Published online Dec 20, 2018. doi: 10.5306/wjco.v9.i8.188
Peer-review started: June 17, 2018
First decision: July 19, 2018
Revised: August 24, 2018
Accepted: November 4, 2018
Article in press: November 4, 2018
Published online: December 20, 2018
Processing time: 188 Days and 17.8 Hours
To investigate the therapeutic potential of two recombinant proteins, Survivin and luteinizing hormone-releasing hormone (LHRH) fusion protein [LHRH(6leu)-LTB] for immunotherapy of breast cancer.
Murine 4T-1 breast cancer model was used to evaluate the efficacy of recombinant proteins in vivo. Twenty four Balb/c mice were divided into 4 groups of 6 mice each. Recombinant Survivin and LHRH fusion protein, alone or in combination, were administered along with immunomodulator Mycobacterium indicus pranii (MIP) in Balb/c mice. Unimmunized or control group mice were administered with phosphate buffer saline. Each group was then challenged with syngeneic 4T-1 cells to induce the growth of breast tumor. Tumor growth was monitored to evaluate the efficacy of immune-response in preventing the growth of cancer cells.
Preventive immunization with 20 µg recombinant Survivin and MIP was effective in suppressing growth of 4T-1 mouse model of breast cancer (P = 0.04) but 50 µg dose was ineffective in suppressing tumor growth. However, combination of Survivin and LHRH fusion protein was more effective in suppressing tumor growth (P = 0.02) as well as metastasis in vivo in comparison to LHRH fusion protein as vaccine antigen alone.
Recombinant Survivin and MIP suppress tumor growth significantly. Combining LHRH fusion protein with Survivin and MIP enhances tumor suppressive effects marginally which provides evidence for recombinant Survivin and LHRH fusion protein as candidates for translating the combination cancer immunotherapy approaches.
Core tip: Targeting Survivin for treatment of cancer is an emerging trend in cancer immunotherapy. In this study we optimized the dose of recombinant full-length Survivin for tumor growth inhibition. Since luteinizing hormone-releasing hormone (LHRH) is also known to support breast cancer in case of premenopausal women, we combined recombinant LHRH fusion protein with Survivin and Mycobacterium indicus pranii and obtained a positive anti-tumor response. We report our results of using recombinant Survivin protein and LHRH fusion protein as potential vaccine antigens for immunotherapy of murine model of breast cancer which was developed by injecting 4T-1 mammary cell line in syngeneic Balb/c mice. Immunized mice challenged with syngeneic 4T-1 cells exhibited suppression of tumor growth and metastasis in lungs in comparison to control mice.
- Citation: Garg H, Hada RS, Gupta JC, Talwar GP, Dubey S. Combination immunotherapy with Survivin and luteinizing hormone-releasing hormone fusion protein in murine breast cancer model. World J Clin Oncol 2018; 9(8): 188-199
- URL: https://www.wjgnet.com/2218-4333/full/v9/i8/188.htm
- DOI: https://dx.doi.org/10.5306/wjco.v9.i8.188
Combination methods that combine two or more therapeutic agents have become a principal treatment modality for breast cancer, especially for metastatic breast cancer[1]. The rationale behind combination approach is to enhance therapeutic response by targeting multiple pathways involved in the complex process of oncogenesis. However, none of the empirically defined combination of drugs such as taxanes/anthracyclins has been able to overcome the problems of drug resistance and metastasis in breast cancer and impart a satisfactory toxicity profile when administered to patients[2,3]. In order to overcome the drawbacks associated with prevalent drug combinations, immunotherapy is proposed as a viable approach for developing targeted and safe treatment options for cancer[4,5]. Advancements in molecular mechanisms of cancer cell survival have led to identification of new targets which can be employed for immunotherapy[6].
Cancer immunotherapy approach using immunomodulators, monoclonal antibodies or vaccines against tumor growth promoters, have shown benefit in preclinical models of many cancers and clinical trials[7,8]. However since tumor cells often develop immune evasion mechanisms[9], combination of tumor antigens may be used to counteract immune resistance by tumor cells. We hypothesize that combination immunotherapy based on potent tumor antigen and hormone, may present a viable approach to enhance the therapeutic landscape and effectively counteract the heterogeneous process of tumor development in breast cancer.
Survivin is a tumor antigen exclusively expressed on tumor cells, essential for cancer cell survival, and its overexpression is associated with aggressiveness of the disease[10,11]. It has been widely explored as a candidate antigen for cancer immunotherapy[12]. Similarly, many tumor cells from breast, prostate, ovary or endometrial origin are hormone dependent for their survival[13]. Therefore, immune system mediated neutralization of hormones is also a key therapeutic strategy for hormone dependent cancers[14]. Immunization against hormones such as luteinizing hormone-releasing hormone (LHRH) using LHRH based peptide vaccines have shown efficacy as vaccine antigens in preclinical models of hormone dependent cancers such as breast and prostate cancer[15].
We have previously shown that preventive immunization with a combination of recombinant Survivin protein and an immunomodulator Mycobacterium indicus pranii (MIP) provides protection to Balb/c mice when challenged with syngeneic mammary tumor 4T-1 cells[16]. This communication describes the studies undertaken to determine the optimum dose of Survivin antigen for maximum tumor suppressive effect. Also investigated is the synergy between Survivin and LHRH fusion protein as vaccine antigens for immunotherapy of murine model of breast cancer. 4T-1 murine breast cancer cells not only display Survivin as tumor antigen but also have receptors for LHRH[17,18]. Thus active immunization against both the Survivin and LHRH, is likely to neutralize Survivin and also make LHRH unavailable for uptake by the cancer cells, hence generate enhanced protective immune response directed against the cancer cells.
Survivin and LHRH fusion protein, were used as vaccine antigens. These antigens were expressed as recombinant proteins using E. coli based host-vector systems as described previously[16,19]. Survivin and LHRH fusion protein were purified as approximately 17 kDa and 14 kDa size proteins, respectively using affinity based Ni-NTA chromatography. Purified proteins were adsorbed on alum before administration as immunogens in mice. MIP was used as immunomodulator along with the recombinantly made vaccine antigens.
Eight-10 week old inbred Balb/c mice were used for conducting the immunogenicity and efficacy studies of the Survivin vaccine alone in vivo. For determining the optimal Survivin dose, study was carried out in 3 groups of mice. Mice were divided into 3 groups randomly with 6 animals in each group. Group 1 was administered phosphate buffer saline (PBS) buffer. While group 2 mice was administered alum adsorbed 20 µg Survivin vaccine (+ MIP), group 3 mice received alum adsorbed 50 µg Survivin dose along with MIP. Five × 106 cells of heat-killed MIP were used in each dose wherever required. Each group of mice was given 2 boosters of its respective dose at 15 d interval after the primary immunization. Sixty days after the last immunization, mice in all the groups were challenged with 3.0 × 105 4T-1 murine breast cancer cells subcutaneously. Third booster of the vaccine was administered on day 18th after the challenge, when tumors were palpable. Tumor size was measured every alternate day till the end of the study. Tumor volume was measured bi-dimensionally with digital vernier calipers and tumor volume calculated using the formula: (a × b2) /2, where “a” is the largest diameter and “b” is the perpendicular diameter. Tumor volumes were measured till day 35 post challenge with 4T-1 cells. Results are expressed as mean ± SD of tumor volume for each group.
Studies were designed in 4 groups of mice each consisting of 6 of 8-10 week old inbred Balb/c mice. Recombinant antigen(s) were administered intramuscularly along with heat-killed 5.0 × 106 cells of MIP. Group 1 of mice received 20 µg of Survivin protein alone, group 2 received 20 µg of LHRH(6leu)-LTB (LTB- heat-labile enterotoxin of E. coli) protein alone and group 3 received combination of 20 µg of Survivin and 20 µg of LHRH(6leu)-LTB proteins. Each group of mice was given 2 boosters of its respective vaccine dose at 15 d interval after the primary immunization. A control group of mice was administered PBS buffer. Fifty-two days after first dose, mice were inoculated with 4T-1 breast cancer cells (3.0 × 105 cells in 100 µL of PBS buffer) subcutaneously on right flank. It was recorded as day 0 of tumor challenge. Tumors were palpable in mice in 18 d after tumor cell inoculation. Third booster of vaccine antigen(s) along with immunomodulator MIP was given intramuscularly at the same day when tumors were palpable. Tumor volume was measured till the end of the study. Mice were sacrificed at the end of the study and lungs were examined for the presence of pulmonary metastases.
Estimation of Interferon-γ levels in sera
Sera were collected from mice and assayed for Interferon gamma levels using commercially available enzyme linked immunosorbent assay (ELISA) kit (E Biosciences, United States) as per the manufacturer’s protocol. Briefly, 96-well microtitre plate was coated with 100 µL/well of IFN-γ capture antibody at a dilution of 1:1000 and incubated overnight at 4 ºC. Plates were then washed thrice with PBST buffer (PBS buffer supplemented with 0.05% Tween-20) and then blocked with ELISA diluent (250 µL/well) at 37 oC for 2 h. Standard was serially diluted in ELISA diluent to obtain concentration in the range of 15-2000 pg/mL and 100 µL of standard of each concentration was added to the corresponding wells. Serum samples were pooled for each group of mice, diluted in ELISA diluent and added to the respective wells at 100 µL/well. Plate was then incubated for 2 h at 37 oC. Biotinylated anti-IFN-γ detection antibody was added to each well (100 µL/well) at a dilution of 1:1000 followed by incubation of 100 µL/well of Avidin-HRP diluted at 1:250 for 30 min. After washing, TMB was added in each well and incubated in dark for 15 min. Reaction was stopped with 2N H2SO4 and absorbance was read at 450 nm. Standard curve was prepared by plotting the average absorbance of each standard on the vertical axis versus the corresponding IFN-γ standard concentration on the horizontal axis and fitted using 4 parameter logistic (4PL) regression analysis. Actual concentration of IFN-γ in mouse serum samples was calculated by extrapolating OD values using the 4PL curve.
ELISA technique was used for the analysis of immunogenicity of the recombinant proteins in mice. ELISA plate (Nunc, Thermo Fisher Scientific, United States) was coated with 100 µL (5 µg/mL concentration) of recombinant Survivin or LHRH(6leu)-LTB protein, and incubated overnight at 4 oC. Plate was washed thrice with 10 mmol/L PBS, pH 7.4 supplemented with 0.05% Tween 20 followed by blocking with 2% Bovine serum albumin (BSA) in PBS buffer. Plate was washed with PBST buffer thrice. One hundred micro litre of the serum diluted at 1:5000 in PBS buffer, was added to the wells of the plate, and incubated for 1 h at 37˚C. HRP-labeled goat anti-mouse antibody was added at a dilution of 1:10000 to each well and color was developed by adding 100 µL of O-phenylenediamine (OPD) solution containing Hydrogen Peroxide. The reaction was stopped by addition of 50 µL of 2N H2SO4. Plate was read at 492 nm in an ELISA plate reader (ELX 800MS, Biotek, United States) and results were expressed as mean ± SD of absorbance values.
Pathogen free female Balb/c mice used in the study were procured from National Institute of Nutrition, Hyderabad. Animals were housed at animal facility of Amity University Uttar Pradesh and were given ad libitum access to water and food. All experimental procedures were in strict agreement with committee for the purpose of control and supervision of experiments on animals (CPCSEA) guidelines for the care and use of laboratory animals and approved by institutional animal ethics committee (IAEC) of Amity University Uttar Pradesh.
Data was analyzed using two tailed student’s t test. P value < 0.05 was reported as significant. Data was analyzed using graph pad prism software.
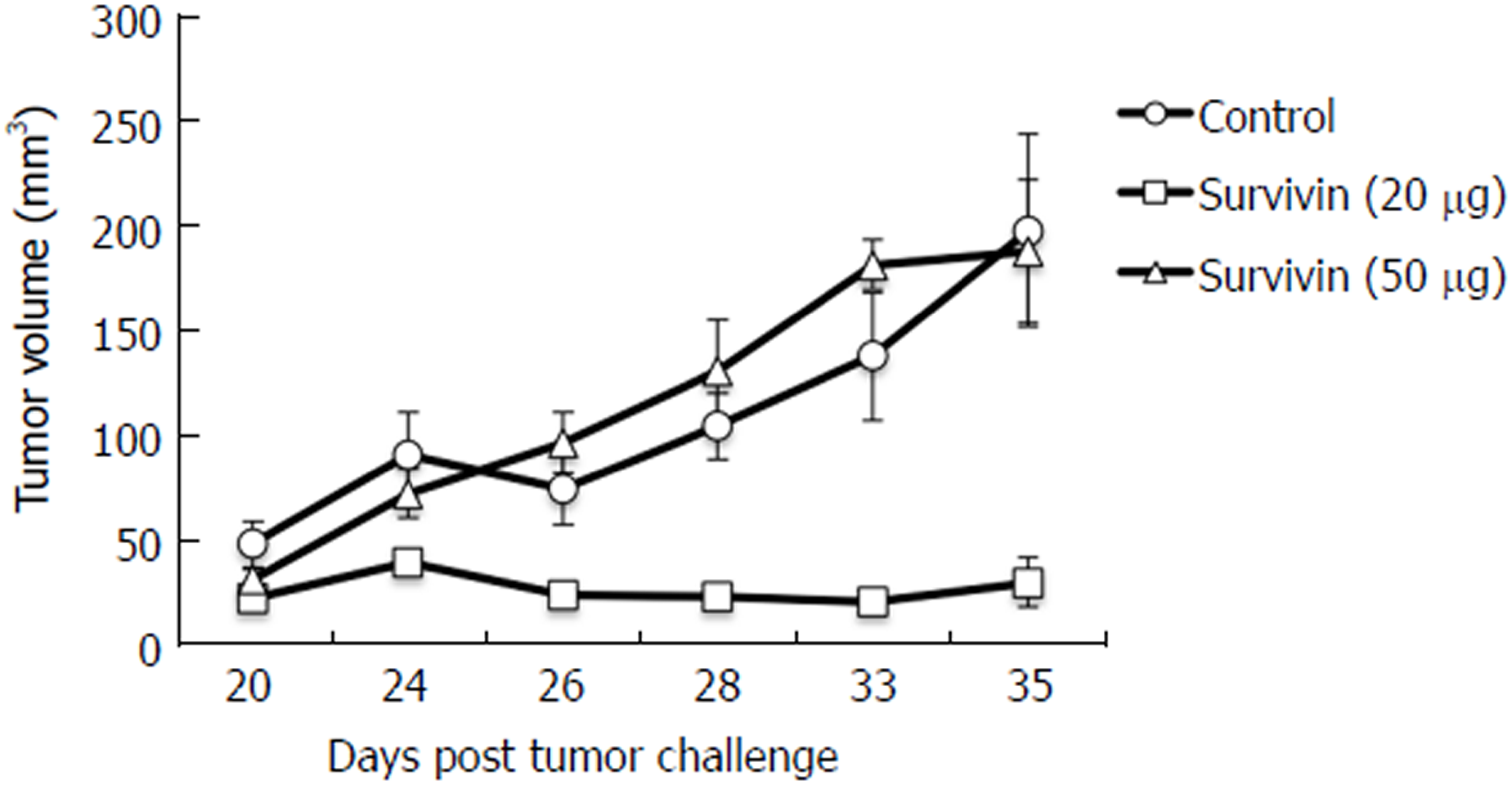
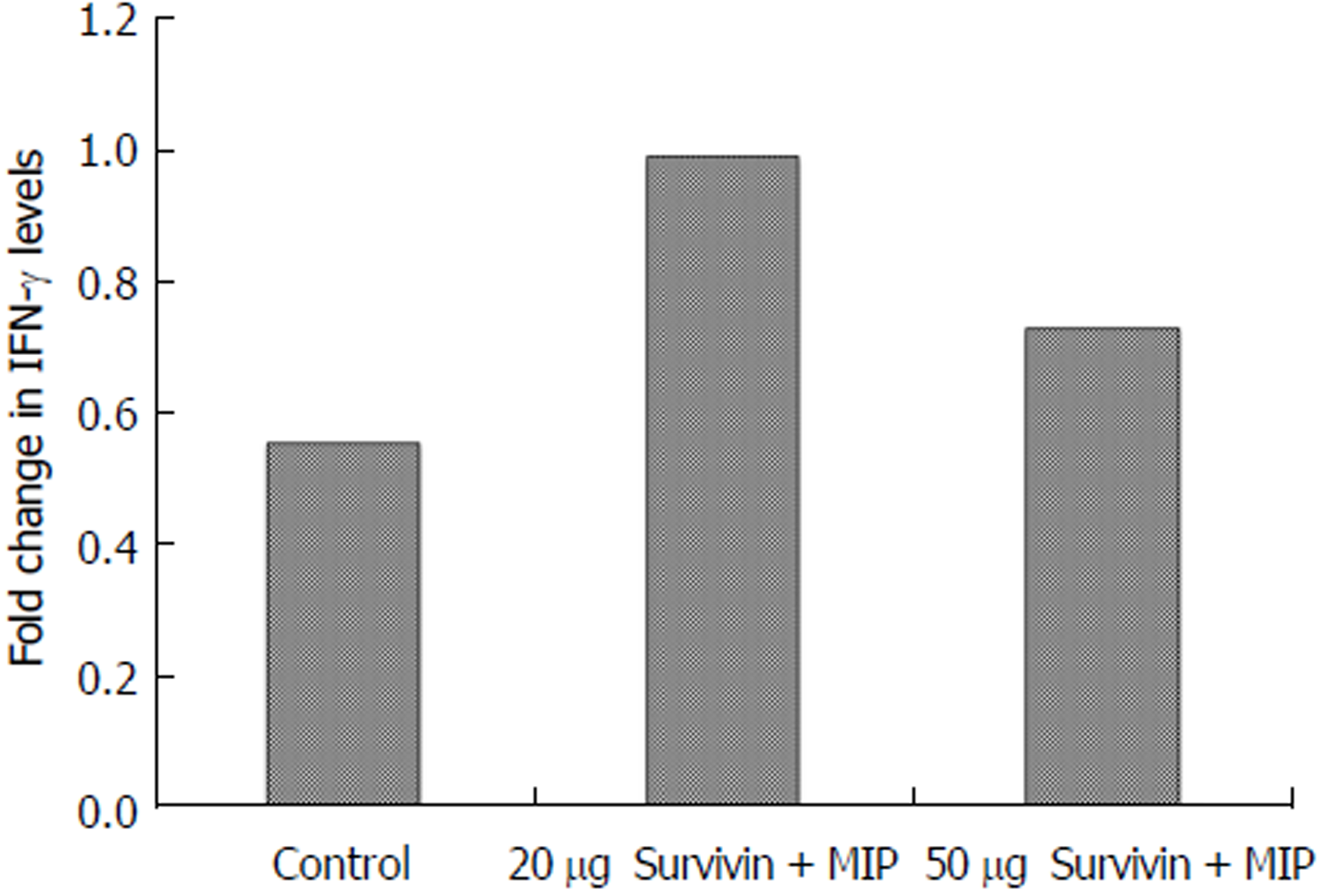
We reported previously that the recombinant Survivin vaccine is efficacious in preventing the growth of 4T-1 based murine model of breast cancer[16]. Increase in vaccine dose from 5 µg to 10 µg, led to better immunogenicity which resulted in significant reduction in tumor suppression[16]. Therefore, it was decided to test if it was possible to employ doses higher than previously used 10 µg dose to inhibit the tumor maximally. Thus the studies were designed to evaluate the efficacy of the vaccine at 20 µg and 50 µg doses. Pathogen-free mice of similar weight (6 mice/group) were first immunized with alum adsorbed recombinant Survivin vaccine along with immune-modulator MIP. Mice were then challenged with syngeneic 4T-1 cells and observed for the tumor growth in presence of pre-formed anti-Survivin antibodies. Three immunizations of alum adsorbed recombinant Survivin and MIP were administered at 15 d interval following which 4T-1 cancer cells were injected at day 90. A booster of the Survivin vaccine was given at day 108 when tumors were palpable. Tumor volume was measured and continued till 35 d post injection of cancer cells. Figure 1 shows the volume of the tumor developed in mice immunized with 20 µg and 50 µg of Survivin protein administered along with MIP. It is evident from the Figure that significant decrease in tumor volume was observed in mice immunized with 20 µg dose of the vaccine (P = 0.04) in comparison to control mice. However increase in vaccine dose to 50 µg did not lead to any significant inhibition in tumor volume thus reflecting dose-inhibition related effects. Figure 2 shows the IFN-γ levels determined in the sera collected from immunized mice. Survivin at 20 µg dose induced higher levels of IFN-γ in comparison to 50 µg dose, which is in accordance with the observed efficacy of the anti-Survivin antibodies in preventing the growth of tumor at 20 µg dose. The mice in each group did not show any visible adverse effects of the treatment.
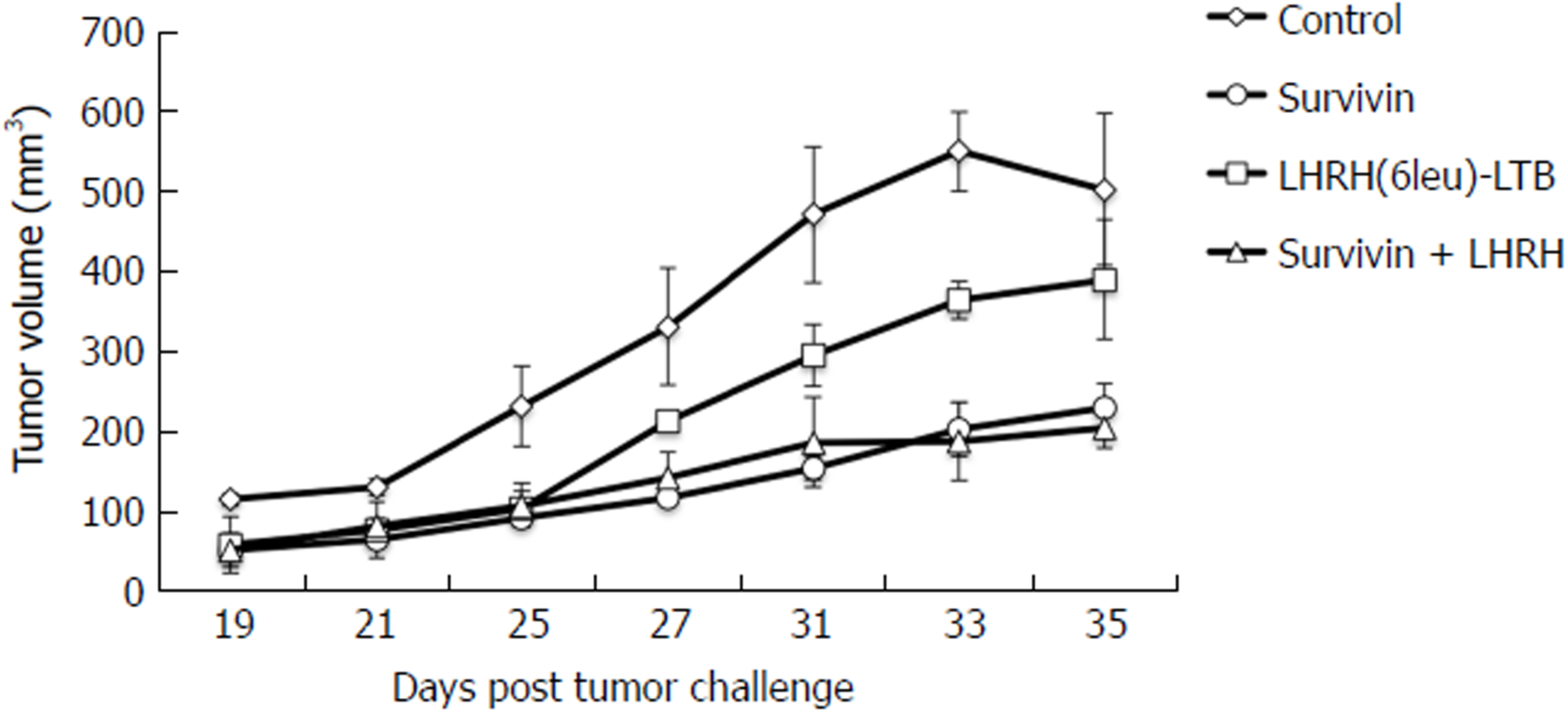
After finding the optimal dose of Survivin vaccine, studies were advanced to investigate the additive effect of LHRH vaccine, if any, in terms preventing the 4T-1 breast cancer cells. Studies were carried out in 4 groups of pathogen free mice (6 mice/group). A group of mice was immunized by combination of alum adsorbed Survivin and LHRH(6leu)-LTB proteins along with immunomodulator MIP. Two groups of mice were injected with Survivin alone (+ MIP) and LHRH(6leu)-LTB alone (+ MIP), respectively. Mice receiving the PBS buffer formed the control group. Mice were immunized thrice at 15 d interval with combination or individual antigens following which 4T-1 tumor cells were injected at 50th day. Boosters of the vaccine antigens were administered when the tumors became palpable. The tumor size was measured post 35 d of tumor cell challenge. Though LHRH(6leu)-LTB alone prevented the growth of tumor size in comparison to control group mice but did not reach statistical significance, inhibition was more significant with Survivin alone (P = 0.04). Combining it with LHRH fusion protein led to marginally better tumor suppression, P = 0.02 (Figure 3). There were no adverse effects of the treatment observed in the mice of each group.
Mice challenged with the tumor cells were followed to evaluate the efficacy of the active immunization by Survivin and LHRH fusion protein alone or in combination in terms of preventing cancer metastasis. The secondary growth of the cancer was studied by counting the number of nodules in the lungs of the mice challenged with the tumor cells. Table 1 shows the mean of number of nodules counted in various groups at the end of the study. Mice immunized with the combination of Survivin and LHRH(6leu)-LTB along with MIP did not show any visible nodules in the lungs 30 d after challenge with 4T-1 cells whereas mice immunized with either Survivin + MIP or LHRH(6leu)-LTB + MIP showed presence of tumor nodules in lungs. Thus combination of Survivin and LHRH(6leu)-LTB along with immunomodulator MIP is capable of controlling the pulmonary metastasis besides suppressing the primary tumor growth.
| Immunization group | Average No. of lung nodules |
| PBS (untreated) | > 20 |
| Survivin | 9 |
| LHRH(6leu)-LTB | 9 |
| Survivin + LHRH(6leu)-LTB | 0 |
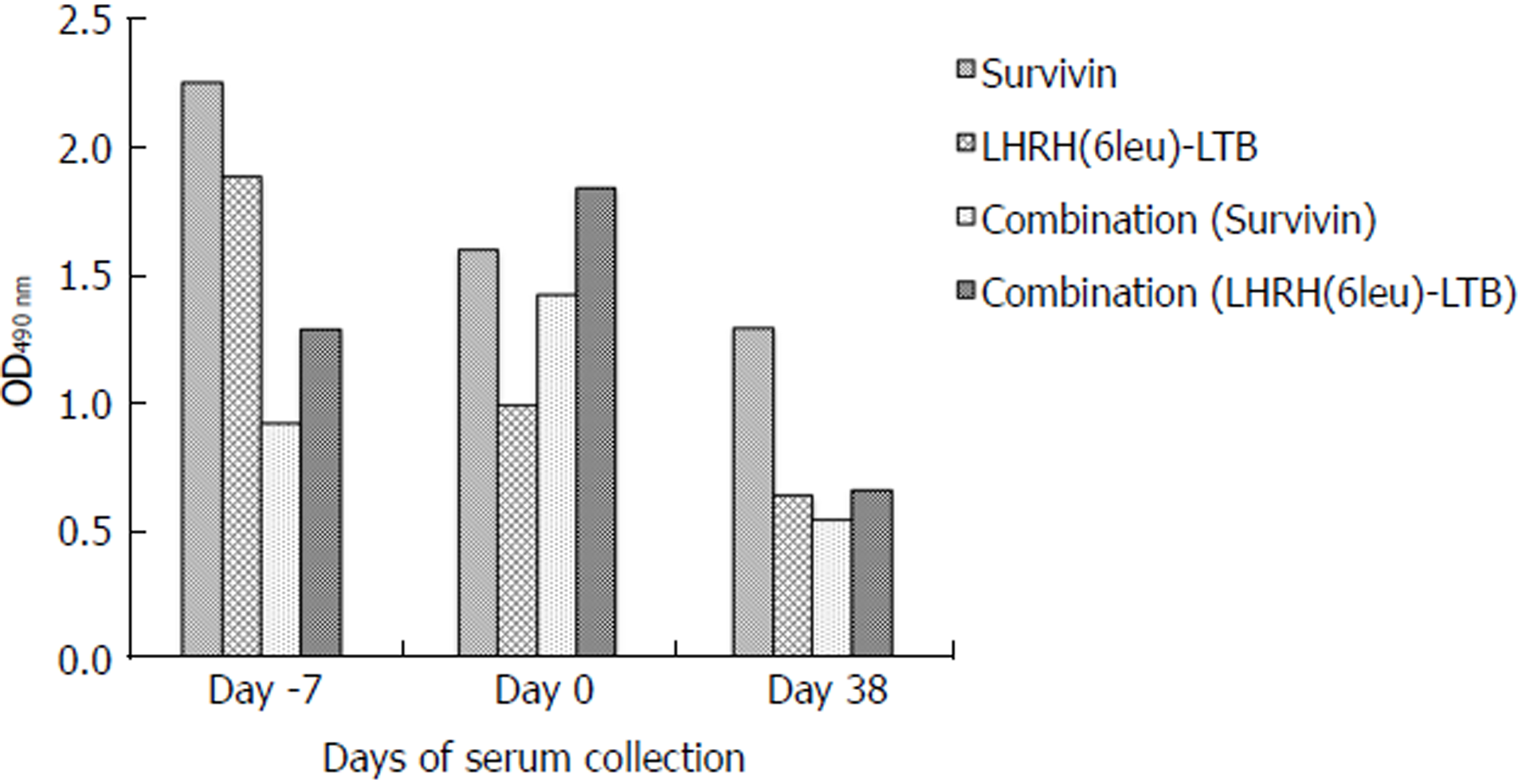
Sera collected from each group of mice were checked for the total IgG titre against the respective antigen(s) pre and post challenge with tumour cells. Both Survivin and LHRH(6leu)-LTB antigens were able to induce antibody response when used to immunize the mice alone. Combined immunization with both the antigens, also led to induction of both anti-Survivin and anti-LHRH antibodies thus confirming their immunogenicity (Figure 4).
Tumor antigens have been proposed as important therapeutic targets for cancer immunotherapy[20]. Immunotherapy approaches incorporating Survivin as tumor antigen have shown efficacy for elimination of cancer cells. Various approaches such as peptide vaccines[21-23], multi epitope vaccines[24,25] or DNA vaccines incorporating Survivin as vaccine antigen[26,27], have shown efficacy in preclinical studies. It has also been suggested that combination of Survivin derived peptides with immunomodulators or immunomodulatory cytokines such as IFN alpha induces a significant overall survival in patients with advanced or recurrent urothelial cancer[28]. We have also previously reported that combination of Survivin along with MIP has potent dose dependent anti-tumor effects in a mouse model of breast carcinoma[16]. Our data suggests that MIP alone is not effective in preventing tumor growth (data not shown) however combining MIP with tumor antigen such as Survivin gives significant tumor suppression. MIP may work as immunomodulator and serves to enhance the immune response against Survivin antigen.
We have demonstrated in the present study that increasing the dose of recombinant Survivin to 20 µg induces most effective tumor suppression in murine 4T-1 model of breast cancer. Interestingly, further increasing the dose of antigen does not induce a tumor suppressive effect. Suppression of immune response at supraoptimal doses of antigen has also been previously reported[29-31]. We hypothesize that high dose Survivin antigen may induce a suboptimal or anergic immune response which may not be able to inhibit the tumor growth.
Interferon-γ secreted by Th1 CD4+ T, NK cells, CD8+ T cells, APCs and B-cells is known for its anti-tumor effects[32]. It has role in rejection of transplanted tumors and inhibition of formation of spontaneous tumors[33,34]. Data presented in the present studies also show higher levels of IFN-γ at 20 µg dose of Survivin than the 50 µg dose which is in agreement with observed better suppression of tumor with 20 µg dose. Previous studies have confirmed the important role of humoral immune response in providing anti-tumor immunity[35]. Our studies also indicate that antigen specific antibodies were induced after immunization with tumor antigens which possibly have contributed to tumor suppression. A recent study by Fenstermaker et al[36] has also demonstrated therapeutic effect of monoclonal antibody against Survivin in vivo in murine glioma model.
Many of the premenopausal breast cancers are also hormone-dependent which rationalizes the use of LHRH agonists as an effective treatment option in breast cancers[37]. Various combinations of LHRH agonists such as cisplatin loaded LHRH nanoparticles which have shown good anti-tumor response in 4T1 breast tumor model by accumulating higher cisplatin in tumors[18]. Another conjugate of LHRH, Pt-Mal-LHRH (activated cisplatin linked to LHRH peptide with a malonate linker) showed targeted delivery to cells expressing LHRH receptors. Treatment of 4T1 breast cancer with Pt-Mal-LHRH reduced tumor volume and metastasis to lungs[38].
We evaluated the possibility of using an immunotherapy based on a combination of Survivin and LHRH fusion protein in 4T-1 murine model of breast cancer. LHRH(6leu)-LTB, a vaccine developed for the immunotherapy of androgen dependent cancers, was used as an immunogen in combination with Survivin along with immunomodulator, MIP. The idea was to generate anti-LHRH antibodies to neutralize LHRH making it unavailable for intake by the breast cancer cells through the receptors. It was observed that efficacy of the combination was marginally better than the Survivin alone but significantly better when only LHRH(6leu)-LTB was used along with MIP. More importantly, combination was best at preventing metastasis of primary tumors to lungs.
Our results show promising outcome in a prophylactic scenario. We undertook the prophylactic approach for two reasons: (1) to investigate if the immunization induces a tumor protective response in vivo; and (2) this prophylactic approach also closely mimics the clinical situation where primary tumors have been removed or adjuvant treatment for cancer patients has been initiated. In both these settings, the patients undergo surgery. Though patients are rendered free of tumor, chances of recurrence are very high. If translation of LHRH fusion protein and Survivin based combination approach is successful, it may find applications in such clinical scenario. We have also investigated the therapeutic potential of this approach by immunizing the mice carrying palpable 4T-1 tumor, which has showed similar suppression of tumor growth (data not shown).
In conclusion, we have shown the Survivin (+ MIP) at a dose of 20 µg is most effective in preventing the growth of 4T-1 breast tumor cells in mice. Incorporation of anti-LHRH vaccine exercises a synergistic effect. A schematic diagram depicting the strategy used in the present study has been shown as Figure 5. Although our results show the protective role of combination immunotherapy with Survivin and LHRH antigens in murine tumor model, yet the study is limited by the fact the results need to be validated in other tumor models and further translational development needs to be undertaken for appropriate human application. Though it did not lead to much benefit in preventing the growth of primary tumor, it was highly effective in blocking the pulmonary metastasis. Our data suggests that combination of Survivin and LHRH fusion protein may hold immense promise for further development of immunotherapeutic approaches in management of breast cancers. The combination enhances immune response that is involved in inhibiting tumor growth, thus it will be effective in immune competent organisms and has to be supplemented with other therapies for use in immune compromised individuals. Furthermore, investigating the molecular mechanism of action of the combination leading to tumor inhibition may also lead to development of novel targeted therapies for cancer.
Tumor cells often develop immune evasion mechanisms, thus combination of tumor antigens may be used to counteract immune resistance. Survivin is a tumor antigen exclusively expressed on tumor cells, essential for cancer cell survival, and its overexpression is associated with aggressiveness of the disease. Similarly, many tumor cells from breast, prostate, ovary or endometrial origin are hormone dependent for their survival. Immunization against hormones such as luteinizing hormone-releasing hormone (LHRH) using LHRH based peptide vaccines have shown efficacy as vaccine antigens in preclinical models of hormone dependent cancers such as breast and prostate cancer. This communication describes the studies undertaken to determine the optimum dose of Survivin antigen for maximum tumor suppressive effect and the synergy between Survivin and LHRH as vaccine antigens for immunotherapy of murine model of breast cancer, 4T-1.
We undertook the prophylactic approach for two reasons: (1) to investigate if the immunization induces a tumor protective response in vivo and (2) this prophylactic approach also closely mimics the clinical situation where primary tumors have been removed or adjuvant treatment for cancer patients has been initiated. Major issues in cancer treatment are the recurrence of tumor after surgery and chemoresistance. Our study was aimed at targeting these issues and developing a strategy, which may prevent tumor spread and recurrence and can overcome resistance. Generating immune response in the body against the tumor antigens will be a safer way of treatment. Anti-Survivin approach may help in overcoming the problem of resistance to therapy.
The aim of this study was to evaluate the possibility of using an immunotherapy based on a combination of Survivin and LHRH fusion protein in 4T-1 murine model of breast cancer. LHRH(6leu)-LTB was used as an immunogen in combination with Survivin along with an immunomodulator, Mycobacterium indicus pranii (MIP). It was observed that efficacy of the combination was marginally better than the Survivin alone but significantly better when only LHRH(6leu)-LTB was used along with MIP. More importantly, combination was best at preventing metastasis of primary tumors to lungs. In most cancer cases, the patients undergo surgery. Though patients are rendered free of tumor, chances of recurrence are very high. If translation of LHRH fusion protein and Survivin based combination approach is successful, it may find applications in such clinical scenario.
Survivin and LHRH fusion protein were used as vaccine antigens. These antigens were expressed as recombinant proteins using E. coli based host-vector systems. Purified proteins were adsorbed on alum before administration as immunogens in mice. MIP was used as immunomodulator along with the recombinantly made vaccine antigens. Inbred Balb/c mice were used for conducting the immunogenicity and efficacy studies of the Survivin vaccine in vivo. Tumor volume was measured bi-dimensionally with digital vernier calipers and results are expressed as mean ± SD of tumor volume for each group. Anti-tumor efficacy of combination of Survivin and LHRH fusion protein in comparison to Survivin and LHRH fusion protein alone was also determined. Sera were collected from mice and assayed for Interferon gamma levels using ELISA kit. ELISA was also performed for the analysis of immunogenicity of the recombinant proteins in mice.
We have shown that Survivin (+ MIP) at a dose of 20 µg is most effective in preventing the growth of 4T-1 breast tumor cells in mice and incorporation of anti-LHRH vaccine exercises a synergistic effect. The study is limited by the fact that the results need to be validated in other tumor models and further translational development needs to be undertaken for appropriate human application. The combination will be effective in immune competent organisms and has to be supplemented with other therapies for use in immune compromised individuals. Furthermore, investigating the molecular mechanism of action of the combination leading to tumor inhibition may also lead to development of novel targeted therapies for cancer.
Our results show the protective role of combination immunotherapy with Survivin and LHRH antigens in murine tumor model. Though it did not lead to much benefit in preventing the growth of primary tumor, it was highly effective in blocking the pulmonary metastasis. Combination of Survivin and LHRH fusion protein may hold immense promise for further development of immunotherapeutic approaches in management of breast cancers.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Kok VC, Kupeli S, Yang T S- Editor: Ji FF L- Editor: A E- Editor: Bian YN
| 1. | Zanardi E, Bregni G, de Braud F, Di Cosimo S. Better Together: Targeted Combination Therapies in Breast Cancer. Semin Oncol. 2015;42:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Biganzoli L, Cufer T, Bruning P, Coleman R, Duchateau L, Calvert AH, Gamucci T, Twelves C, Fargeot P, Epelbaum R. Doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: The European Organization for Research and Treatment of Cancer 10961 Multicenter Phase III Trial. J Clin Oncol. 2002;20:3114-3121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Jassem J, Pieńkowski T, Płuzańska A, Jelic S, Gorbunova V, Mrsic-Krmpotic Z, Berzins J, Nagykalnai T, Wigler N, Renard J. Doxorubicin and paclitaxel versus fluorouracil, doxorubicin, and cyclophosphamide as first-line therapy for women with metastatic breast cancer: final results of a randomized phase III multicenter trial. J Clin Oncol. 2001;19:1707-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 217] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Morrissey KM, Yuraszeck TM, Li CC, Zhang Y, Kasichayanula S. Immunotherapy and Novel Combinations in Oncology: Current Landscape, Challenges, and Opportunities. Clin Transl Sci. 2016;9:89-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 6. | Wang RF, Wang HY. Immune targets and neoantigens for cancer immunotherapy and precision medicine. Cell Res. 2017;27:11-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (1)] |
| 7. | Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 732] [Cited by in RCA: 815] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 8. | Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 1086] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 9. | Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35 Suppl:S185-S198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 1108] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 10. | Cheng KY, Wang ZL, Gu QY, Hao M. Survivin Overexpression Is Associated with Aggressive Clinicopathological Features in Cervical Carcinoma: A Meta-Analysis. PLoS One. 2016;11:e0165117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Chuwa AH, Sone K, Oda K, Ikeda Y, Fukuda T, Wada-Hiraike O, Inaba K, Makii C, Takeuchi M, Oki S. Significance of survivin as a prognostic factor and a therapeutic target in endometrial cancer. Gynecol Oncol. 2016;141:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Boullosa LF, Savaliya P, Bonney S, Orchard L, Wickenden H, Lee C, Smits E, Banham AH, Mills KI, Orchard K. Identification of survivin as a promising target for the immunotherapy of adult B-cell acute lymphoblastic leukemia. Oncotarget. 2017;9:3853-3866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Capper CP, Rae JM, Auchus RJ. The Metabolism, Analysis, and Targeting of Steroid Hormones in Breast and Prostate Cancer. Horm Cancer. 2016;7:149-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Junco JA, Basalto R, Fuentes F, Bover E, Reyes O, Pimentel E, Calzada L, Castro MD, Arteaga N, López Y. Gonadotrophin releasing hormone-based vaccine, an effective candidate for prostate cancer and other hormone-sensitive neoplasms. Adv Exp Med Biol. 2008;617:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Junco JA, Peschke P, Zuna I, Ehemann V, Fuentes F, Bover E, Pimentel E, Basulto R, Reyes O, Calzada L. Immunotherapy of prostate cancer in a murine model using a novel GnRH based vaccine candidate. Vaccine. 2007;25:8460-8468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Garg H, Gupta JC, Talwar GP, Dubey S. Immunotherapy approach with recombinant survivin adjuvanted with alum and MIP suppresses tumor growth in murine model of breast cancer. Prep Biochem Biotechnol. 2018;48:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Taheri A, Dinarvand R, Ahadi F, Khorramizadeh MR, Atyabi F. The in vivo antitumor activity of LHRH targeted methotrexate-human serum albumin nanoparticles in 4T1 tumor-bearing Balb/c mice. Int J Pharm. 2012;431:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Li M, Tang Z, Zhang Y, Lv S, Li Q, Chen X. Targeted delivery of cisplatin by LHRH-peptide conjugated dextran nanoparticles suppresses breast cancer growth and metastasis. Acta Biomater. 2015;18:132-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Gupta JC, Hada RS, Sahai P, Talwar GP. Development of a novel recombinant LHRH fusion protein for therapy of androgen and estrogen dependent cancers. Protein Expr Purif. 2017;134:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Srinivasan R, Wolchok JD. Tumor antigens for cancer immunotherapy: therapeutic potential of xenogeneic DNA vaccines. J Transl Med. 2004;2:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Ciesielski MJ, Kozbor D, Castanaro CA, Barone TA, Fenstermaker RA. Therapeutic effect of a T helper cell supported CTL response induced by a survivin peptide vaccine against murine cerebral glioma. Cancer Immunol Immunother. 2008;57:1827-1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Fenstermaker RA, Ciesielski MJ, Qiu J, Yang N, Frank CL, Lee KP, Mechtler LR, Belal A, Ahluwalia MS, Hutson AD. Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immunol Immunother. 2016;65:1339-1352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 23. | Yang Z, Wang L, Wang H, Shang X, Niu W, Li J, Wu Y. A novel mimovirus vaccine containing survivin epitope with adjuvant IL-15 induces long-lasting cellular immunity and high antitumor efficiency. Mol Immunol. 2008;45:1674-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Idenoue S, Hirohashi Y, Torigoe T, Sato Y, Tamura Y, Hariu H, Yamamoto M, Kurotaki T, Tsuruma T, Asanuma H. A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res. 2005;11:1474-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Lennerz V, Gross S, Gallerani E, Sessa C, Mach N, Boehm S, Hess D, von Boehmer L, Knuth A, Ochsenbein AF. Immunologic response to the survivin-derived multi-epitope vaccine EMD640744 in patients with advanced solid tumors. Cancer Immunol Immunother. 2014;63:381-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Lladser A, Ljungberg K, Tufvesson H, Tazzari M, Roos AK, Quest AF, Kiessling R. Intradermal DNA electroporation induces survivin-specific CTLs, suppresses angiogenesis and confers protection against mouse melanoma. Cancer Immunol Immunother. 2010;59:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Xiang R, Mizutani N, Luo Y, Chiodoni C, Zhou H, Mizutani M, Ba Y, Becker JC, Reisfeld RA. A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res. 2005;65:553-561. [PubMed] |
| 28. | Tanaka T, Kitamura H, Inoue R, Nishida S, Takahashi-Takaya A, Kawami S, Torigoe T, Hirohashi Y, Tsukamoto T, Sato N. Potential survival benefit of anti-apoptosis protein: survivin-derived peptide vaccine with and without interferon alpha therapy for patients with advanced or recurrent urothelial cancer--results from phase I clinical trials. Clin Dev Immunol. 2013;2013:262967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Gotsman I, Israeli D, Alper R, Rabbani E, Engelhardt D, Ilan Y. Induction of immune tolerance toward tumor-associated-antigens enables growth of human hepatoma in mice. Int J Cancer. 2002;97:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Suzuki G, Kawase Y, Koyasu S, Yahara I, Kobayashi Y, Schwartz RH. Antigen-induced suppression of the proliferative response of T cell clones. J Immunol. 1988;140:1359-1365. [PubMed] |
| 31. | Michallet MC, Saltel F, Flacher M, Revillard JP, Genestier L. Cathepsin-dependent apoptosis triggered by supraoptimal activation of T lymphocytes: a possible mechanism of high dose tolerance. J Immunol. 2004;172:5405-5414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 687] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 33. | Blankenstein T, Qin Z. The role of IFN-gamma in tumor transplantation immunity and inhibition of chemical carcinogenesis. Curr Opin Immunol. 2003;15:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2010] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 35. | Tsou P, Katayama H, Ostrin EJ, Hanash SM. The Emerging Role of B Cells in Tumor Immunity. Cancer Res. 2016;76:5597-5601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 36. | Fenstermaker RA, Figel SA, Qiu J, Barone TA, Dharma SS, Winograd EK, Galbo PM, Wiltsie LM, Ciesielski MJ. Survivin Monoclonal Antibodies Detect Survivin Cell Surface Expression and Inhibit Tumor Growth In Vivo. Clin Cancer Res. 2018;24:2642-2652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Goel S, Sharma R, Hamilton A, Beith J. LHRH agonists for adjuvant therapy of early breast cancer in premenopausal women. Cochrane Database Syst Rev. 2009;4:CD004562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Calderon LE, Keeling JK, Rollins J, Black CA, Collins K, Arnold N, Vance DE, Ndinguri MW. Pt-Mal-LHRH, a Newly Synthesized Compound Attenuating Breast Cancer Tumor Growth and Metastasis by Targeting Overexpression of the LHRH Receptor. Bioconjug Chem. 2017;28:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |