthroplasty (THA). As highly cross-linked polyethylene (HXLPE) is presumed to give lower wear rates, in vivo studies are needed to confirm this.
To compare the wear of REXPOL, a HXPLE, with conventional PE within the first five years after implantation using Roentgen stereophotogrammetric analysis (RSA).
Patients were randomised to receive either a HXLPE (REXPOL) or a conventional PE insert during primary THA. RSA images were obtained directly postoperative and after 6 wk, 12 wk, 6 mo, 12 mo, 24 mo and five years. Functional outcomes were assessed using the Hip Injury and Osteoarthritis Outcome Score and Harris Hip Score at baseline and five years after surgery.
The HXLPE (REXPOL) showed less wear in the latero-medial direction. Significant wear rates of conventional PE were seen in the latero-medial and center-proximal direction and in volume and corrected volume, whereas the REXPOL did not show these outcomes over time. Improvement from baseline in functional outcome did not significantly differ.
Total 3D wear is less in THAs inserted with a REXPOL inlay than a conventional PE inlay after five years. This study confirms, for the first, that the REXPOL HXLPE inlay is preferred to standard PE.
Core Tip: Polyethylene (PE) particles produced by wear of the acetabular insert can cause osteolysis and thereby aseptic loosening of the implant in total hip arthroplasty (THA). As highly cross-linked polyethylene (HXLPE) is presumed to result in lower wear rates, we performed a randomised controlled trial (RCT) using Roentgen stereophotogrammetric analysis with a five-year follow-up period. This RCT showed, for the first time, that five-year total 3D wear was less in THAs inserted with REXPOL HXLPE and hereby confirms that this inlay is preferred to standard PE.
- Citation: van Loon J, Hoornenborg D, Sierevelt I, Opdam KT, Kerkhoffs GM, Haverkamp D. Highly cross-linked versus conventional polyethylene inserts in total hip arthroplasty, a five-year Roentgen stereophotogrammetric analysis randomised controlled trial. World J Orthop 2020; 11(10): 442-452
- URL: https://www.wjgnet.com/2218-5836/full/v11/i10/442.htm
- DOI: https://dx.doi.org/10.5312/wjo.v11.i10.442
Since the introduction of total hip arthroplasty (THA) in the 1960s, the incidence of this procedure has been increasing. Although THA is one of the most successful orthopaedic procedures, the main causes of late revisions are wear, and the resulting osteolysis causing aseptic loosening of the implant[1].
Therefore, the search to minimise wear continues and several bearing couplings over time have been tried, of which polyethylene (PE) with a ceramic head still remains the best option[2]. However, wear still occurs due to existing friction, resulting in progressive loss of material and the presence of microparticles. These PE particles induce a foreign-body reaction, which results in osteolysis[3]. The number of wear particles produced, the material used, and its morphological form determine the severity of the aforementioned reaction[4]. In response to this problem of PE wear, a highly cross-linked PE (HXLPE) has been developed. Following irradiation, free-radicals are formed, creating cross-links in the PE, which are increased by heating and reduce wear[5]. Depending on the type and dose of irradiation and the type of PE used as the control group, wear can be decreased by 42%-100% compared to traditional PE[6].
To determine the performance of an implant, a standardised and reliable method is required to measure wear. Stilling et al[7] demonstrated that wear in different directions combined with volume wear of acetabular inserts can be calculated accurately using Roentgen stereophotogrammetric analysis (RSA). As wear is one of the most important reasons for revision in THA, and therefore an indicator of long-term survival, HXLPE could reduce the number of revisions needed in the future. To prove this, in vivo analyses with RSA are needed to confirm that the in vitro results are confirmed in the real setting.
The objective of this randomised controlled trial (RCT) was to compare the wear of two different inlays, the HXLPE (REXPOL) and conventional PE acetabular inserts with similar ceramic head articulation, within the first five years after implantation.
Our hypothesis was that total 3D wear after five years in the REXPOL group would be less than that in the conventional PE group.
This single center RCT was granted ethical approval by the local ethics committee review board of the Slotervaart Medical Center (registration number: NL23524.048.08; Dutch trial register: NL5605). The design and reporting of this study were conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) principles.
This was a single center, double-blind RCT comparing HXLPE (REXPOL, Smith and Nephew) to standard PE acetabular inserts (Standard PE, Smith and Nephew) with similar ceramic head (Biolox, Smith and Nephew) articulation. Both the patients and investigators were blinded with regard to the group patients were assigned to. RSA analysis was performed in a blinded mode. Randomisation was performed by the use of numbered opaque envelopes, containing the prescribed PE insert. The orthopaedic surgeon randomly received those envelopes and opened them prior to the procedure.
Between January 2011 and January 2014, patients undergoing THA in the Slotervaart Medical Center were included in this study after completing an informed consent if they met the inclusion criteria (Table 1).
| Inclusion | Exclusion |
| Primary arthroplasty due to: | Patients who recently suffered: |
| Primary osteoarthritis | Post-operative osteoarthritis |
| Avascular necrosis | Charnley C osteoarthritis |
| Femoral neck fracture | Infection of the hip |
| Hip dysplasia | |
| Age between 60 - 75 yr at surgery | Prior osteotomy or arthroplasty of the affected hip |
| Willing to comply with the post-operative review program | Under treatment for osteoporosis |
| Body mass index > 35 kg/m2 | |
| Requiring cortisone medication |
Previous RSA studies showed a high degree of sensitivity and accuracy of measurements of migration; relatively small patient groups showed a statistically significant outcome[8]. Standard PE has a linear wear rate of around 0.06-0.08 mm/year, whereas REXPOL is expected to show almost no wear over five years. A recent publication on five-year wear results in THA measured by RSA, revealed a mean 3D wear of 0.23 mm (95%CI: 0.17-0.29) for HXLPE vs 0.41 mm (95%CI: 0.32-0.50) for conventional PE[9]. Based on this difference in wear of 0.18 mm, a SD of 0.21 and a power of 80%, a sample size of 21 patients was required in each group, to identify a statistically significant difference at the 0.05 significance level.
All THAs were performed in the Slotervaart Medical Center in the standardised way using a straight lateral approach, according to the surgical technique described by the manufacturer of the implants. All patients received the same uncemented acetabular cup (EP-FIT PLUS, Smith and Nephew) and a titanium uncemented Zweymuller femoral stem implant (SL-PLUS, Smith and Nephew) with the same ceramic head articulation (Biolox, Smith and Nephew). As inclination of > 45˚ gives more wear, the navigated position of the cup is aimed to be between 40 and 45˚ of inclination and 15 to 25˚ of anteversion[10,11]. In these series, computer navigation was used to determine this position (CT free navigation Galileo, Plus Orthopedic AG, Switzerland). The liner used was either a HXLPE liner (REXPOL, Smith and Nephew) or a standard PE liner (Standard PE, Smith and Nephew). Leg length and femoral offset were aimed to be identical to the contralateral side. In addition to this procedure, at least five well-scattered tantalum markers were installed (ø 1.0 mm) with a specially designed insertion instrument into the bone around the stem component to obtain skeletal landmarks.
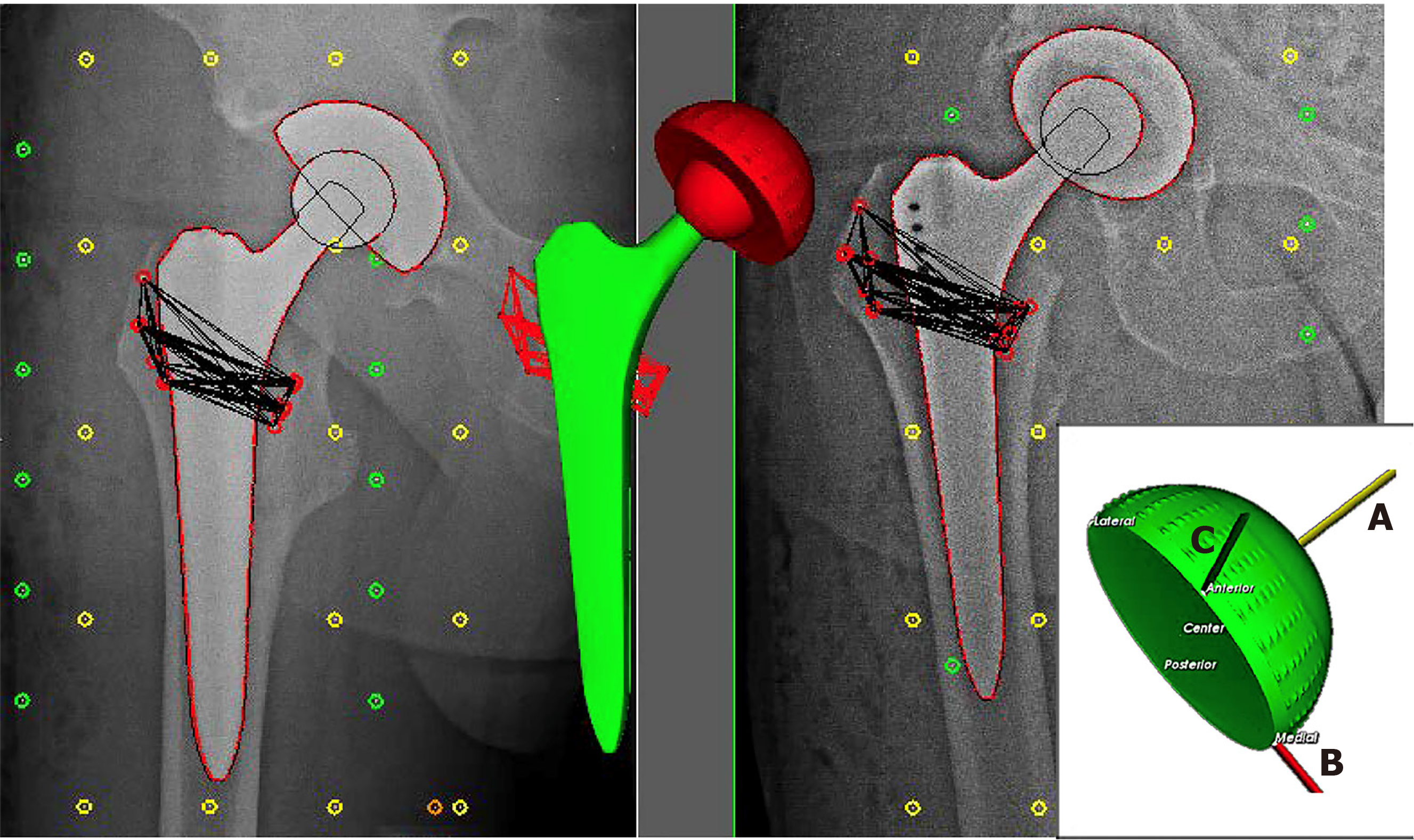
Patient demographics were recorded at baseline. RSA evaluations were performed postoperatively, after receiving the same standard rehabilitation program, within one week, at 6 wk, 3 mo, 6 mo, 12 mo, 24 mo and 60 mo after implantation. RSA measurements were performed as described in the guidelines of Valstar et al[8], in the supine position using a uniplanar calibration box (Medis CarbonBox nr. 011, Medis Specials, Leiden, Netherlands). Analysis of the radiographic images was carried out with the model-based RSA Software, version 4.1 (RSAcore, Dept. of Orthopaedics, LUMC, Netherlands). The RSA system resulted in anteroposterior and lateral views of the hip simultaneously. The RSA at four to seven days postoperatively was used as a baseline. By using the implanted tantalum balls that were fixed in the bone around the implant, the position of the implant relative to the bone was accurately assessed using a model-based RSA technique (Figure 1). With this technique the 2D head penetration as a measure of linear wear was measured in millimetres by the proximal-distal migration (A-axis) and medial-lateral migration (B-axis). Using this penetration, the thickness of the inlay could be calculated in millimetres. Additionally, the anterior-posterior migration (C-axis) was measured to calculate 3D head penetration, to determine the volume. The volume of the PE inlay was determined (in mm3/year) to measure the number of millimetres of linear wear/year. Normally wear occurs in the upward direction, in a cylindrical shape. However, as the wear is not only in a neat upwards direction, but also in other angles or directions, a corrected volume was also calculated, according to the formula of Hashimoto[12]. This formula has been validated as the most accurate way to determine volume wear from linear wear[13].
The pain and activity of daily living (ADL) domains of the Hip Injury and Osteoarthritis Outcome Score (HOOS) were assessed pre-operatively, and after five years by a research nurse[14]. The HOOS was constructed to assess patient-relevant outcomes in five separate subscales: Pain, symptoms, ADL, sport and recreation function and hip-related quality of life. The sum scores of the domains in this questionnaire are transformed into a zero to 100, worst to best scale. Another functional questionnaire assessed, was the Harris Hip Score (HHS)[15]. This questionnaire was focussed on pain and function, completed by range of motion and deformity. The maximum of 100 points is the best possible outcome.
Statistical analyses were performed with IBM SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, United States). After confirmation of normal distribution, continuous variables are presented as mean ± SD. Categorical data are described as numbers with accompanying proportions. A mixed model analysis was performed to evaluate the amount of wear between both groups during follow-up. The effect of the different inlay was considered as a model factor and interaction with the follow-up time was evaluated to assess the differences in progression of wear in both inlays. The difference in wear at final follow-up was assessed by the Student’s t-test. To assess the differences of the PROMs between the inlay groups after five years, univariate as well as multivariate regression analyses were performed to adjust for potential confounders such as demographics. The differences were significant if the P values were less than 0.05. All statistical methods in this study were performed by a biomedical statistical expert (Inger N Sierevelt).
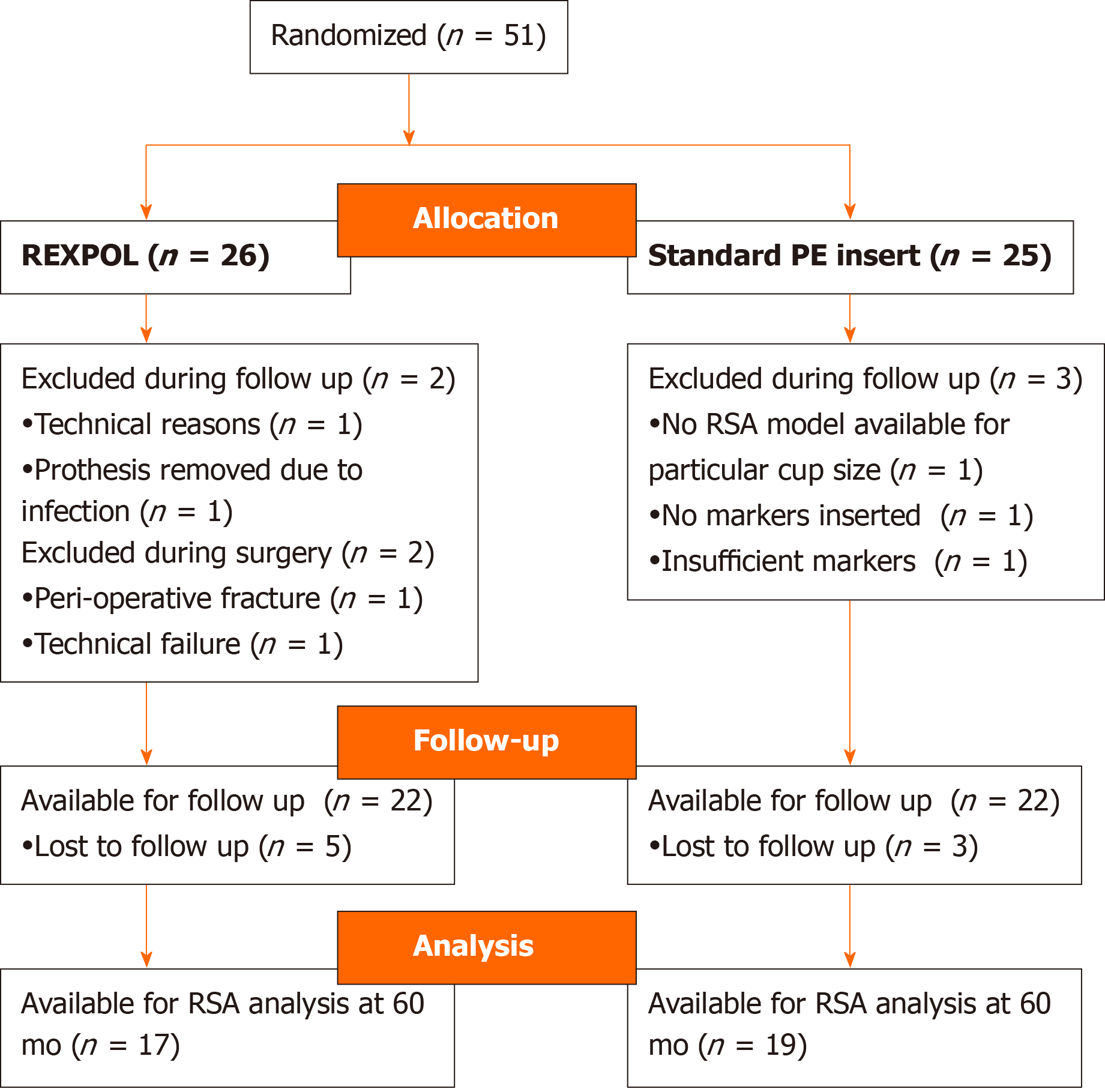
A total of 51 consecutive patients were included in this study at baseline. Figure 2 shows a flow chart of the patients during this study. Seven patients were excluded, and the remaining 44 patients were included in our analysis; 22 in the REXPOL and 22 in the Standard PE insert group. During follow-up, five patients in the REXPOL group and three in the Standard PE group were lost to follow-up. The patient demographics and baseline characteristics of both groups were comparable and are shown in Table 2. No significant differences were seen in cup sizes between the two groups and no revisions were needed during follow-up in either group.
| Conventional PE | REXPOL | |
| Number of patients, n (%) | 25 (49) | 26 (51) |
| Gender, n (%) | ||
| Male | 10 (40) | 10 (39) |
| Female | 15 (60) | 16 (59) |
| BMI, mean ± SD kg/m2 | 26.7 ± 2.9 | 27.2 ± 3.4 |
| Age at operation in years, mean ± SD | 68.5 ± 4.6 | 68.6 ± 5.1 |
| HOOS pain, mean ± SD | 46.5 ± 21.6 | 51.1 ± 17.7 |
| HOOS ADL, mean ± SD | 41.1 ± 16.6 | 46.0 ± 17.2 |
| HHS, mean ± SD | 50.6 ± 12.7 | 54.6 ± 10.9 |
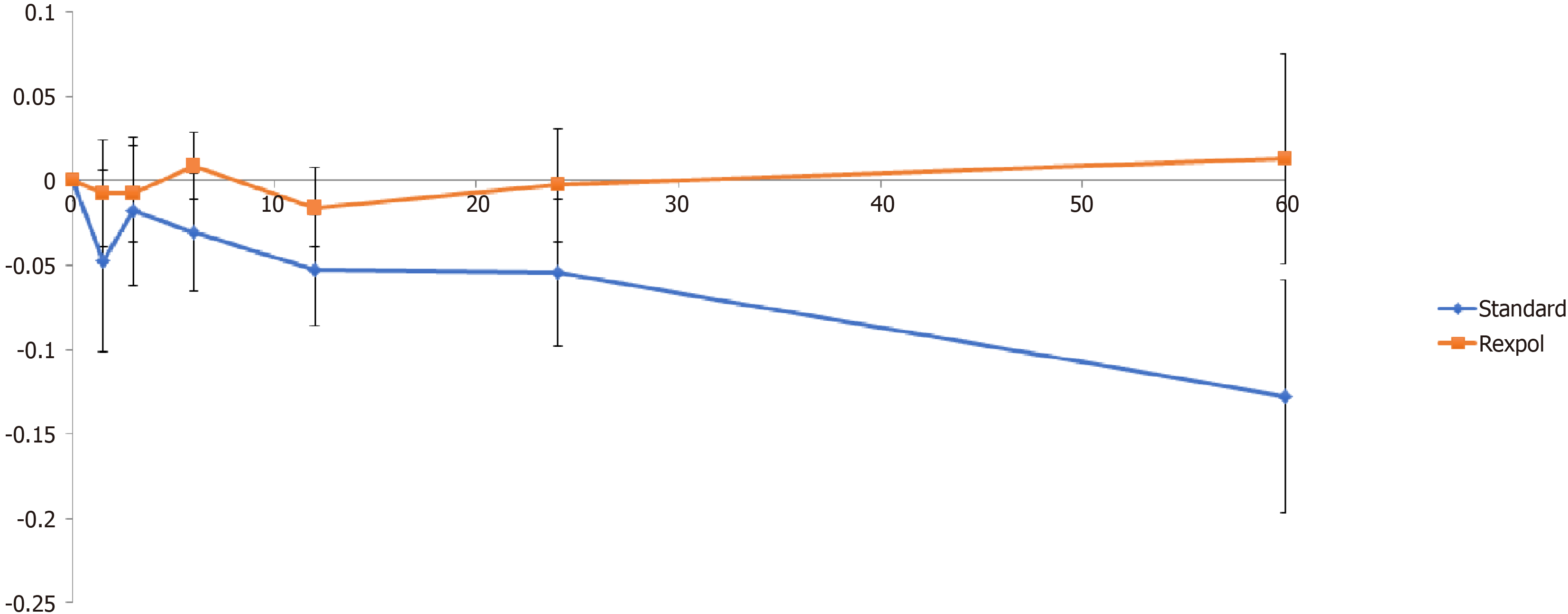
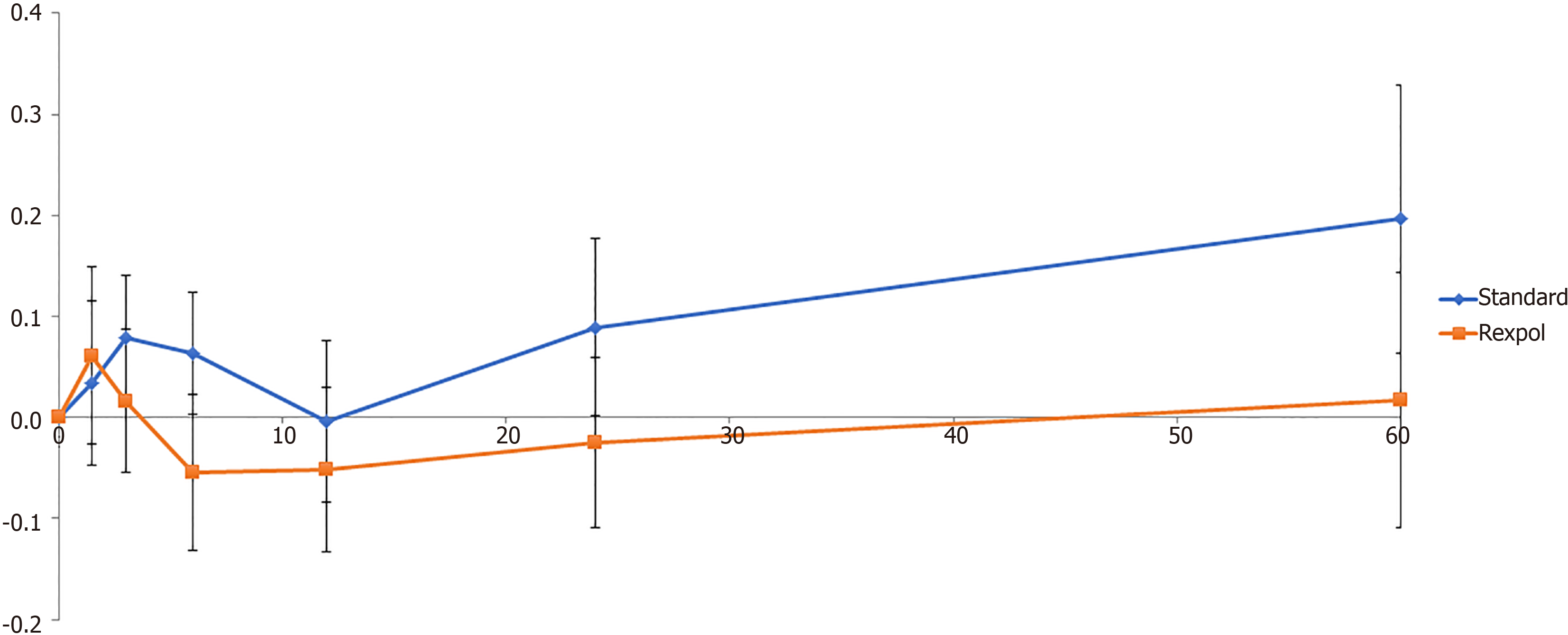
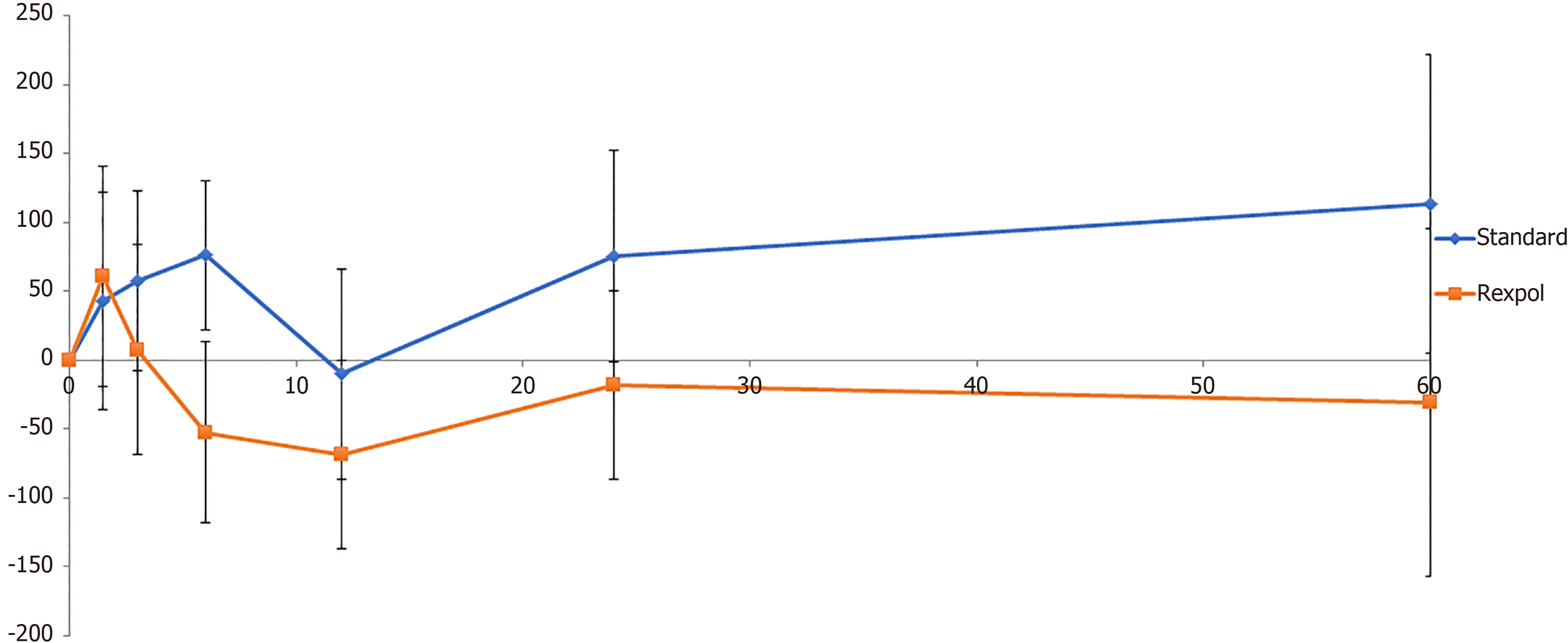
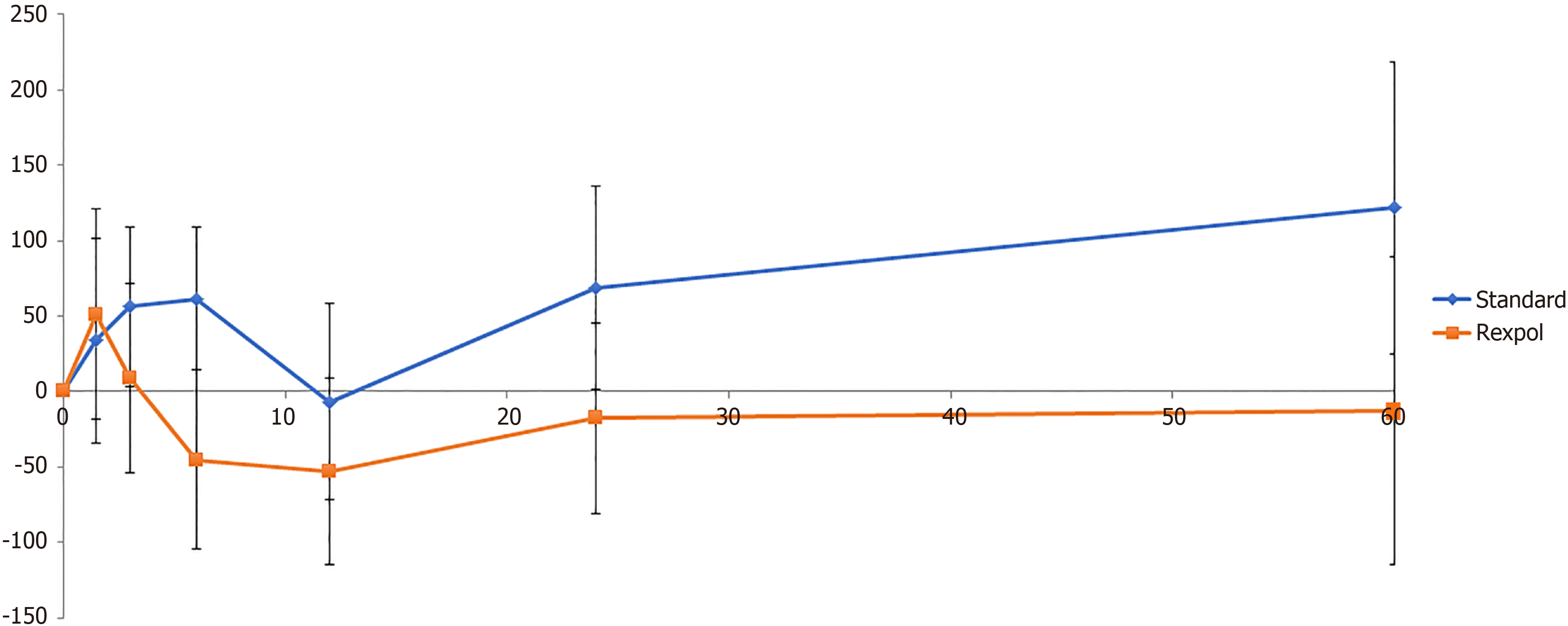
The total wear of the inlay measured from baseline showed less wear in all directions in the REXPOL group, which was significant in the REXPOL group in the latero-medial direction. All results of total wear measured from baseline are shown in Table 3. Due to a significant interaction between cup type and follow-up time, the wear pattern during follow-up of the REXPOL and Standard PE inlay were analysed separately. These wear patterns over the years showed greater wear in all directions in the conventional PE group, which is shown in Figures 3-6. The corresponding wear rates over this time period in Table 4 show that in all directions and volumes calculated, conventional PE had significant wear rates, whereas REXPOL did not show this outcome over time. The RSA images showed no signs of osteolysis.
| Conventional PE (Polyethylene) | REXPOL | P value | |
| Medial (mm) | -0.128 (-0.202 to -0.054) | 0.013 (-0.054 to 0.081) | 0.006 |
| Proximal (mm) | 0.196 (0.054 to 0.338) | 0.017 (-0.120 to 0.154) | 0.07 |
| Volume (mm3) | 113.39 (-2.48 to 229.26) | -30.59 (-167.16 to 105.98) | 0.10 |
| Corrected volume (mm3) | 121.6 (17.76 to 225.46) | -12.45 (-122.94; 98.03) | 0.07 |
| Conventional PE (Polyethylene) | P value | REXPOL | P value | |
| Medial (mm/yr) | -0.021 (-0.028 to -0.015) | < 0.001 | 0.004 (-0.002 to 0.009) | 0.21 |
| Proximal (mm/yr) | 0.033 (0.018 to 0.047) | < 0.001 | 0.003 (-0.011 to 0.017) | 0.66 |
| Volume (mm3/yr) | 15.94 (2.729 to 29.15) | 0.02 | -5.545 (-20.36 to 9.269) | 0.46 |
| Corrected volume (mm3/yr) | 18.53 (7.188 to 29.86) | 0.002 | -2.142 (-14.13 to 9.841) | 0.72 |
The functional questionnaires were obtained at five years, to detect potential differences in functional outcomes. These results are shown in Table 5, with no significant differences observed.
| Univariate | Multivariate | ||||
| Conventional PE (n = 17) | REXPOL (n = 17) | P value | Adjusted β-coefficient | P value | |
| HOOS pain | 93.8 (86.8; 100) | 85.9 (77.1; 94.7) | 0.15 | -3.3 (-14.9; 8.3) | 0.57 |
| HOOS ADL | 89.0 (81.2: 96.8) | 77.6 (66.6; 88.5) | 0.08 | -8.6 (-23.3; 6.1) | 0.24 |
| HHS | 89.7 (83.0; 96.4) | 86.5 (78.8; 94.2) | 0.51 | 0.15 (-10.1; 10.4) | 0.98 |
The main finding of this study is that total 3D wear was less in the REXPOL group than in the standard PE group, with significant less wear in the medial direction after five years. Moreover, the wear rates in the medial and proximal direction and in both volume and corrected volume were significant in the standard PE group, but not in the REXPOL group.
Several in vivo studies have shown that HXLPE can reduce wear in comparison with normal PE inlays in THA[16]. However, only one study investigated the results of REXPOL in vivo, without randomisation and RSA analysis[17]. The outcomes in that study supported our findings of reduced wear in the REXPOL group, with approximately 70% less wear at the five-year follow-up. Therefore, this study is the first to present randomised clinical RSA data regarding the REXPOL liner.
In other in vivo studies using RSA, a systematic review performed by Callary et al[18] showed that only 12 cohorts comprising 260 THAs have compared the outcomes of HXLPE vs normal PE. Their recommendations on standardisation of reporting RSA outcomes are applied in our study. However, the studies included in their review assessed different inlays and not all of them were randomised. Thus, our study contributes to their statement that more longer-term standardised studies are needed to improve our understanding of the factors related to wear. Moreover, this will provide a better indication of the chance of osteolysis and as a result loosening of the cup and revision in the longer term.
A literature review by Dumbleton et al[19] showed that a threshold for wear of 0.05 mm/year would eliminate osteolysis. Although both standard PE and REXPOL showed wear rates below this threshold in our study, the long-term wear of REXPOL is still unclear. Long-term results were reported in the study by Broomfield et al[20] using another brand of HXPLE with the same low wear rates at 12 years. Rates of 0.03 mm/year were seen in the standard PE group and 0.003 mm/year in the HXLPE group, with higher wear rates in patients with osteolysis. Their long-term outcomes were supported by several studies showing ten-year or longer wear rates in favour of HXLPE[21-23]. Moreover, the study by Oparaugo et al[24] clarified the correlation between wear debris-induced osteolysis, volumetric wear-rates and revision.
Despite this correlation, subsequent concern was raised that HXLPE microparticles would show increased bioactivity in vivo as this had been observed in in vitro studies, since these particles are smaller than conventional PE[25-27]. However, the in vivo study by Lachiewicz et al[28] showed that at 10 to 14 years, small osteolytic lesions were also seen with HXLPE. Broomfield et al[20] supported this outcome and showed 50% osteolysis after 12-years with conventional PE vs 4% with HXPLE, which was statistically significant. This shows that HXLPE wear particles are not more biologically active than conventional PE and may not elevate the risk of osteolysis.
As osteolysis is one of the main reasons for loosening of the cup, the aforementioned results on long-term reduction of osteolysis become even more clinically relevant if a reduction in revisions is seen over time. The study by Hanna et al[29] showed less wear in the HXLPE group and as a result no osteolysis or revisions at 13-years in the HXPLE group with an implant survival rate of 100% vs 86% in the conventional PE group. De Steiger et al[30] also confirmed this in a large observational study and showed a 16-year cumulative revision rate of 11.7% with conventional PE vs 6.2% with HXLPE. The aforementioned outcomes confirm that the lower wear rates of HXLPE as seen in our study can reduce the risk of osteolysis when compared to conventional PE and can also reduce revision rates in the longer term.
By measuring wear as the slope of the amount of penetration in the different directions, some negative results on wear are seen in this study. These negative wear rate outcomes have been reported in previous studies[1,31-35]. However, this may be due to lower wear rates of HXLPE being harder to accurately measure compared to conventional PE. Although RSA is considered the best way to measure wear of a prothesis, it has an accuracy range of 0.022 mm to 0.086 mm, depending on the direction of measurement. In the case of HXPLE with even lower wear rates, it becomes more challenging to determine small amounts of wear[36]. Therefore, it becomes more important to have large cohorts to detect significant differences. As our study was carried out with small cohorts, the wear results should be interpreted while bearing this in mind. The expectation is that in the longer term these wear rates can be calculated more accurately for HXLPE, because they will be determined outwith the threshold. Since our RSA analysis was performed while in the supine position, another explanation of the negative wear rates is subluxation of the femoral head while lying. However, this was not confirmed by the review conducted by Callary et al[18] who showed no differences between the studies on supine or standing RSA. To overcome problems of negative wear results, long-term results of HXLPE wear are needed.
According to the outcomes in favour of HXLPE, this study confirms that the use of inlays such as the REXPOL, is preferred in THAs.
The clinical outcomes of our study were measured to assess any major drawbacks of standard PE or HXLPE at the five-year follow-up. As improvement from baseline was seen in both groups with no significant differences between the groups, no practical disadvantages were seen by preferring one inlay over the other. As the study was not powered by clinical outcomes, further research is needed to investigate these outcomes.
This study showed, for the first time, that REXPOL resulted in less wear in the short-term in a randomised setting by RSA. Therefore, further investigation of wear over a longer period should be performed, to confirm that REXPOL can reduce the risk of osteolysis and consequently reduce revision rates in THA. Also, more research needs to be carried out to overcome problems of minimal differences in wear rates. In addition, research on other variables that influence wear such as activity, weight and surgical factors such as inclination of the acetabular component should be performed.
Total 3D wear is less with REXPOL inlay than with conventional PE inlay in THAs after five years. This study confirmed, for the first time, that the REXPOL HXLPE inlay is preferred to the standard PE inlay.
Highly cross-linked polyethylene (HXLPE) inlay in total hip arthroplasty (THA) is thought to result in lower wear rates in vivo, compared to conventional polyethylene.
More in vivo studies are needed, especially those using Roentgen stereo-photogrammetric analysis (RSA), to confirm the advantage of HXLPE over conventional polyethylene (PE).
The objective of the study was to compare wear of the HXLPE (REXPOL) and conventional PE acetabular inlay with similar ceramic head articulation, within the first five years after implantation.
A double-blind randomised controlled trial was performed to compare wear of REXPOL, a HXLPE, with conventional PE within the first five years after implantation using RSA.
The HXLPE (REXPOL) inlay showed less wear in the latero-medial direction. Significant wear rates of the conventional PE inlay were seen in the latero-medial and center-proximal direction and in volume and corrected volume, whereas the REXPOL inlay did not show this outcome over time.
Total 3D wear is less with the REXPOL (HXLPE) inlay than with the conventional PE inlay in THAs after five years.
Further investigations into the long-term wear and factors that might influence wear rates should be conducted, to confirm that HXLPE (REXPOL) can reduce the risk of osteolysis and consequently reduce revision rates in THA. In addition, an investigation of the impact of wear reduction by HXPLE on the functional outcomes of patients is required.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: ESSKA-AFAS.
Specialty type: Orthopedics
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cho MR, Cankaya D S-Editor: Zhang H L-Editor: Webster JR E-Editor: Xing YX
| 1. | McCalden RW, MacDonald SJ, Rorabeck CH, Bourne RB, Chess DG, Charron KD. Wear rate of highly cross-linked polyethylene in total hip arthroplasty. A randomized controlled trial. J Bone Joint Surg Am. 2009;91:773-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Haverkamp D. The latest information on total hip arthroplasty bearing surfaces. Minerva Ortopedica e Traumatologica. 2009;60:233-240. |
| 3. | Green TR, Fisher J, Stone M, Wroblewski BM, Ingham E. Polyethylene particles of a 'critical size' are necessary for the induction of cytokines by macrophages in vitro. Biomaterials. 1998;19:2297-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 331] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Chuter GS, Cloke DJ, Mahomed A, Partington PF, Green SM. Wear analysis of failed acetabular polyethylene: a comparison of analytical methods. J Bone Joint Surg Br. 2007;89:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 453] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 6. | Atienza C, Maloney WJ. Highly cross-linked polyethylene bearing surfaces in total hip arthroplasty. J Surg Orthop Adv. 2008;17:27-33. [PubMed] |
| 7. | Stilling M, Kold S, de Raedt S, Andersen NT, Rahbek O, Søballe K. Superior accuracy of model-based radiostereometric analysis for measurement of polyethylene wear: A phantom study. Bone Joint Res. 2012;1:180-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Valstar ER, Gill R, Ryd L, Flivik G, Börlin N, Kärrholm J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop. 2005;76:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 462] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 9. | Digas G, Kärrholm J, Thanner J, Malchau H, Herberts P. The Otto Aufranc Award. Highly cross-linked polyethylene in total hip arthroplasty: randomized evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis. Clin Orthop Relat Res. 2004;6-16. [PubMed] |
| 10. | Hirakawa K, Mitsugi N, Koshino T, Saito T, Hirasawa Y, Kubo T. Effect of acetabular cup position and orientation in cemented total hip arthroplasty. Clin Orthop Relat Res. 2001;135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Patil S, Bergula A, Chen PC, Colwell CW, D'Lima DD. Polyethylene wear and acetabular component orientation. J Bone Joint Surg Am. 2003;85-A Suppl 4:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 263] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. |
Hashimoto Y. Polyethylene wear in total hip arthroplasty: volumetric wear measurement of retrieved acetabular components.
41th Annual Meeting Orthopaedic Research Society 1995; |
| 13. | Mizoue T, Yamamoto K, Masaoka T, Imakiire A, Akagi M, Clarke IC. Validation of acetabular cup wear volume based on direct and two-dimensional measurements: hip simulator analysis. J Orthop Sci. 2003;8:491-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Nilsdotter AK, Lohmander LS, Klässbo M, Roos EM. Hip disability and osteoarthritis outcome score (HOOS)--validity and responsiveness in total hip replacement. BMC Musculoskelet Disord. 2003;4:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 780] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 15. | Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737-755. [PubMed] |
| 16. | Kurtz SM, Gawel HA, Patel JD. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clin Orthop Relat Res. 2011;469:2262-2277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 369] [Cited by in RCA: 322] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 17. | Orradre Burusco I, Romero R, Brun M, López Blasco JJ. Cross-linked ultra-high-molecular weight polyethylene liner and ceramic femoral head in total hip arthroplasty: a prospective study at 5 years follow-up. Arch Orthop Trauma Surg. 2011;131:1711-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Callary SA, Solomon LB, Holubowycz OT, Campbell DG, Munn Z, Howie DW. Wear of highly crosslinked polyethylene acetabular components. Acta Orthop. 2015;86:159-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty. 2002;17:649-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 430] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 20. | Broomfield JA, Malak TT, Thomas GE, Palmer AJ, Taylor A, Glyn-Jones S. The Relationship Between Polyethylene Wear and Periprosthetic Osteolysis in Total Hip Arthroplasty at 12 Years in a Randomized Controlled Trial Cohort. J Arthroplasty. 2017;32:1186-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Johanson PE, Digas G, Herberts P, Thanner J, Kärrholm J. Highly crosslinked polyethylene does not reduce aseptic loosening in cemented THA 10-year findings of a randomized study. Clin Orthop Relat Res. 2012;470:3083-3093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Röhrl SM, Nivbrant B, Nilsson KG. No adverse effects of submelt-annealed highly crosslinked polyethylene in cemented cups: an RSA study of 8 patients 10 yaers after surgery. Acta Orthop. 2012;83:148-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Teeter MG, Lanting BA, Naudie DD, McCalden RW, Howard JL, MacDonald SJ. Highly crosslinked polyethylene wear rates and acetabular component orientation: a minimum ten-year follow-up. Bone Joint J. 2018;100-B:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Oparaugo PC, Clarke IC, Malchau H, Herberts P. Correlation of wear debris-induced osteolysis and revision with volumetric wear-rates of polyethylene: a survey of 8 reports in the literature. Acta Orthop Scand. 2001;72:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Fisher J, McEwen HM, Tipper JL, Galvin AL, Ingram J, Kamali A, Stone MH, Ingham E. Wear, debris, and biologic activity of cross-linked polyethylene in the knee: benefits and potential concerns. Clin Orthop Relat Res. 2004;114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Ingram JH, Stone M, Fisher J, Ingham E. The influence of molecular weight, crosslinking and counterface roughness on TNF-alpha production by macrophages in response to ultra high molecular weight polyethylene particles. Biomaterials. 2004;25:3511-3522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Ries MD, Scott ML, Jani S. Relationship between gravimetric wear and particle generation in hip simulators: conventional compared with cross-linked polyethylene. J Bone Joint Surg Am. 2001;83-A Suppl 2 Pt 2:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Lachiewicz PF, Soileau ES, Martell JM. Wear and Osteolysis of Highly Crosslinked Polyethylene at 10 to 14 Years: The Effect of Femoral Head Size. Clin Orthop Relat Res. 2016;474:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 29. | Hanna SA, Somerville L, McCalden RW, Naudie DD, MacDonald SJ. Highly cross-linked polyethylene decreases the rate of revision of total hip arthroplasty compared with conventional polyethylene at 13 years' follow-up. Bone Joint J. 2016;98-B:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 30. | de Steiger R, Lorimer M, Graves SE. Cross-Linked Polyethylene for Total Hip Arthroplasty Markedly Reduces Revision Surgery at 16 Years. J Bone Joint Surg Am. 2018;100:1281-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 31. | Bragdon CR, Barrett S, Martell JM, Greene ME, Malchau H, Harris WH. Steady-state penetration rates of electron beam-irradiated, highly cross-linked polyethylene at an average 45-month follow-up. J Arthroplasty. 2006;21:935-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Callary SA, Field JR, Campbell DG. Low wear of a second-generation highly crosslinked polyethylene liner: a 5-year radiostereometric analysis study. Clin Orthop Relat Res. 2013;471:3596-3600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Campbell DG, Field JR, Callary SA. Second-generation highly cross-linked X3™ polyethylene wear: a preliminary radiostereometric analysis study. Clin Orthop Relat Res. 2010;468:2704-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Engh CA, Stepniewski AS, Ginn SD, Beykirch SE, Sychterz-Terefenko CJ, Hopper RH, Engh CA. A randomized prospective evaluation of outcomes after total hip arthroplasty using cross-linked marathon and non-cross-linked Enduron polyethylene liners. J Arthroplasty. 2006;21:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Manning DW, Chiang PP, Martell JM, Galante JO, Harris WH. In vivo comparative wear study of traditional and highly cross-linked polyethylene in total hip arthroplasty. J Arthroplasty. 2005;20:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 36. | Bragdon CR, Malchau H, Yuan X, Perinchief R, Kärrholm J, Börlin N, Estok DM, Harris WH. Experimental assessment of precision and accuracy of radiostereometric analysis for the determination of polyethylene wear in a total hip replacement model. J Orthop Res. 2002;20:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |