Published online Oct 28, 2019. doi: 10.5312/wjo.v10.i11.394
Peer-review started: May 8, 2019
First decision: July 31, 2019
Revised: September 6, 2019
Accepted: October 7, 2019
Article in press: October 7, 2019
Published online: October 28, 2019
Processing time: 201 Days and 15.7 Hours
Magnetically controlled growing rods (MCGR) are a novel treatment option for early onset scoliosis (EOS). Although the complication profile with MCGR use has been reviewed, these reviews do not take into account important implants modifications, termed iterations, that were made due to early on postoperative complications is not well reported or understood.
To assess the effect of MCGR implant iterations on post-operative complications in EOS.
A systematic review was performed to identify studies investigating MCGR specifically for the treatment of EOS, refined to those reporting the implant iteration, specifically the incorporation of the keeper plate to the implant design. Articles with mixed implant iteration usage were excluded. Complications following surgery were recorded as well as potential risk factors and compared between implant cohorts.
Although 20 articles were identified for inclusion, 5 included mixed implant iteration leaving a total of 271 patients identified through 15 clinical studies that met inclusion criteria. The average follow-up was 25.4-mo. Pre-keeper plate implants were utilized in 3 studies with a total of 49 patients. Overall, 115 (42.4%) post-operative complications were identified, with 87% defined as major. The addition of the keeper plate significantly decreased the rate of post-operative complications per study (35.7% vs 80.6%, P = 0.036), and the rate of distraction failure (8.1% vs 40.8%, P = 0.02). Unplanned reoperation occurred in 69 (26.7%) patients but was not different between implant iteration cohorts (25.5% without keeper plate vs 27.1% with keeper plate, P = 0.92).
MCGR for EOS has a cumulative complication rate of 42.4% but this is significantly reduced to 35.7% when reviewing only keeper-plate enabled implants. However, 25% of published articles included mixed implant iterations. Future studies should discern between implants iterations when reporting on the usage of MCGR for EOS.
Core tip: Magnetically controlled growing rods (MCGR) are a novel treatment approach for early onset scoliosis which is gaining increases clinical usage. Since its introduction, numerous modifications have been implemented to improve the performance of the construct, however, these modifications are often over-looked in current published series. This study evaluated the effect of the addition of the keeper plate to MCGR, finding that it had a significant impact on decreasing the rate of post-operative distraction failures. Despite the impact of this modification, 25% of published articles included mixed implant designs in their series, potentially inflating reported complication rates.
- Citation: Shaw KA, Hire JM, Kim S, Devito DP, Schmitz ML, Murphy JS. Magnetically controlled growing instrumentation for early onset scoliosis: Caution needed when interpreting the literature. World J Orthop 2019; 10(11): 394-403
- URL: https://www.wjgnet.com/2218-5836/full/v10/i11/394.htm
- DOI: https://dx.doi.org/10.5312/wjo.v10.i11.394
Early onset scoliosis (EOS) is a complex entity that has seen an evolution in its approach to surgical intervention from early definitive fusion, to non-fusion technique that allow and facilitate continued spinal growth[1]. Magnetically controlled growing rods (MCGR) are one such non-fusion approach that has gained interest and support since its introduction in 2007[2]. MCGR has been found to be a safe and effective non-fusion treatment for EOS[3-5], with equivalent curve correction and thoracic height growth as compared with traditional growing rods (TGR)[6]. Clinical reports, however, on the outcomes and complications of MCGR have been limited to case series and cohort studies with limited patient numbers[2-23].
Thakar et al[24] preformed a retrospective review of reported studies using MCGR for the treatment of EOS. From an identified 15 studies including 336 children undergoing MCGR insertion, they identified a mean complication rate of 44.5%, with 33% of children undergoing an unplanned reoperation. However, the timeline of these studies included spanned a seven year period since the introduction of the implant[24]. Over this period, the manufacturers made several alterations to the implant design, consisting first of the addition of a keeper plate in 2010 to the actuator to decrease the incidence of lost distraction, followed by alterations to the welding process in 2012, as well as expanded size options in the rod and actuator[2,25].
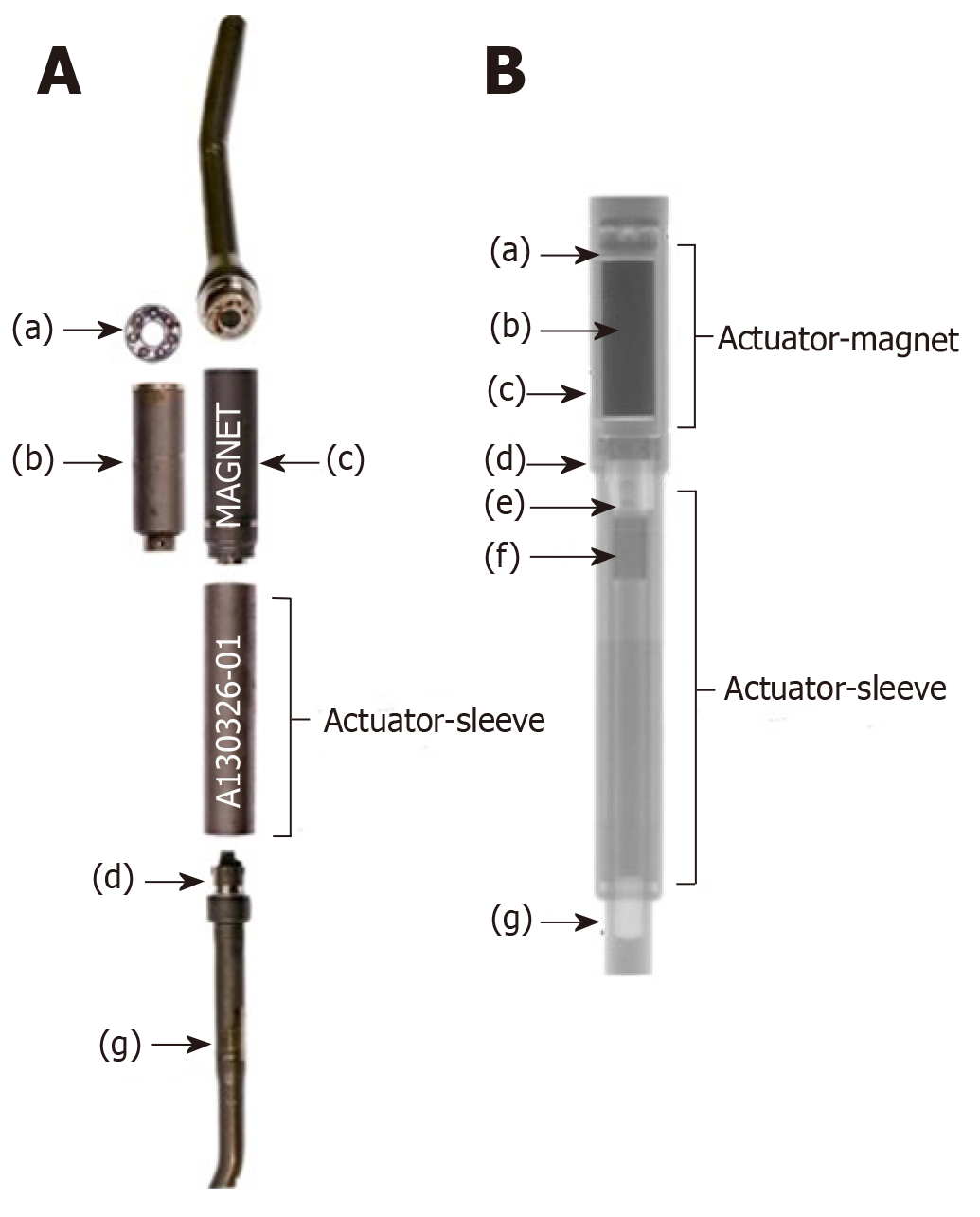
Early reports identified a high rate of loss of distraction due to the magnetic lengthening mechanism being unable to maintain the rod in the lengthened position. Due to the rotatory mechanism of lengthening, this inability to lock the rod in the lengthened position, the actuator was prone to unwind and shorten resulted in a loss of distraction[2,25]. To combat this, a magnetic lock, the keeper plate, was applied around the lengthening mechanism to maintain the rod in place at its desired length and prevent the rod collapse identified in the early implant iterations, Figure 1. However, the efficacy of the keeper plate to decrease the rate of loss of distraction has not been previously reported.
The aim of this study is to examine the reported literature on the reporting of implant iterations as well as its effect on the post-operative complication rates following MCGR implantation for the treatment of EOS, specifically the effect of the addition of the keeper plate. We hypothesized that the reporting of implant iteration would be limited and the rate of postoperative complications, specifically the rate of distraction loss, would be significantly lower in children treated with implants containing a keeper plate.
After obtaining institutional review board approval, a comprehensive systematic review was conducted using an internet-based search beginning with queries into the MEDLINE database for all articles between January 1, 1967 and February 1, 2018. The search terms included: (1) “early onset scoliosis”; (2) “magnetically controlled growing rods”; (3) “scoliosis”; and (4) “magnetically controlled growing rods complications”. The preferred reporting items for systematic reviews and meta-analyses protocol was followed for data analysis and synthesis[26].
The abstracts of all identified articles were subsequently analyzed to determine relevance to complications associated with MCGR for early-onset scoliosis. Articles were excluded for one or more of the following criteria: Literature review or expert opinion, publication in non-English language, published prior to the year 1967, did not include pediatric patients, included fewer than 3 patients, implanted instrumentation other than MCGR. Studies reported from the same institution were further scrutinized to determine if overlapping patient cohorts were reported, excluding studies with shorter average follow-up.
A total of 49 articles were identified for further review. The full manuscripts of the remaining studies were then reviewed for the following inclusion criteria: Peer-reviewed clinical studies of level I to IV evidence, involving pediatric patients undergoing surgery for implantation of MCGR, and reporting the number of perioperative complications and unplanned procedures. The references of all articles were cross-referenced as well for any additional articles that were not found on the initial search. The patient cohorts of studies with the same authors and/or institutions were scrutinized to ensure that no redundant data was collected.
Articles were further reviewed to determine the iteration of implant utilized. Since its introduction, there have been 7 main alterations to the implant design with the earliest change being the addition of a keeper plate, introduced in 2010, to correct early issues with loss of distraction[2,25]. Articles were reviewed to delineate between series with and without the keeper plate based upon either direct report or time period reviewed in each study. For studies that did not specify the iteration of implant used, surgical dates were reviewed with years before 2010 defined as pre-Keeper plate series. Studies with mixed implants utilized were included in the analysis if they included > 80% of procedures with a specific implant. Studies with overlapping surgical dates were excluded.
Patient demographics (age, gender, curve etiology), construct design (number of rods implanted, technique, anchors placed), and the frequency and number of lengthening’s were extracted from each article. Complication rates were recorded for each study. Complications were classified as either major or minor, with major complications defined as complications necessitating cessation of treatment (failure of distraction) or revision surgery (implant failure to include rod breakage, screw pull-out, proximal junctional kyphosis, deep surgical site infection, or sequela that did not resolve without significant interventions). Minor complications were defined as prominent hardware, superficial surgical site infection, or issues that required minimal intervention without repeat surgical intervention. Reoperation or need for revision surgery was recorded as a separate variable.
Data analysis was performed using SPSS statistical package version 24 (SPSS Inc, Chicago, IL, United States). Significance was set at P < 0.05. Descriptive statistics were generated. Univariate analyses were used to compare overall complication rates by implant iteration, specific complication rates, and to identify risk factors for post-operative complications.
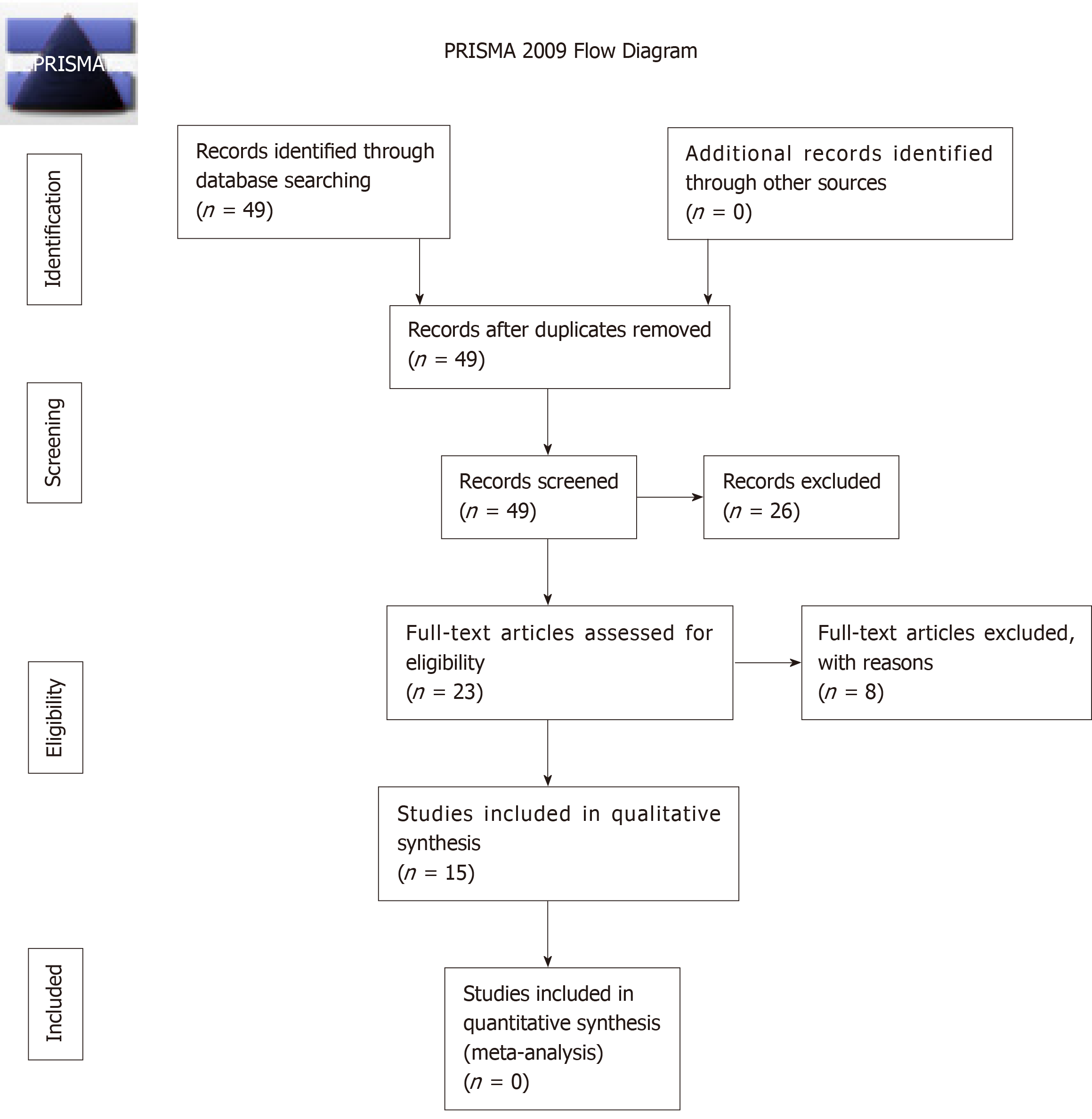
A total of 49 studies were identified for manuscript review. After review of the manuscripts, 26 were excluded (7 mechanical failure studies, 6 cost comparison studies, 3 imaging studies, 2 case reports, 2 editorial, 2 non-human studies, 2 animal studies, 1 case series, and 1 review article). Of the remaining 23 clinical articles, 3 additional studies were excluded (1 each with insufficient patient number, overlapping patient samples, combined MCGR/Shilla technique) leaving 20 clinical studies for review. Of these 20 studies, an additional 5 studies were excluded due to mixed implant iterations leaving 15 studies that met inclusionary criteria, consisting of 11 case series and 4 cohort studies, Figure 2.
From the 15 clinical articles, a total of 271 children were identified (7.87 years ± 1.54 years, 46.8% male) with an average of 26.4-mo follow-up. Curve etiology is summarized in Table 1, with idiopathic (32.8%) reported as the most common, and an average curve magnitude of 61.3 degrees. Pre-keeper plate implants were utilized in 3 studies with remaining 12 post-Keeper plate implants. The majority of cases were primary MCGR implantations (74.7%) vs conversion procedures (25.2%). Dual rod instrumentation (76.4%) was the most common construct, with children undergoing an average of 7.85 lengthening’s.
| Items | |
| Curve etiology | |
| Idiopathic | 89 (32.8) |
| Congenital | 43 (15.9) |
| Syndromic | 68 (25.1) |
| Neuromuscular | 63 (23.2) |
| Neurofibromatosis | 8 (2.9) |
| Type of surgery | |
| Primary | 195 (74.7) |
| Conversion | 66 (25.3) |
| Unspecified | 10 |
| Type of instrumentation | |
| Single rod | 64 (23.6) |
| Dual rod | 207 (76.4) |
From the identified 271 children, 115 (42.4%) experienced a post-operative complication, Table 2. Of the 115 complications, 95 (82.6%) were defined as major, with an average major complication rate of 80% per study. Complications were not subdivided according to curve etiology. Failure of distraction was the most common complication, occurring in 14% of children, followed by implant failure (including rod breakage and implant failure not otherwise characterized) in 8.86%, and screw/hook pullout (8.12%), Table 2. Of the 115 children with a postoperative complication, 69 patients (27.9% of overall cohort) required an unplanned reoperation. The most common reason for reoperation was the inability to distract (n = 20), followed by proximal instrumentation pullout with or without proximal junctional kyphosis (n = 19), rod breakage (n = 19), wound dehiscence/infections (n = 6), prominent hardware (n = 2), and 3 unlisted procedures.
| Complication rate | Without keeper plate | With keeper plate | |
| Overall complication rate/study | 35.6% (n = 115) | 80.61% (n = 38) | 35.65% (n = 77) |
| Major complications | n = 95 | n = 32 | n = 63 |
| Cumulative Complications | |||
| Distraction failure | 14.0% (n = 38) | 40.8% (n = 20) | 8.1% (n = 18) |
| Implant failure | 8.86% (n = 24) | 18.36% (n = 9) | 6.76% (n = 15) |
| Screw pull-out | 8.12% (n = 22) | 4.1% (n = 2) | 9.0% (n = 20) |
| Infection | 2.2% (n = 6) | 2.04% (n = 1) | 2.25% (n = 5) |
| Prominent hardware | 2.58% (n = 7) | 14.28% (n = 7) | 0% (n = 0) |
| Proximal junctional kyphosis | 2.58% (n = 7) | 0% (n = 0) | 3.15% (n = 7) |
| Wound dehiscence | 0.74% (n = 2) | 0% (n = 0) | 0.9% (n = 2) |
Univariate analysis of complications between implant iterations identified that complication rates significantly decreased with the addition of the keeper plate (35.7% vs 80.6%, P = 0.036, Table 2). Additionally, there was a statistically significant decrease in the rate of distraction failure in the keeper plate cohort (8.1% vs 40.8%, P = 0.02). There was not difference in reoperation rates between implant iteration cohorts (25.5% without keeper plate vs 27.1% with keeper plate, P = 0.92). Identified studies did not provide information for revision surgeries according to type of instrumentation (single rod vs dual road), or by proximal anchor type (rib vs spine) or number of proximal anchor points. Given the paucity of available data, a subgroups analysis was foregone. Summary of articles included for analysis is shown in the Table 3.
| First author | Yr | Keeper plate? | # Of patients | Primary surgeries | Revisions | % Male | Age at surgery (yr) | Curve magnitude |
| Hickey[8] | 2014 | Y | 8 | 4 | 4 | 75% | 4.5 | 59.25 |
| Akbarnia[6] | 2014 | N | 12 | 12 | 0 | 42% | 6.8 | 59 |
| Lebon[4] | 2017 | Y | 30 | 25 | 5 | 53% | 9.1 | 66 |
| Akbarnia[2] | 2013 | N | 14 | 14 | 0 | 50% | 8.83 | 60 |
| Thompson[17] | 2016 | Y | 19 | 11 | 8 | 53% | 9.1 | 62 |
| Heydar[14] | 2017 | Y | 16 | 16 | 0 | 37.5% | 7.83 | 62 |
| Heydar[3] | 2016 | Y | 18 | 18 | 0 | 39% | 7.3 | 68 |
| Yılmaz[18] | 2016 | Y | 8 | 5 | 3 | 25% | 10.6 | --- |
| Keskinen[16] | 2016 | Y | 50 | 27 | 23 | 38.4% | 55.2 | |
| Hosseini[15] | 2016 | N | 23 | 15 | 8 | 29.2% | 7.45 | 55.35 |
| La Rosa[21] | 2017 | Y | 10 | 10 | 0 | 50% | 7.2 | 64.7 |
| Teoh[11] | 2016 | Y | 8 | 4 | 4 | --- | 8.2 | 60 |
| Rolton[22] | 2016 | Y | 21 | 10 | 11 | 52% | 7.8 | 54 |
| Nnadi[23] | 2018 | Y | 10 | 10 | 0 | 50% | 6.2 | 57.7 |
| Ridderbusch[5] | 2017 | Y | 24 | 24 | 0 | 33% | 8.9 | 63 |
Through this systematic review, we identified that children treated with all types of MCGR implants for EOS have a 42.4% rate of postoperative complications at an average of 26.4-mo follow-up after implantation, with failure of distraction being the most common complication seen in 14%. The implant iteration was found to significantly affect complication rates with the keeper plate-enabled implants significantly decreasing the rate of postoperative complications (35.7% vs 80.6%). However, of the 20 studies published at the time of this review, 25% included mixed implants iterations in their retrospective reviews.
Complications in the treatment of EOS are not infrequent, given the patient age and the necessity to accommodate continued growth of the thorax and spine. TGR instrumentation preceded MCGR in the treatment of EOS, with well-reported complication profiles. Bess et al[27] reported that 58% of patients developed at least one complication during their treatment duration, with higher rates of complications with the use of single rod fixation, decreasing patient age, and with each additional lengthening procedure. Yang et al[28] identified underlying scoliosis etiology, prior rod failure, single rod constructs, stainless steel rods, small diameter rods, and tandem connector variables as risk factors for rod failure with TGR. Additionally, the requirement for repeat surgical interventions for lengthening increase the rate of wound and other complications 24% for each additional lengthening procedure[28].
MCGR was developed in an attempt to meet the need for continued spinal growth and curve correction while attempting to decrease the risk of post-operative complications. MCGR functionally lengthens the spinal construct through the application of an external magnet which induces a rotatory motion to the actuator, which is threaded, resulting in elongation[2]. Akbarnia et al[6] performed a case-matched comparison of children with EOS treated with MCGR and TGR, finding equivalent curve correction and thoracic height gain. Although the MCGR cohort had less overall surgical procedures, the incidence of unplanned reoperation secondary to post-operative complications was not affected, with 75% of MCGR reoperations occurring secondary to unspecified implant failures.
Unique to MCGR is the risk of rod distraction failure[29], which accounts for between 25%-35% of unplanned surgical procedures[4,29]. The current findings reinforce previous studies[24], that these instances are not isolated, with loss of distraction accounting for 33% of all complications, and 28.9% of reoperations. Numerous mechanisms for distraction failure have been identified in the literature, to include: Fracture of the actuator pin, wear of the extending bar, debris in the actuator, damage to the radial bearings, and O-ring seal failure[30,31]. Loss of distraction ranged in the reported articles, accounting for between 0% to 100% of complications, and affecting between 0% and 100% of patients/series (average 14.86% patients/series)[2-22,29,32].
The only identified risk factor for complication was the use of a pre-keeper plate implant, with an 80.6% complication rate compared with 35.7% in keeper plate enabled implants. The necessity for the keeper plate was identified early following the induction of MCGR due to tendency for the actuator to unwind and shorten resulted in a loss of distraction[2,25]. To combat this, a magnetic lock, the keeper plate, was applied around the lengthening mechanism to maintain the actuator in the desired lengthen position and prevent rod collapse[25]. With regard to distraction failure, this decreased to a rate of 8.1% from 40.8% with the introduction of the keeper plate. This data indicates that the keeper plate was successful as designed to lock the magnetic actuator in its lengthening position, resisting the tendency to unwind and shorten following distraction.
An important implication of this data is in the future reporting of clinical outcomes of MCGR and the synthesis of the current published literature in systematic reviews. Since the introduction of MCGR technology, the product has gone through a continual process of quality improvement, evident by the seven iteration changes to date[1,9]. This study is the first to report on the effect these iteration changes have on post-operative complications, specifically the introduction of the keeper plate to reduce rod distraction failure. Despite this fact, 25% of the published clinical articles included mixed implant iterations in their analysis. Given these significant differences, future studies and systematic reviews need to include implant iterations in their data reporting and analysis for postoperative complications to avoid contaminating the results of more recent MCGR implant iterations.
This study is not without its limits. As a systematic review, the strength of the findings are solely dependent on the quality and rigor of the studies included in the analysis, which in this instance is comprised largely of level IV case series and four level II cohort studies. As a newer surgical technique, there is also the risk for performance bias between the 2 study cohorts, which could also impact the rate of postoperative complications. This is further confounded by the temporal relationships between included studies. The concern for overlapping patients in the identified studies was mitigated by close inspection of the study methods. However, several studies reported data from multi-center databases[1,2,15,18] and as such, the risk for overlapping information is present.
A number of the identified risk factors for post-operative complications, include patient age, curve etiology, number, and type of proximal and distal fixation points, as well as type of implantation (primary vs conversion), were not able to be investigated due to a lack of reporting in the original studies. The average follow-up in this review consisted of 26 mo. Given that the average patient age at time of MCGR implantation was 7.87 years, these results do not account for the full extent of the child’s treatment course and may underestimate the long-term complication profile. Additionally, there is no standard method for reporting complications for children treated with MCGR, leading to variable methods of reporting in the identified studies.
Given these identified deficiencies in standardized complication reporting, we recommend future studies also consider MCGR complication reporting according to patient and treatment variables (underlying diagnosis, number of rods, type of implantation, type and number of proximal anchorage points, occurrence of complication by number of lengthenings) and classify complications into the following categories: Permanent mechanical distraction failure, temporary distraction failure, rod breakage unrelated to the distraction mechanism, proximal anchorage failure, infectious/wound complication, and hardware prominence. These six categories represent the most common post-operative complications, while also identifying complications requiring an alteration in the planned treatment course.
In conclusion, this systematic review identified that 271 children undergoing MCGR implantation for the treatment of EOS, resulting in a cumulative 42.4% rate of post-operative complications, 87% of which required a cessation in the planned treatment course or a reoperation. The introduction of the keeper plate significantly decreased the rate of post-operative complications to 35.7% and the rate of distraction failure. However, of the 20 clinical articles reporting on the outcomes of MCGR in EOS, 25% included mixed implant iterations highlighting the need for strict. Further research is needed to investigate the effects of subsequent implant iterations as well as the long-term outcomes of treatment.
Although the outcomes of using magnetically controlled growing rods (MCGR) to treat early onset scoliosis (EOS) has been reviewed, these studies do not take into account important implants modifications, termed iterations, that were made due to early on postoperative complications is not well reported or understood.
To gain a deeper understanding of how modification to MCGR after affected patients outcomes for the treatment of EOS and the implications of these effects on the reporting of future MCGR.
To assess the effect of MCGR implant iterations on post-operative complications in EOS.
A systematic review was performed to identify studies investigating MCGR specifically for the treatment of EOS, refined to those reporting the implant iteration, specifically the incorporation of the keeper plate to the implant design. Articles with mixed implant iteration usage were excluded. Complications following surgery were recorded as well as potential risk factors and compared between implant cohorts.
Although 20 articles were identified for inclusion, 5 (25%) included mixed implant iteration leaving a total of 271 patients identified through 15 clinical studies that met inclusion criteria. Pre-keeper plate implants were utilized in 3 studies with a total of 49 patients. Overall, 115 (42.4%) post-operative complications were identified, with 87% defined as major. The addition of the keeper plate significantly decreased the rate of post-operative complications per study (35.7% vs 80.6%, P = 0.036), and the rate of distraction failure (8.1% vs 40.8%, P = 0.02). Unplanned reoperation occurred in 69 (26.7%) patients but was not different between implant iteration cohorts (25.5% without keeper plate vs 27.1% with keeper plate, P = 0.92).
MCGR implant with Keeper plates have less post-operative distraction failures. Of the currently published studies, 25% include mixed implant designs. Future studies reporting on MCGR outcomes should include implant iteration in their analysis. MCGR implant with Keeper plates have less post-operative distraction failures. Of the currently published studies, 25% include mixed implant designs. Studies included mixed implant iterations could be artificially inflating postoperative complication rates. Have more recent implant modification exhibited similar effects on MCGR outcomes. Twenty-five percent of currently published studies on MCGR outcomes included mixed implant iterations which could be artificially inflating complication rates. The addition of the keeper plate has decreased the incidence of distraction failure in the treatment of EOS. Understanding implant design gives important insight to understanding how they affect patient outcomes.
Future studies should include implant iterations in the reporting of MCGR outcomes for the treatment of EOS. Long-term follow-up of children treated with MCGR for EOS. Subdividing MCGR outcomes by implant iteration will help ensure complications rates are not artificially inflated.
Manuscript source: Invited Manuscript
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Peng BGS-Editor: Tang JZ L-Editor: A E-Editor: Liu MY
| 1. | Yang S, Andras LM, Redding GJ, Skaggs DL. Early-Onset Scoliosis: A Review of History, Current Treatment, and Future Directions. Pediatrics. 2016;137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 2. | Akbarnia BA, Cheung K, Noordeen H, Elsebaie H, Yazici M, Dannawi Z, Kabirian N. Next generation of growth-sparing techniques: preliminary clinical results of a magnetically controlled growing rod in 14 patients with early-onset scoliosis. Spine (Phila Pa 1976). 2013;38:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Heydar AM, Şirazi S, Bezer M. Magnetic Controlled Growing Rods as a Treatment of Early Onset Scoliosis. Spine. 2016;41:E1336-E1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Lebon J, Batailler C, Wargny M, Choufani E, Violas P, Fron D, Kieffer J, Accadbled F, Cunin V, De Gauzy JS. Magnetically controlled growing rod in early onset scoliosis: a 30-case multicenter study. Eur Spine J. 2017;26:1567-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Ridderbusch K, Rupprecht M, Kunkel P, Hagemann C, Stücker R. Preliminary Results of Magnetically Controlled Growing Rods for Early Onset Scoliosis. J Pediatr Orthop. 2017;37:e575-e580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Akbarnia BA, Pawelek JB, Cheung KM, Demirkiran G, Elsebaie H, Emans JB, Johnston CE, Mundis GM, Noordeen H, Skaggs DL, Sponseller PD, Thompson GH, Yaszay B, Yazici M; Growing Spine Study Group. Traditional Growing Rods Versus Magnetically Controlled Growing Rods for the Surgical Treatment of Early-Onset Scoliosis: A Case-Matched 2-Year Study. Spine Deform. 2014;2:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Dannawi Z, Altaf F, Harshavardhana NS, El Sebaie H, Noordeen H. Early results of a remotely-operated magnetic growth rod in early-onset scoliosis. Bone Joint J. 2013;95-B:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Hickey BA, Towriss C, Baxter G, Yasso S, James S, Jones A, Howes J, Davies P, Ahuja S. Early experience of MAGEC magnetic growing rods in the treatment of early onset scoliosis. Eur Spine J. 2014;23 Suppl 1:S61-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Skov ST, Wijdicks SPJ, Bünger C, Castelein RM, Li H, Kruyt MC. Treatment of early-onset scoliosis with a hybrid of a concave magnetic driver (magnetic controlled growth rod) and a contralateral passive sliding rod construct with apical control: preliminary report on 17 cases. Spine J. 2018;18:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Teoh KH, Winson DM, James SH, Jones A, Howes J, Davies PR, Ahuja S. Do magnetic growing rods have lower complication rates compared with conventional growing rods? Spine J. 2016;16:S40-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Teoh KH, Winson DM, James SH, Jones A, Howes J, Davies PR, Ahuja S. Magnetic controlled growing rods for early-onset scoliosis: a 4-year follow-up. Spine J. 2016;16:S34-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Cheung JP, Bow C, Samartzis D, Kwan K, Cheung KM. Frequent small distractions with a magnetically controlled growing rod for early-onset scoliosis and avoidance of the law of diminishing returns. J Orthop Surg (Hong Kong). 2016;24:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Cheung KM, Cheung JP, Samartzis D, Mak KC, Wong YW, Cheung WY, Akbarnia BA, Luk KD. Magnetically controlled growing rods for severe spinal curvature in young children: a prospective case series. The Lancet. 2012;379:1967-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 14. | Heydar AM, Şirazi S, Okay E, Kiyak G, Bezer M. Short Segment Spinal Instrumentation in Early-onset Scoliosis Patients Treated With Magnetically Controlled Growing Rods: Surgical Technique and Mid - Short-term Outcomes. Spine (Phila Pa 1976). 2017;42:1888-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Hosseini P, Pawelek J, Mundis GM, Yaszay B, Ferguson J, Helenius I, Cheung KM, Demirkiran G, Alanay A, Senkoylu A, Elsebaie H, Akbarnia BA. Magnetically controlled Growing Rods for Early-onset Scoliosis: A Multicenter Study of 23 Cases With Minimum 2 years Follow-up. Spine (Phila Pa 1976). 2016;41:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Keskinen H, Helenius I, Nnadi C, Cheung K, Ferguson J, Mundis G, Pawelek J, Akbarnia BA. Preliminary comparison of primary and conversion surgery with magnetically controlled growing rods in children with early onset scoliosis. Eur Spine J. 2016;25:3294-3300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Thompson W, Thakar C, Rolton DJ, Wilson-MacDonald J, Nnadi C. The use of magnetically-controlled growing rods to treat children with early-onset scoliosis: early radiological results in 19 children. Bone Joint J. 2016;98-B:1240-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Yılmaz B, Ekşi MŞ, Işik S, Özcan-Ekşi EE, Toktaş ZO, Konya D. Magnetically Controlled Growing Rod in Early-Onset Scoliosis: A Minimum of 2-Year Follow-Up. Pediatr Neurosurg. 2016;51:292-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Yoon WW, Sedra F, Shah S, Wallis C, Muntoni F, Noordeen H. Improvement of pulmonary function in children with early-onset scoliosis using magnetic growth rods. Spine (Phila Pa 1976). 2014;39:1196-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Doany ME, Olgun ZD, Kinikli GI, Bekmez S, Kocyigit A, Demirkiran G, Karaagaoglu AE, Yazici M. Health-Related Quality of Life in Early-Onset Scoliosis Patients Treated Surgically: EOSQ Scores in Traditional Growing Rod Versus Magnetically Controlled Growing Rods. Spine (Phila Pa 1976). 2018;43:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | La Rosa G, Oggiano L, Ruzzini L. Magnetically Controlled Growing Rods for the Management of Early-onset Scoliosis: A Preliminary Report. J Pediatr Orthop. 2017;37:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Rolton D, Thakar C, Wilson-MacDonald J, Nnadi C. Radiological and clinical assessment of the distraction achieved with remotely expandable growing rods in early onset scoliosis. Eur Spine J. 2016;25:3371-3376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Nnadi C, Thakar C, Wilson-MacDonald J, Milner P, Rao A, Mayers D, Fairbank J, Subramanian T. An NIHR-approved two-year observational study on magnetically controlled growth rods in the treatment of early onset scoliosis. Bone Joint J. 2018;100-B:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Thakar C, Kieser DC, Mardare M, Haleem S, Fairbank J, Nnadi C. Systematic review of the complications associated with magnetically controlled growing rods for the treatment of early onset scoliosis. Eur Spine J. 2018;27:2062-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 25. | Jenks M, Craig J, Higgins J, Willits I, Barata T, Wood H, Kimpton C, Sims A. The MAGEC system for spinal lengthening in children with scoliosis. Appl Health Econ Health Policy. 2014;12:587-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13305] [Article Influence: 831.6] [Reference Citation Analysis (0)] |
| 27. | Bess S, Akbarnia BA, Thompson GH, Sponseller PD, Shah SA, El Sebaie H, Boachie-Adjei O, Karlin LI, Canale S, Poe-Kochert C, Skaggs DL. Complications of growing-rod treatment for early-onset scoliosis: analysis of one hundred and forty patients. J Bone Joint Surg Am. 2010;92:2533-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 428] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 28. | Yang JS, Sponseller PD, Thompson GH, Akbarnia BA, Emans JB, Yazici M, Skaggs DL, Shah SA, Salari P, Poe-Kochert C; Growing Spine Study Group. Growing rod fractures: risk factors and opportunities for prevention. Spine (Phila Pa 1976). 2011;36:1639-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Kwan KYH, Alanay A, Yazici M, Demirkiran G, Helenius I, Nnadi C, Ferguson J, Akbarnia BA, Cheung JPY, Cheung KMC. Unplanned Reoperations in Magnetically Controlled Growing Rod Surgery for Early Onset Scoliosis With a Minimum of Two-Year Follow-Up. Spine (Phila Pa 1976). 2017;42:E1410-E1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Joyce TJ, Smith SL, Rushton PRP, Bowey AJ, Gibson MJ. Analysis of Explanted Magnetically Controlled Growing Rods From Seven UK Spinal Centers. Spine (Phila Pa 1976). 2018;43:E16-E22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Panagiotopoulou VC, Tucker SK, Whittaker RK, Hothi HS, Henckel J, Leong JJH, Ember T, Skinner JA, Hart AJ. Analysing a mechanism of failure in retrieved magnetically controlled spinal rods. Eur Spine J. 2017;26:1699-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Choi E, Yaszay B, Mundis G, Hosseini P, Pawelek J, Alanay A, Berk H, Cheung K, Demirkiran G, Ferguson J, Greggi T, Helenius I, La Rosa G, Senkoylu A, Akbarnia BA. Implant Complications After Magnetically Controlled Growing Rods for Early Onset Scoliosis: A Multicenter Retrospective Review. J Pediatr Orthop. 2017;37:e588-e592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |