Published online Jun 10, 2017. doi: 10.5306/wjco.v8.i3.230
Peer-review started: February 14, 2017
First decision: March 7, 2017
Revised: March 18, 2017
Accepted: April 18, 2017
Article in press: April 20, 2017
Published online: June 10, 2017
Processing time: 122 Days and 5.1 Hours
Pancreatic cancer is the third leading cause of cancer mortality in both men and women in the United States, with poor response to current standard of care, short progression-free and overall survival. Immunotherapies that target cytotoxic T lymphocyte antigen-4, programmed cell death protein-1, and programmed death-ligand 1 checkpoints have shown remarkable activities in several cancers such as melanoma, renal cell carcinoma, and non-small cell lung cancer due to high numbers of somatic mutations, combined with cytotoxic T-cell responses. However, single checkpoint blockade was ineffective in pancreatic cancer, highlighting the challenges including the poor antigenicity, a dense desmoplastic stroma, and a largely immunosuppressive microenvironment. In this review, we will summarize available clinical results and ongoing efforts of combining immune checkpoint therapies with other treatment modalities such as chemotherapy, radiotherapy, and targeted therapy. These combination therapies hold promise in unleashing the potential of immunotherapy in pancreatic cancer to achieve better and more durable clinical responses by enhancing cytotoxic T-cell responses.
Core tip: Pancreatic cancer is the third leading cause of cancer mortality in both men and women in the United States. Pancreatic cancer is one of nonimmunogenic cancers that lacks of optimal treatments especially from immunotherapy prospective. Therefore, combining immune checkpoint therapies with other treatment modalities in pancreatic cancer will be the best strategy to achieve better and more durable clinical responses by enhancing cytotoxic T-cell responses.
- Citation: Guo S, Contratto M, Miller G, Leichman L, Wu J. Immunotherapy in pancreatic cancer: Unleash its potential through novel combinations. World J Clin Oncol 2017; 8(3): 230-240
- URL: https://www.wjgnet.com/2218-4333/full/v8/i3/230.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i3.230
Pancreatic cancer is the third leading cause of cancer mortality in both men and women in the United States[1]. The vast majority of patients with pancreatic cancer are diagnosed with advanced disease, and there has been a lack of optimal treatment option as the cancer is highly refractory to standard chemotherapy. Recently, two chemotherapy regimens, FOLFIRINOX and gemcitabine plus albumin-bound paclitaxel (nab-paclitaxel), have emerged as the standard of care for metastatic pancreatic cancer. These two regimens showed improved overall and progression-free survival (PFS) compared to gemcitabine alone in two phase III randomized controlled trials[2,3]. Nevertheless, only up to 30% of patients showed response to either of these two regimens. The median PFS and overall survival (OS) remain poor, under 6 and 12 mo, respectively. Thus, there is still an urgent need to develop therapies that deliver more effective and durable clinical responses.
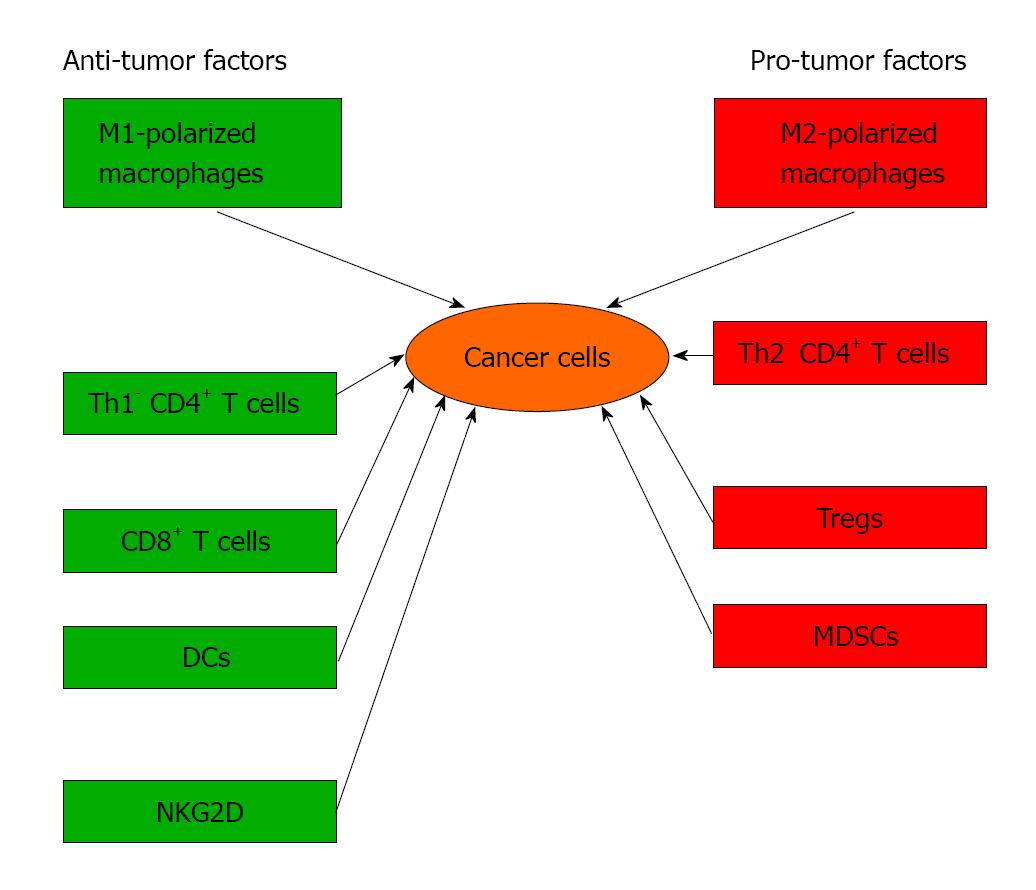
Observations in human disease and murine modeling has suggested that pancreatic cancer is almost invariably associated with a robust inflammatory infiltrate which can have divergent influences on disease progression by either combating cancer growth via antigen-restricted tumoricidal immune responses or by promoting tumor progression via induction of immune suppression (Figure 1)[4-6]. For example, cluster of differentiation 8 (CD8+) and T-helper type 1 cells (Th1)-polarized cluster of differentiation 4 (CD4+) T cells mediate antitumor effects in murine models of pancreatic cancer and are associated with increased survival in patients with pancreatic cancer[7-10]. Conversely, we recently reported that T- helper type 2 cells (Th2)-polarized CD4+ T cells promote pancreatic cancer progression in mice and intra-tumoral CD4+ Th2 cells infiltrates correlate with reduced survival in human disease[7-9,11-13]. Similarly, Foxp3+ T-regulatory cells (Tregs) facilitate tumor immune escape in pancreatic cancer[14]. Myeloid cells can influence T cells differentiation and cytotoxicity in pancreatic cancer. We reported that tumor-infiltrating myeloid-derived suppressor cells (MDSCs) negate cytotoxic CD8+ T cells anti-tumor responses, accelerates pancreatic cancer growth and metastasis[8,15-17]. Similar to T cells, macrophages also have cell types with different properties such as classically activated (M1) macrophages induce immunogenic responses, whereas alternatively activated (M2) macrophages have permissive influences on tumor growth by recruiting Tregs and Th2 cells[18]. However, the drivers of immunosuppressive cell differentiation in pancreatic cancer are based on comprehensive understanding of regulation of the balance between immunogenic and immune-suppressive T cell populations.
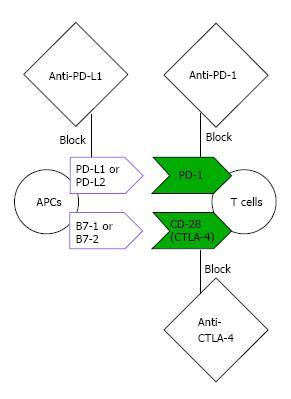
The last few years witnessed a paradigm shift in cancer treatment strategy incorporating immunotherapy. Unprecedented clinical success has been observed for therapies targeting two major checkpoints of T cell response (Figure 2): Cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1). Both checkpoints are expressed on activated T cells, but they act in distinct pathways. CTLA-4 blocks the essential cluster differentiation 28 (CD28) costimulation by competing and depleting the ligand of CD28 (B7-1 and B7-2) on antigen presenting cells (APCs). On the other hand, PD-1 interferes with the signaling pathways mediated by the T cell receptor and serves as a more distal block of T cell response by binding to its ligands (programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2) which are present on many cell types including tumors cells[19].
Monoclonal antibodies targeting CTLA-4 or PD-1 have shown durable clinical responses and prolonged OS in patients with melanoma, a highly immunogenic cancer. While single agent PD-1/PD-L1 inhibitors demonstrate impressive clinical benefits in many cancers such as non small cell lung cancer (NSCLC), renal cell carcinoma, bladder cancer, and Hodgkin’s lymphoma[20-29]. These results have led to FDA approval of lpilimumab (anti-CTLA-4) in 2011 in melanoma[30]. PD-1 inhibitors such as pembrolizumab and nivolumab were approved later in melanoma as well[23,28,29]. PD-1 inhibitors (nivolumab and pembrolizumab), along with PD-L1 inhibitors such as atezolizumab have been approved in NSCLC, another example of immunogenic cancer[21,22,24,29]. The activity of CTLA-4 and PD-L1 inhibitors are being explored in pancreatic cancer as well[22,31].
In early clinical trials single agent therapy with anti-CTLA-4 or anti-PD-1/anti-PD-1 pathway (anti-PD-L1) alone were largely ineffective in pancreatic cancer[22,31,32]. In a single-arm phase II study, lpilimumab failed to induce tumor response in patients with advanced pancreatic cancer[32]. Similarly, single agent BMS-936559, an anti-PD-L1 monoclonal antibody, did not show any activity in 14 patients with advanced pancreatic cancer in a phase I study[22].
The efficacy of immunotherapy in pancreatic cancer is handicapped by small number of cumulative mutational load that can lead to expression of non-self-antigens, or “neoantigens” which are recognized by the immune system as foreign. Cancers with higher number of mutational load are associated with more neoantigens that are easier to be recognized by the immune system, compared to cancer with lower number of mutational load[33-35]. There are 3 major barriers for the utility of immunotherapy in pancreatic cancer. First, the mutational load in pancreatic cancer is very low as compared with melanoma and lung cancers[36,37]. Second, pancreatic cancer features a largely immunosuppressive microenvironment, characterized by a dense desmoplastic reaction with prominent infiltration of tumorigenic macrophages and myeloid derived suppressor cells (MDSCs)[38]. Third, there are very few infiltrating T cells in the microenvironment of pancreatic cancer, therefore could not provide sufficient T cell responses. Pancreatic cancer creates a nonimmunogenic (or “cold”) tumor microenvironment, limiting the activity of immune checkpoint therapies[31].
On the other hand, there is still evidence of T cell-mediated immunity in pancreatic cancer. An analysis of resected surgical samples of pancreatic cancer patients has shown that higher levels of CD4+ and CD8+ tumor infiltrating T cells are associated with better prognosis[10]. In addition, since immunosuppression occurs early during tumorigenesis as shown in Pdx1Cre;KrasG12D;Tp53R172H (KPC) mouse model, the tumor cells may have been shielded from immune pressure, thus preserving their sensitivity to T cell attack[38].
In addition, downstream signals are also critical in the T cell immune responses. Interferon-gamma (IFN-γ) promotes inhibition of melanoma cell growth and induces apoptosis of tumor cells by regulating T-cells responses[39-44]. Immune checkpoint inhibitors increase production of IFN-γ from T-cell[45-50]. However its effect will be suboptimal if there is a defect in the IFN-γ pathway[51]. Studies in patients with melanoma showed that a defect in the IFN-γ pathway can lead to resistance to anti-CTLA4 and anti-PD-1 therapies[51,52]. Several genomic biomarkers of IFN-γ pathways such as interferon gamma receptor 1 (IFNGR1), janus kinase 1 (JAK1), and JAK2 have been identified in melanoma patients with good response to immune checkpoint therapies[41-43,51,52]. On the other hand, genes such as suppressor of cytokine signaling 1 (SOCS1) and protein inhibitor of activated signal transducer and activator of transcription 4 (PIAS4) have demonstrated the opposite effects by inhibiting IFN-γ signaling pathway[51,53,54].
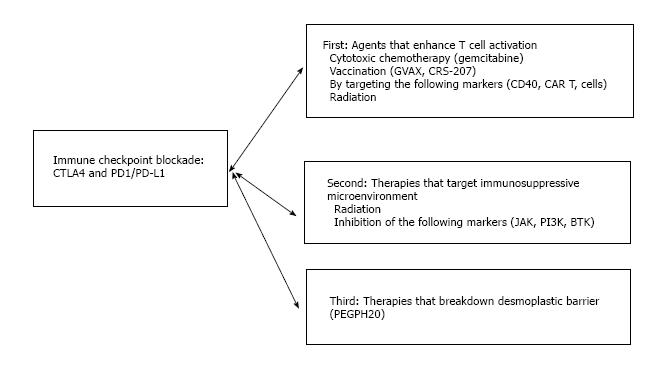
Thus, the incorporation of additional therapies that can turn a “cold” tumor microenvironment into a “hot” one presents an important strategy to elicit clinical activity of immune checkpoint therapies. These additional therapies mainly fall into three categories (Figure 3): First, therapies that enhance tumor antigen presentation to help T cell priming/activation; second, therapies that modulate tumor microenvironment to relieve immunosuppression. Third, therapies which breakdown the desmoplastic barrier surrounding pancreatic cancer to bring infiltrating T cells. Below we will summarize the combination therapies that have already been assessed clinically and provide future directions of new combinations that may hold promise.
Gemcitabine is one of the backbone chemotherapy agents for the treatment of pancreatic cancer. It has been suggested that gemcitabine is not immunosuppressive in pancreatic cancer patients and may be able to enhance naïve T cells activation[55]. Combination of gemcitabine and immune checkpoint blockade has been evaluated for their potential synergistic activity.
Gemcitabine plus CTLA-4 blockade: A phase I clinical study evaluated the combination of gemcitabine and an anti-CTLA-4 antibody (tremelimumab) in treatment naive patients with metastatic pancreatic cancer. This combination showed a tolerable side effect. Among 28 out of 34 evaluable patients, 2 achieved partial response (PR) and 7 showed stable disease (SD) for > 10 wk[4]. In another ongoing phase Ib study of unresectable pancreatic cancer, preliminary results showed that, among 11 evaluable patients (out of 13 enrolled), ipilimumab and gemcitabine resulted in 2 PR and 5 SD[56,57].
Gemcitabine plus PD-1/PD-L1 blockade: An immunohistochemistry analysis has shown that positive PD-L1 expression in resected pancreatic cancer was correlated with worse OS[58]. In a mouse model of pancreatic cancer, combining gemcitabine with either anti-PD-1 or anti-PD-L1 antibody enhanced tumor infiltration of CD8+ T cells and resulted in complete responses in treated mice[58]. A clinical pilot study of combination of gemcitabine and anti-PD-1 antibody has closed to enrollment (NCT01313416).
The most extensively studied pancreatic cancer vaccine is GVAX. GVAX is a whole cell vaccine composed of irradiated, allogeneic pancreatic tumor cells genetically engineered to secret granulocyte macrophage-colony stimulating factor (GM-CSF), a cytokine that stimulates dendritic cell activation and T cell priming. When used as part of adjuvant therapy in the post-resection setting, GVAX was able to induce pancreatic cancer specific CD8+ T cell expansion as shown in a phase II study[59]. Also, when used as neoadjuvant and adjuvant therapy, GVAX and low dose cyclophosphamide (an alkylating agent with an ability to deplete Tregs) resulted in formation of intratumoral tertiary lymphoid aggregates and T cell infiltration, suggesting the ability of GVAX in the conversion of pancreatic cancer from a “non-immunogenic” into an “immunogenic” state[60].
GVAX plus CTLA-4 blockade: In a small phase Ib study, GVAX in combination with anti-CTLA-4 antibody ipilimumab was evaluated in 30 patients with advanced, refractory pancreatic cancer that were previously treated with gemcitabine-based chemotherapy. Compared to ipilimumab alone, the combination therapy resulted in improved survival (27% vs 7% at 1 year). Also, a longer survival was associated with an increase in peak mesothelin-specific T cells and a larger T cell repertoire (the percentage of mesothelin peptides for which enhanced T-cell responses were measured), indicating a positive role of T cell response[61].
GVAX plus PD-1/PD-L1 blockade: Detailed analysis of lymphoid aggregates formed after GVAX therapy revealed elevated expression of PD-L1 on monocytes/macrophages[60,62], suggesting the potential benefit of targeting PD-1/PD-L1 checkpoint. This concept was supported by experiments in a pancreatic cancer mouse model, where the combination of GVAX and an anti-PD-1 antibody resulted in better survival than anti-PD-1 antibody alone, and this activity was correlated with increased CD8+ T cells and elevated IFN-γ production in the tumor microenvironment[62]. Currently, a randomized clinical study (NCT02451982) is ongoing to evaluating GVAX with or without anti-PD-1 antibody (nivolumab) as neoadjuvant and adjuvant treatment in patients with resectable pancreatic cancer.
GVAX and CRS-207 plus PD-1/PD-L1 blockade: CRS-207 is a bacterial vaccine composed of live-attenuated, double deleted Listeria monocytogenes expressing human mesothelin, an antigen commonly overexpressed in pancreatic cancer cells. CRS-207 can induce robust innate as well as mesothelin-specific adaptive immune response, therefore allowing for a “boost” to the immune response initiated by GVAX. In a randomized, phase II study, GVAX prime followed by CRS-207 boost resulted in prolonged OS compared to GVAX alone in patients with metastatic, refractory pancreatic cancer. This study also showed that mesothelin-specific CD8+ T cell response was correlated with better survival[63,64]. On the basis of these findings, a randomized phase II study (NCT02243371) was to evaluate whether adding anti-PD-1 therapy (nivolumab) will further enhance the activity of this prime-boost strategy[65]. This study has closed to enrollment.
In a phase IIb study (NCT02004262) in refractory and metastatic pancreatic cancer, 303 patients were randomized between GVAX and CRS-207 (arm A), only CRS-207 (arm B), and single agent chemotherapy (arm C)[66]. No OS advantage was seen in arm A when compared to arm C[66]. A large number of patient drop out prior to treatment was observed in both arm A and C (40% versus 60%, respectively), indicating the challenge of therapeutic benefit in refractory pancreatic cancer. It also hints that these patients in the refractory setting may be too sick to benefit from immunotherapy due to rapid deterioration of disease.
CD40 agonist: CD40 is a member of the tumor necrosis factor receptor family. Ligation of CD40 can occur on dendritic or B cells, or at CD40 ligand (CD154) on activated T cells, such effect can enhance T cell immunity[67]. In a 22 patients series with unresectable pancreatic cancer, a CD40 agonist (CP-870, 893) and gemcitabine led to an encouraging clinical response[7,11]. Rather unexpectedly, it showed that tumor infiltration by macrophages played a larger role for depletion of tumor stroma and killing of tumor cells[7]. In a more recent study in the KPC mouse model, however, the use of CD40 agonist monoclonal antibody (mAb) with gemcitabine and nab-paclitaxel induced macrophage-independent T cell immunity. This study also found that CD40 agonist in addition to chemotherapy was able to sensitize the tumors to anti-CTLA-4 and/or anti-PD-1 therapies, leading to tumor regression and improved survival[31]. A recent study using an orthotopic pancreatic cancer mouse model also demonstrated tumor regression and enhanced immune response with the combination of CD40 agonist antibody with gemcitabine/Nab-paclitaxel[68]. It is yet to be seen whether these pre-clinical results can translate into clinical benefits.
CAR T cells: Autologous T cells genetically engineered to express a chimeric antigen receptor (CAR) have been developed to trigger cancer-specific T cell immunity and have shown impressive activity in acute lymphoblastic leukemia[69]. For the treatment of pancreatic cancer, the CARs are engineered to recognize mesothelin, a specific membrane protein antigen overexpressed on pancreatic cancer cells. Mesothelin-specific CAR T cells are currently under phase I clinical evaluation, with preliminary results suggesting acceptable safety profiles and potential clinical activity against advanced pancreatic cancer. This study demonstrated that 2 out of 6 patients achieved SD and one patient with liver metastasis at baseline showed no fluorodeoxyglucose (FDG) uptake within 1 mo of treatment[12,70,71]. Therefore, CAR T cells represent another treatment modality to combine with immune checkpoint therapies.
The effects of radiotherapy (RT) on the immunology of pancreatic cancer have not been intensively studied. However, work in other cancers has suggested that RT should be considered an immune adjuvant as evidenced by radiotherapy (RT) induced enhancement of both innate and adaptive immunity. For example, the immunogenicity of dendritic cells (DCs) is reportedly improved by RT-induced necrotic tumor cell release of high mobility group box 1 protein (HMGB1) which ligates toll-like receptor 4 (TLR4) and toll-like receptor 9 (TLR9) on DCs. Such events promote DCs’ cellular maturation and enhance their antigen processing capabilities[72]. Another consequence of RT-induced necrotic cell death is the translocation of calreticulin from the endoplasmic reticulum to the plasma membrane which facilitates assembly of major histocompatibility-1 (MHC I)-peptide complexes. Calreticulin also enhances DCs cross presentation of antigens to cytotoxic T lymphocytes. In addition to upregulating the antigen-presentation machinery in DCs, RT can reportedly enhance immunogenicity by inducing the release of tumor antigens, upregulating the expression of T-cell co-activating ligands, and sensitizing tumor cells to antigen-independent cell death via the Fas receptor[72]. RT is further thought to augment diverse aspects of T cell immunity via adenosine triphosphate release from apoptotic cells which induces secretion of Interleukin-1-beta (IL-1β). A consequence of this cascade is T helper1 (Th1) polarization of antigen-restricted CD4+ T cell responses and activation of cytotoxic T cells. Additionally, activation of cytotoxic T cells can be further activated by irradiation, via natural killer group 2 member D (NKG2D) receptor on cytotoxic T cells. NKG2D receptor can be induced in a stress event such as DNA damage which can be achieved by RT[72]. Therefore, ionizing radiation can result in “immunogenic cell death’, in which the dying tumor cells trigger “danger signals” (a signal of releasing HMGB1 and binding to TLR4 and TLR9 on DCs to process the antigen) to boost T cell activation[72,73].
As described earlier, an important barrier to the success of immunotherapy in pancreatic cancer is an immunosuppressive tumor microenvironment, enriched with immunosuppressive cells such as tumor associated macrophages (TAMs) and MDSCs. In animal models of pancreatic cancer, blockade of immunosuppressive MDSCs could promote antitumor T-cell responses and block protumor macrophage responses[6,74-76]. Therefore, drugs that block these immunosuppressive cells in the tumor microenvironment represent attractive strategies to sensitize pancreatic cancer to immune checkpoint therapies.
RT’s theoretical potential ability to convert the tumor microenvironment from a “cold” to a “hot” state suggests the opportunity of RT combination with immune checkpoint therapy. In the KPC pancreatic mouse model, any combination of immune checkpoint inhibitor with RT substantially increased OS, when compared to anti-CTLA-4 antibody or anti-PD-L1 antibody alone without RT. In particular, the triple therapy (RT + CTLA-4 antibody + PD-1 antibody) resulted in the highest response rate and longest OS among any of the immunotherapy group as single therapy or in combinations[77].
However, our recent preclinical studies on RT in pancreatic cancer suggest caution as we found that RT induced the programming and recruitment of immuno-suppressive M2-like macrophages which lead to the expansion of tumor promoting Th2-polarized CD4+ T cells and Tregs. We also found that combining RT with either macrophage neutralization or M-CSF blockade resulted in synergistic efficacy in mice model, suggesting another treatment strategy for pancreatic cancer utilizing RT combining with colony stimulating factor-1 receptor inhibitor[76,78].
So far there have been no published clinical results on RT plus checkpoint blockade for the treatment of pancreatic cancer. Currently, an open-label, three-cohort, multi-institutional phase Ib study is ongoing at New York University (NCT02868632) to assess stereotactic body radiation therapy (SBRT) in combination with either MEDI4736 (an anti-PD-L1 antibody) alone, tremelimumab (an anti-CTLA4 antibody) alone, or the combination of MEDI4736 and tremelimumab in patients with unresectable/locally advanced previously untreated pancreatic cancer. A study with similar design that tests the combination of radiation with checkpoint blockade in second line setting is also ongoing (NCT02311361).
JAK inhibitors: The Janus kinase (JAK) and its downstream factor signal transducer and activator of transcription (STAT) are important mediators of signaling pathways initiated from cytokine and growth factor receptors. Excessive JAK/STAT signaling can lead to production and release of inflammatory cytokines, promote recruitment, expansion of MDSCs and Tregs which induce an immunosuppressive tumor microenvironment[79]. Also, JAK/STAT pathway has been shown to induce the expression of PD-L1 on cells in the tumor microenvironment[14,80]. In pre-clinical studies, JAK inhibitors led to decreased numbers of Tregs, TAMs and MDSCs, with enhanced number of activity of CD4+ and CD8+ T cells[18]. The study of JAK inhibitor Ruxolitinib and capecitabine for the treatment of advanced pancreatic cancer has closed to enrollment (JANUS study; NCT02117479)[81].
PI3K inhibitors: Phosphoinositide-3-kinase (PI3K) is a family of lipid kinases that catalyze the production of second messenger phosphatidylinositol-3,4,5-triphosphate (PIP3), which leads to activation of downstream kinases. PI3K was known to play an important role in signaling pathways in B cells, which were found to contribute to an immunosuppressive microenvironment that dampens T cell immunity[82]. Inactivation of PI3K was associated with a decrease in Tregs and MDSCs and an increase in CD8+ cytotoxic T cell activity, indicating a role of PI3K in regulating tumor microenvironment[5]. PI3K inhibitors could shift immunosuppressive microenvironment in pancreatic cancer into a more immunogenic one. Therefore PI3K inhibitors could help potentiate the activity of immune checkpoint inhibitors.
BTK inhibitors: BTK is a cytoplasmic, Tec family tyrosine kinase important in B-lymphocyte development, differentiation, and signaling. In pancreatic cancer, the BTK inhibitor (ibrutinib) was shown to inhibit mast cells, and as a result, to reduce fibrosis in the tumor microenvironment both in a KPC mouse model and patient-derived xenograft[83]. Ibrutinib was also known to inhibit interleukin-2-inducible T-cell kinase (ITK), an important enzyme for the survival of Th2 cells; thus ibrutinib may be able to shift the balance away from the Th2 cells protumor response and toward the Th1 cells antitumor immune responses. A phase I/II clinical study assessing ibrutinib in combination with anti-PD-L1 antibody MEDI4736 in relapsed or refractory solid tumors, including pancreatic cancer has closed to enrollment (NCT02403271)[84].
PEGPH20: In pancreatic cancer, high levels of hyaluronan in the extracellular matrix contribute to a high interstitial pressure in the tumor stroma, leading to vascular compression and hypoperfusion. Pegylated hyaluronidase PEGPH20 is an enzyme that can degrade hyaluronan, and has been shown in a KPC mouse model to deplete hyaluronan in the tumor stroma and enhance the activity of gemcitabine[85]. In a phase I (28 patients) and a phase II (135 patients) studies, patients with previously untreated advanced pancreatic cancer, PEGPH20 along with chemotherapy (gemcitabine, or gemcitabine/nab-paclitaxel) resulted in good tumor response and PFS, but only in patients with high levels of hyaluronan[15,86]. Therefore, in pancreatic cancers with high levels of hyaluoran, PEGPH20 therapy may allow more effective T cell infiltration and enhance the activity of immune checkpoint therapies.
Both challenges and opportunities exist for the development of effective immunotherapy for pancreatic cancer. Given that single agent therapies against CLTA-4 or PD-1 or PD-L1 immune checkpoint were largely ineffective in pancreatic cancer, ongoing investigations and future directions lie in the field of combination therapies, where additional treatment modalities may unleash durable anti-tumor immune responses by enhancing tumor-specific T cell activation and antagonizing the immunosuppressive microenvironment in pancreatic cancer.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aung W, Avci E, Peters GJ, Takao S S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12991] [Article Influence: 1443.4] [Reference Citation Analysis (2)] |
| 2. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5640] [Article Influence: 402.9] [Reference Citation Analysis (1)] |
| 3. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4889] [Article Influence: 407.4] [Reference Citation Analysis (0)] |
| 4. | Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH, Bagalà C, Colombi F, Cagnazzo C, Gioeni L, Wang E. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;25:1750-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Ali K, Soond DR, Piñeiro R, Hagemann T, Pearce W, Lim EL, Bouabe H, Scudamore CL, Hancox T, Maecker H. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 421] [Cited by in RCA: 421] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 6. | Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 754] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 7. | Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1302] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 8. | Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, Barilla R, Torres-Hernandez A, Hundeyin M, Mani VR. γδ T Cells Support Pancreatic Oncogenesis by Restraining αβ T Cell Activation. Cell. 2016;166:1485-1499.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 9. | De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 554] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 10. | Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26-e31. [PubMed] |
| 11. | Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286-6295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 359] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 12. | Beatty GL, O’Hara MH, Nelson AM, McGarvey M, Torigian DA, Lacey SF, Melenhorst JJ, Levine B, Plesa G, June CH. Safety and antitumor activity of chimeric antigen receptor modified T cells in patients with chemotherapy refractory metastatic pancreatic cancer. 2015 ASCO Annual Meeting. J Clin Oncol. 2015;Abstracts 33:3007. |
| 13. | Ochi A, Nguyen AH, Bedrosian AS, Mushlin HM, Zarbakhsh S, Barilla R, Zambirinis CP, Fallon NC, Rehman A, Pylayeva-Gupta Y. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med. 2012;209:1671-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 14. | Bellucci R, Martin A, Bommarito D, Wang K, Hansen SH, Freeman GJ, Ritz J. Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. Oncoimmunology. 2015;4:e1008824. [PubMed] |
| 15. | Hingorani SR, Harris WP, Hendifar AE, Bullock AJ, Wu XW, Huang Y, Jiang P. High response rate and PFS with PEGPH20 added to nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients with high-HA tumors: Interim results of a randomized phase II study. 2015 ASCO Annual Meeting. J Clin Oncol. 2015;Abstracts 33:4006. |
| 16. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11786] [Article Influence: 785.7] [Reference Citation Analysis (0)] |
| 17. | Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 565] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 18. | Koblish HK, Hansbury M, Wang LCS, Yang G, Huang T, Xue CB, Li YL, Yue E, Combs A, Yao W. Novel immunotherapeutic activity of JAK and PI3Kδ inhibitors in a model of pancreatic cancer. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; 2015 Apr 18-22; Philadelphia, PA: AACR;. Cancer Res. 2015;75:Abstract nr 1336. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2870] [Cited by in RCA: 3582] [Article Influence: 358.2] [Reference Citation Analysis (0)] |
| 20. | Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2598] [Cited by in RCA: 2805] [Article Influence: 280.5] [Reference Citation Analysis (0)] |
| 21. | Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6945] [Cited by in RCA: 7537] [Article Influence: 753.7] [Reference Citation Analysis (0)] |
| 22. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6290] [Article Influence: 483.8] [Reference Citation Analysis (0)] |
| 23. | Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2712] [Cited by in RCA: 2725] [Article Influence: 227.1] [Reference Citation Analysis (0)] |
| 24. | Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3965] [Cited by in RCA: 4187] [Article Influence: 380.6] [Reference Citation Analysis (0)] |
| 25. | Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4375] [Cited by in RCA: 4605] [Article Influence: 460.5] [Reference Citation Analysis (0)] |
| 26. | Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1915] [Article Influence: 191.5] [Reference Citation Analysis (0)] |
| 27. | Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2517] [Cited by in RCA: 2894] [Article Influence: 321.6] [Reference Citation Analysis (0)] |
| 28. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 9912] [Article Influence: 762.5] [Reference Citation Analysis (0)] |
| 29. | Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1801] [Cited by in RCA: 1827] [Article Influence: 166.1] [Reference Citation Analysis (0)] |
| 30. | Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3331] [Cited by in RCA: 3409] [Article Influence: 243.5] [Reference Citation Analysis (0)] |
| 31. | Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res. 2015;3:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 357] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 32. | Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 964] [Cited by in RCA: 978] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 33. | Gubin MM, Schreiber RD. CANCER. The odds of immunotherapy success. Science. 2015;350:158-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6065] [Cited by in RCA: 6337] [Article Influence: 633.7] [Reference Citation Analysis (0)] |
| 35. | Evans RA, Diamond MS, Rech AJ, Chao T, Richardson MW, Lin JH, Bajor DL, Byrne KT, Stanger BZ, Riley JL. Lack of immunoediting in murine pancreatic cancer reversed with neoantigen. JCI Insight. 2016;1:e88328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 36. | Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL. Signatures of mutational processes in human cancer. Nature. 2013;500:415-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7533] [Cited by in RCA: 7316] [Article Influence: 609.7] [Reference Citation Analysis (1)] |
| 37. | Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3848] [Cited by in RCA: 4129] [Article Influence: 344.1] [Reference Citation Analysis (0)] |
| 38. | Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol. 2013;25:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 39. | Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu XY. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997;17:5328-5337. [PubMed] |
| 40. | Detjen KM, Farwig K, Welzel M, Wiedenmann B, Rosewicz S. Interferon gamma inhibits growth of human pancreatic carcinoma cells via caspase-1 dependent induction of apoptosis. Gut. 2001;49:251-262. [PubMed] |
| 41. | Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836-848. [PubMed] |
| 42. | Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95-109. [PubMed] |
| 43. | Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95:7556-7561. [PubMed] |
| 44. | Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 734] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 45. | Alegre ML, Shiels H, Thompson CB, Gajewski TF. Expression and function of CTLA-4 in Th1 and Th2 cells. J Immunol. 1998;161:3347-3356. [PubMed] |
| 46. | Chen H, Liakou CI, Kamat A, Pettaway C, Ward JF, Tang DN, Sun J, Jungbluth AA, Troncoso P, Logothetis C. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci USA. 2009;106:2729-2734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Dulos J, Carven GJ, van Boxtel SJ, Evers S, Driessen-Engels LJ, Hobo W, Gorecka MA, de Haan AF, Mulders P, Punt CJ. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother. 2012;35:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 253] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 48. | Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, Logothetis C, Sharma P. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci USA. 2008;105:14987-14992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 445] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 49. | Paradis TJ, Floyd E, Burkwit J, Cole SH, Brunson B, Elliott E, Gilman S, Gladue RP. The anti-tumor activity of anti-CTLA-4 is mediated through its induction of IFN gamma. Cancer Immunol Immunother. 2001;50:125-133. [PubMed] |
| 50. | Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, Yagita H, Overwijk WW, Lizée G, Radvanyi L. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72:5209-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 348] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 51. | Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell. 2016;167:397-404.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 999] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 52. | Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375:819-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2021] [Cited by in RCA: 2410] [Article Influence: 267.8] [Reference Citation Analysis (0)] |
| 53. | Liu B, Gross M, ten Hoeve J, Shuai K. A transcriptional corepressor of Stat1 with an essential LXXLL signature motif. Proc Natl Acad Sci USA. 2001;98:3203-3207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | Song MM, Shuai K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J Biol Chem. 1998;273:35056-35062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 338] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 55. | Plate JM, Plate AE, Shott S, Bograd S, Harris JE. Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother. 2005;54:915-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Mohindra NA, Kircher SM, Nimeiri HS, Benson AB, Rademaker A, Alonso E, Blatner N, Khazaie K, Mulcahy MF. Results of the phase Ib study of ipilimumab and gemcitabine for advanced pancreas cancer. 2015 GI ASCO Annual Meeting. J Clin Oncol. 2015;33:e15281. |
| 57. | Kalyan A, Kircher SM, Mohindra NA, Nimeiri HS, Maurer V, Rademaker A, Benson AB, Mulcahy MF. Ipilimumab and gemcitabine for advanced pancreas cancer. A phase Ib study. 2016 GI ASCO Annual Meeting. J Clin Oncol. 2016;34:e15747. |
| 58. | Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 701] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 59. | Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 297] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 60. | Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 416] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 61. | Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Donehower RC, Jaffee EM. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 419] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 62. | Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, Wamwea A, Bigelow E, Lutz E, Liu L. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015;38:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 319] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 63. | Le DT, Wang-Gillam A, Picozzi V Jr, Greten TF, Crocenzi TS, Springett GM, Morse M, Zeh H, Cohen DJ, Fine RL, Onners B, Uram JN, Laheru D, Murphy A, Skoble J, Lemmens E, Grous JJ, Dubensky T, Brockstedt DG, Jaffee EM. A phase 2 randomized trial of GVAX pancreas and CRS-107 immunotherapy versus GVAX alone in patients with metastatic pancreaticoadenocarcinoma. 2014 Gastrointestinal Cancers Symposium. J Clin Oncol. 2014;32:177. |
| 64. | Le DT, Whiting CC, Lutz ER, Nair N, Engstrom A, Lemmens E, Tagliaferri MC, Murphy AL, Brockstedt DG, Jaffee EM. Clinical and immune characteristics of rapid dropout and long term survival in a phase II safety and efficacy study of combination CRS-207/GVAX immunotherapy in pancreatic cancer. 2016 Gastrointestinal Cancers Symposium. J Clin Oncol. 2016;34:459. |
| 65. | Le DT, Crocenzi TS, Uram JN, Lutz ER, Laheru D, Sugar EA, Vonderheide RH, Fisher GA, Ko AH, Murphy AL. Randomized phase II study of the safety, efficacy, and immune response of GVAX pancreas (with cyclophosphamide) and CRS-207 with or without nivolumab in patients with previously treated metastatic pancreatic adenocarcinoma (STELLAR). 2015 ASCO Annual Meeting. J Clin Oncol. 2015;33:TPS4148. |
| 66. | Le DT, Ko AH, Wainberg ZA, Picozzi VJ, Kindler HL, Wang-Gillam A, Oberstein PE, Morse M, Zeh H, Weekes CD. Results from a phase 2b, randomized, multicenter study of GVAX pancreas and CRS-207 compared to chemotherapy in adults with previously-treated metastatic pancreatic adenocarcinoma (ECLIPSE Study). 2017 Gastrointestinal Cancers Symposium. J Clin Oncol. 2017;35:345. |
| 67. | Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 415] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 68. | Siolas D, Cullis J, Avanzi A, Byme K, Leichman LP, Vonderheide RH, Bar-Sagi D. Antitumor activity and immune response in CD40 immunotherapy with gemcitabine and nab-paclitaxel in an orthotopic pancreatic cancer mouse model. 2016 Gastrointestinal Cancers Symposium. J Clin Oncol. 2016;34:271. |
| 69. | Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4147] [Cited by in RCA: 4211] [Article Influence: 382.8] [Reference Citation Analysis (0)] |
| 70. | Tanyi JL, Haas AR, Beatty GL, Morgan MA, Stashwick CJ, O’Hara MH, Porter DL, Maus MV, Levine BL, Lacey SF. Safety and feasibility of chimeric antigen receptor modified T cells directed against mesothelin (CART-meso) in patients with mesothelin expressing cancers. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; 2015 Apr 18-22; Philadelphia, PA: AACR;. Cancer Res. 2015;75:CT105. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Kunk PR, Bauer TW, Slingluff CL, Rahma OE. From bench to bedside a comprehensive review of pancreatic cancer immunotherapy. J Immunother Cancer. 2016;4:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 72. | Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers. Int J Mol Sci. 2014;15:927-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 209] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 73. | Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, Tesniere A, Martins I, Ly A, Haynes NM. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol. 2010;22:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 74. | Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 685] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 75. | Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19:3404-3415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 475] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 76. | Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057-5069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 971] [Cited by in RCA: 1015] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 77. | Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1498] [Cited by in RCA: 1901] [Article Influence: 190.1] [Reference Citation Analysis (0)] |
| 78. | Seifert L, Werba G, Tiwari S, Giao Ly NN, Nguy S, Alothman S, Alqunaibit D, Avanzi A, Daley D, Barilla R. Radiation Therapy Induces Macrophages to Suppress T-Cell Responses Against Pancreatic Tumors in Mice. Gastroenterology. 2016;150:1659-1672.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 79. | Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 3398] [Article Influence: 212.4] [Reference Citation Analysis (0)] |
| 80. | Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, Freeman GJ, Ferris RL. Identification of the Cell-Intrinsic and -Extrinsic Pathways Downstream of EGFR and IFNγ That Induce PD-L1 Expression in Head and Neck Cancer. Cancer Res. 2016;76:1031-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 81. | Hurwitz H, Cutsem EV, Bendell JC, Hidalgo M, Li CP, Garrido M, Macarulla TM, Sahai V, Sama AR, Greeno E. Two randomized, placebo-controlled phase 3 study of ruxolitinib (Rux) capecitabine © in patients (pts) with advanced /metastatic pancreatic cancer after failure/intolerance of first-line chemotherapy: JANUS 1 (J1) and JANUS 2 (J2). 2017 Gastrointestinal Cancers Symposium. J Clin Oncol. 2017;35:343. |
| 82. | Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 242] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 83. | Massó-Vallés D, Jauset T, Serrano E, Sodir NM, Pedersen K, Affara NI, Whitfield JR, Beaulieu ME, Evan GI, Elias L. Ibrutinib exerts potent antifibrotic and antitumor activities in mouse models of pancreatic adenocarcinoma. Cancer Res. 2015;75:1675-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 84. | Borazanci EH, Hong DS, Gutierrez M, Rasco DW, Reid TR, Veeder MH, Tawashi A, Lin J, Dimery IW, Piper VG. Ibrutinib durvalumab (MEDI4736) in patients with relapsed or refractory (R/R) pancreatic adenocarcinoma: A phase Ib/II multicenter study. 2016 Gastrointestinal Cancers Symposium. J Clin Oncol. 2016;34:TPS484. |
| 85. | Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1640] [Article Influence: 126.2] [Reference Citation Analysis (0)] |
| 86. | Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, Tjulandin SA, Gladkov OA, Holcombe RF, Korn R. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin Cancer Res. 2016;22:2848-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |