Published online Feb 10, 2017. doi: 10.5306/wjco.v8.i1.54
Peer-review started: August 29, 2016
First decision: November 14, 2016
Revised: December 6, 2016
Accepted: December 27, 2016
Article in press: December 28, 2016
Published online: February 10, 2017
Processing time: 165 Days and 14.7 Hours
To develop a leptin peptide receptor antagonist linked to nanoparticles and determine its effect on viability of breast cancer cells.
The leptin antagonist, LPrA2, was coupled via EDAC [1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide] to iron oxide nanoparticles (IONP-LPrA2) to increase its efficacy. IONP-LPrA2 conjugation was confirmed by Western blot and nanoparticle tracking analysis. Human triple negative breast cancer (TNBC) MDA-MB-231, HCC1806 and estrogen receptor positive (ER+) MCF-7 cells were analyzed for the expression of the leptin receptor, Ob-R. The effects of leptin and antagonist on levels of leptin-induced STAT3 phosphorylation and cyclin D1, cell cycle progression, cell proliferation, and tumorsphere formation in breast cancer cells were determined. Doses of the chemotherapeutics [cisplatin (Cis), cyclophosphamide (CTX), doxorubicin (Dox) and paclitaxel (PTX)] to effectively reduce cell viability were calculated. The effects of combination treatments of IONP-LPrA2 and chemotherapeutics on cell viability were determined.
Western blot analysis of coupling reaction products identified IONP-LPrA2 at approximately 100 kD. IONP-LPrA2 significantly decreased leptin-induced pSTAT3 levels in HCC1806 cells and drastically decreased cyclin D1 levels in all cell lines. IONP-LPrA2 significantly reduced leptin-induced S phase progression and cell proliferation in all breast cancer cell lines and the formation of tumorspheres in MDA-MB-231 cells. Also, IONP-LPrA2 showed an additive effect on the reduction of breast cancer cell survival with chemotherapeutics. Cis plus IONP-LPrA2 produced a significant reduction in the survival of MDA-MB-231 and HCC1806 cells. CTX plus IONP-LPrA2 caused a significant decrease in the survival of MDA-MB-231 cells. Dox plus IONP-LPrA2 caused a marked reduction in the survival of HCC1806 cells. Although, PTX plus IONP-LPrA2 did not have a major effect on the viability of the breast cancer cells when compared to PTX alone.
Present data indicate that IONP-LPrA2 may be a useful adjuvant for chemotherapeutic treatment of breast cancer, particularly for TNBC which lacks targeted therapeutic options.
Core tip: Breast cancer is the second leading cause of cancer deaths in women. Triple negative breast cancer is an aggressive subtype that lacks targeted therapy. Obesity is a risk factor for breast cancer and is associated with high leptin levels. Leptin induces the expression of cell cycle associated proteins advancing cell cycle progression. Leptin also increases breast cancer stem cell growth, which promotes chemotherapeutic resistance. We have developed a leptin antagonist linked to iron oxide nanoparticles (IONP-LPrA2) which significantly inhibits leptin-induced cell proliferation and survival of breast cancer cells treated with chemotherapeutics. IONP-LPrA2 can increase chemotherapeutic efficacy in breast cancer.
- Citation: Harmon T, Harbuzariu A, Lanier V, Lipsey CC, Kirlin W, Yang L, Gonzalez-Perez RR. Nanoparticle-linked antagonist for leptin signaling inhibition in breast cancer. World J Clin Oncol 2017; 8(1): 54-66
- URL: https://www.wjgnet.com/2218-4333/full/v8/i1/54.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i1.54
The American Cancer Society estimates that there will be nearly 300000 new breast cancer cases diagnosed worldwide and approximately 50000 women will die from breast cancer in 2016[1]. Triple negative breast cancer (TNBC) accounts for 15% of all breast cancer diagnoses. TNBC is a subtype of breast cancer characterized by the lack of expression of the estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor-2 (HER2)[1,2]. Due to the absence of receptor expression; this form of breast cancer, which predominantly affects younger, African American and Hispanic patients lacks targeted therapeutic options[3]. TNBC patients are commonly treated with chemotherapy; however these patients make up approximately 30% of breast cancer-related deaths annually[4]. This necessitates the development of targeted therapies for this more aggressive form of the disease.
There are many factors that increase the risk of developing TNBC including environment, genetic susceptibility, and obesity[5]. Obesity has a negative impact on breast cancer patient survival and, like TNBC, is associated with an increased risk of recurrence[6]. Obesity is correlated to high levels of leptin, a cytokine produced by adipose tissue which regulates satiety. The leptin signaling pathway occurs in approximately 80% of breast cancers[7]. The binding of leptin to its receptor, Ob-R, leads to activation of pathways involved in cell proliferation, migration, and survival[8]. Leptin is a survival factor in breast cancer and may have the ability to limit the effectiveness of chemotherapy drugs by activating the JAK2/STAT3, MAPK/ERK, and PI3/Akt signaling pathways[9,10]. Therefore leptin signaling inhibition has become a promising therapeutic area for breast cancer, particularly in the case of TNBC for which there is no targeted therapy[11].
The binding of leptin to Ob-R upregulates Notch, interleukin 1 (IL-1), vascular endothelial growth factor (VEGF), and its receptor VEGFR2; which promote breast cancer cell survival and angiogenesis[12]. The harmful effects of leptin signaling on breast cancer onset and progression have been shown to be diminished by the leptin peptide receptor antagonist 2 (LPrA2)[13]. LPrA2 and the pegylated form (PEG-LPrA2) have been shown to cause a delay in cancer onset and progression as well as a reduction in 4T1-tumor growth in BALB/C mice[14]. Additionally, PEG-LPrA2 has been shown to decrease MDA-MB-231 and MCF-7-tumor growth in SCID mice[15]. In another study, diet-induced obese (DIO) C57BL/6J mice treated with the carcinogen 7, 12-Dimethylbenz (a) anthracene (DMBA) along with PEG-LPrA2 did not develop tumors[16]. The anti-tumor activity of LPrA2 provides mounting evidence for its usefulness in cancer therapy.
The leptin signaling pathway plays a major role in breast cancer cell growth, angiogenesis, as well as metastasis and invasion[8]. Although the leptin antagonist LPrA2 attenuates leptin signaling, it is limited by its insolubility in water and short half-life of 1-2 h[14,17,18]. Here we describe the coupling of LPrA2 to a nanoparticle delivery system which uses iron oxide nanoparticles (IONPs) to capture multiple LPrA2 peptides. We assessed the conjugation of LPrA2 to IONPs (IONP-LPrA2) to determine the inhibitory effect on breast cancer cell growth and survival. Because LPrA2 decreases breast cancer tumor growth and chemotherapy is widely used in the treatment of breast cancer, we sought to assess if combining IONP-LPrA2 and chemotherapeutic drugs would allow for reduction of the effective dose. Thus, we evaluated the survival of human breast cancer cell lines with IONP-LPrA2 and a panel of anti-cancer drugs.
IONPs were obtained from Ocean Nanotech San Diego, CA. 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDAC), Sulfo-NHS, and other chemicals were purchased from Sigma Aldrich St. Louis, MO. Ob-R (sc-8325), Cyclin D1 (sc-246), pSTAT3 (sc-8059), STAT3 (sc-8019) antibodies were purchased from Santa Cruz Biotechnology Santa Cruz, CA. Anti-rabbit and anti-mouse conjugated to horseradish peroxidase were obtained from Bio-Rad Laboratories Hercules, CA. Dulbecco’s Modified Eagles Medium (DMEM), Iscove’s Modified Dulbecco’s Medium (IMEM), Protease and Phosphatase Inhibitor cocktails, Penicillin/Streptomycin, Slide-a-lyzer dialysis cassette, and Western blotting chemiluminescence substrate were purchased from Thermo Fisher Scientific Rockford, IL. Mammocult complete medium was obtained from Stem Cell Technologies Vancouver, BC. Fetal bovine serum was obtained from Med Supply Partners Atlanta, GA. Leptin was purchased from R and D Systems Minneapolis, MN. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) kit was purchased from Molecular Probes Eugene, OR. Annexin V/fluorescein Isothiocyanate (FITC) and propidium iodide (PI) were obtained from Nexcelom Bioscience Boston, MA. Cisplatin (Cis) was purchased from Millipore Billerica, MA. Cyclophosphamide (CTX), paclitaxel (PTX), and doxorubicin (Dox) were obtained from SelleckChem Houston, TX.
LPrA2 was synthesized as described[8,19]. LPrA2 was de-salted using the slide-a-lyzer dialysis cassette (Thermo Fisher). LPrA2 was conjugated to IONPs (Ocean Nanotech) by the outlined method[20].
IONP-LPrA2 was separated by SDS-PAGE. LPrA2 and LPrA2-Scramble (Sc) were used as positive and negative controls, respectively. The peptides were transferred onto nitrocellulose membranes (Bio-Rad). The membranes were probed with an LPrA2 antibody, purified from antigen injected rabbit bleeds. Anti-rabbit IgG conjugated to horseradish peroxidase (Bio-Rad) was used for further detection of the peptides. Chemiluminescent detection of the bands was displayed by Western blotting substrate (Thermo Fisher).
Dilutions of 1:10000 of the bound and unbound particles were sonicated for 30 min. The size and distribution of the conjugated and unconjugated IONPs were determined by the NanoSight (Malvern Instruments Ltd., Worcestershire, United Kingdom).
Human ER+ MCF-7 cells in addition to TNBC MDA-MB-231 and HCC1806 cells (American Type Culture Collection, ATCC, Manassas, VA) were cultured in DMEM (Thermo Fisher) with 10% FBS and 1% penicillin/streptomycin (Thermo Fisher) and maintained in an incubator at 37 °C with 5% CO2.
Cells were seeded at 2 × 105 in 6 well cell culture plates and grown to 70%-80% confluence. The cells were treated with leptin (1.2 nmol/L) (R and D Systems), or IONP-LPrA2 (0.0036 pmol/L) plus leptin (1.2 nmol/L) for 24-48 h. Basal cells served as untreated controls. The cells were lysed with RIPA buffer (Sigma) containing protease/phosphatase inhibitors (Thermo Fisher). Proteins were pulled down by Immunoprecipitation. Immunoblotting analysis was performed as described[21]. The membranes were incubated with Ob-R, cyclin D1, pSTAT3, and STAT3 (Santa Cruz Biotechnology) antibodies overnight at 4 °C. GAPDH (Sigma) was used as a protein loading control. Relative protein levels were determined by Image J software (National Institute of Health, NIH).
Cells were seeded at 2 × 105 in 6 well cell culture plates and grown to 70%-80% confluence. They were treated with IONP-LPrA2 at indicated (pmol/L) concentrations plus leptin (1.2 nmol/L for 24-48 h. Leptin and unconjugated LPrA2 served as controls. The cells were trypsinized, washed with 1 × PBS, and resuspended in cold 100% methanol (Sigma). The were stored at -20 °C prior to analysis (< 1 wk). Afterward, the cells were centrifuged to remove the methanol. They were resuspended in 50 μL PI (Nexcelom) and incubated at 37 °C for 40 min. The cells were centrifuged to remove the PI, resuspended in 1 × PBS, and analyzed by the Nexcelom Cellometer Vision® image based cytometer to determine the percentage of cells in the S phase of the cell cycle.
Cells were seeded at 5 × 103 in 96 well cell culture plates and grown to 70%-80% confluence. The cells were treated with leptin (1.2 nmol/L), or IONP-LPrA2 (0.0036 pmol/L) plus leptin (1.2 nmol/L) for 24-48 h. Basal cells served as untreated controls. The media was removed from the cells, the wells were washed with 1 × PBS, and 200 μL of IMEM (Thermo Fisher) together with 10 μL of sterile 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 5 mg/mL in PBS, (Molecular Probes) were added. The plates were incubated for 4 h at 37 °C. Following incubation the media was removed, 50 μL of Dimethyl sulfoxide was added to the wells, and the plates were incubated at 37 °C for 30 min. The absorbance was read at 540 nm using a microplate reader (Molecular Devices) to measure cell proliferation.
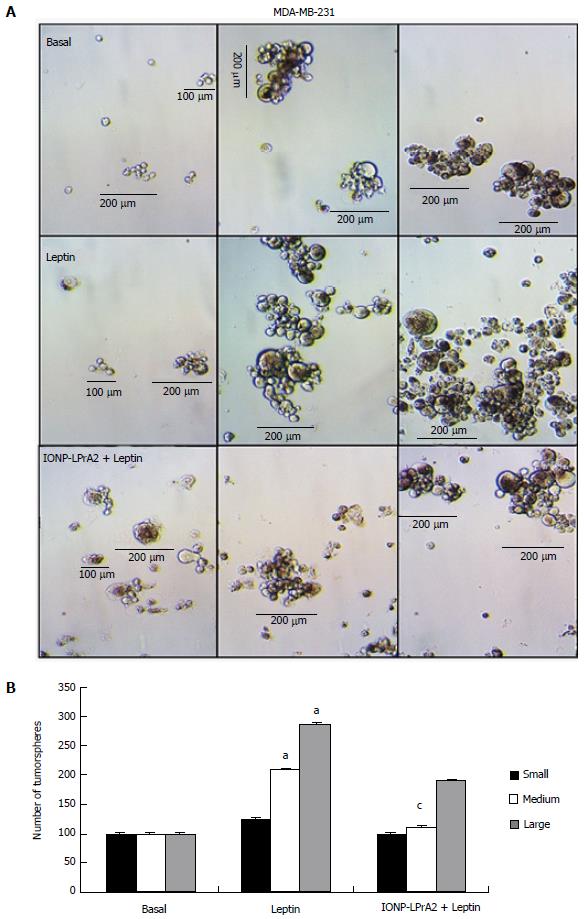
MDA-MB-231 cells were seeded at 5 × 103-2 × 104 cells/mL in low attachment plates and grown for 1-2 wk in Mammocult complete medium (Stem Cell Technologies) supplemented with heparin and hydrocortisone and treated with leptin (1.2 nmol/L), or IONP-LPrA2 (0.0036 pmol/L) plus leptin (1.2 nmol/L). Basal tumorspheres served as untreated controls. The tumorspheres were visually assessed by light microscopy. The size of the tumorspheres were determined and the number of tumorspheres were counted manually in triplicate.
Cells were seeded at 2 × 105 in 6 well cell culture plates and grown to 70%-80% confluence. They were treated with the chemotherapeutic drugs: Cis (Millipore), CTX, PTX, and Dox (SelleckChem) in 5% FBS with or without IONP-LPrA2 for time periods ranging from 1-6 d. Before trypsinizing, the supernatants were transferred into microfuge tubes for subsequent analysis. The trypsinized cells were added to the supernatants and centrifuged. The pellets were washed with 1 × PBS and resuspended in Annexin V binding buffer (Nexcelom). Annexin V/FITC, and PI, 5 μL each (Nexcelom) were added with mixing. The samples were incubated in the dark at room temperature for 15 min. The cells were washed with 1 × PBS, centrifuged, and resuspended in Annexin V binding buffer to a concentration of 3 × 104 cells per 20 μL. The samples were analyzed by the Cellometer Vision. The viability was determined by multiplying the percentage of live cells by the total cell count.
All experiments were performed in triplicate. One-way ANOVA (SigmaPlot) was used to determine statistical significance among treatment groups and controls. Data presented as the average ± standard deviation (SD). P values of P < 0.05 were considered statistically significant.
The statistical review was performed by Ward Kirlin, PhD. The appropriate ANOVA of variance was performed on the data presented in this paper, and levels of statistical significance are based on the F-values and Tukey’s multiple comparisons between group means as determined using SigmaPlot (Systat Software, Inc.). Mean + SDs are indicated in the graphical analysis, based on replicates of densitometry analysis of Western blots, the percentage of cells in S-phase of the cell cycle, or percentage of proliferating cells as indicated in the figures.
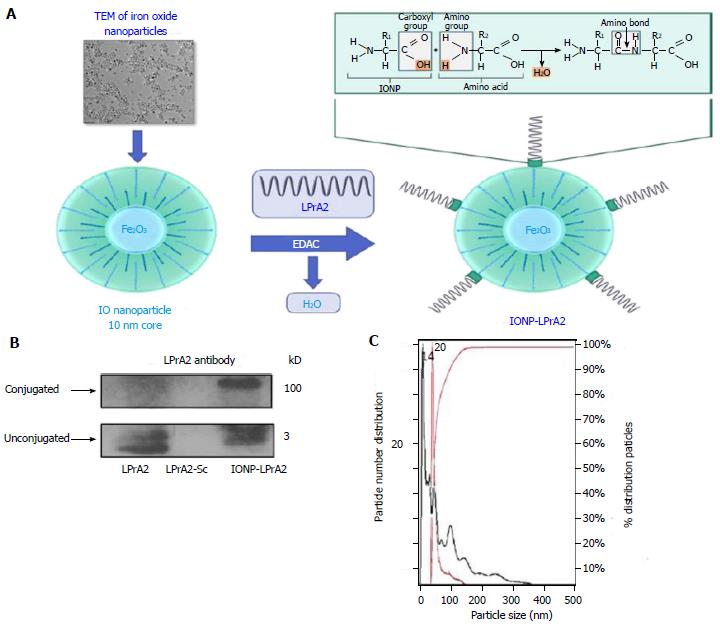
The leptin antagonist, LPrA2, has been shown to inhibit breast cancer growth and progression in vitro as well as in vivo[2,22,23]. To increase its efficacy, LPrA2 was conjugated to IONPs. IONPs are amphiphilic and have a 10 nm core[20]. The binding of LPrA2 to IONPs was facilitated by EDAC, which activates the carboxyl group on the IONP surface and allows the formation of an amide bond with the amino group of LPrA2 (Figure 1A). To confirm the binding of the LPrA2 peptides to the nanoparticles, the conjugates were analyzed by SDS-PAGE and Western blot. With LPrA2 antibody incubation, bands were detected at approximately 100 kD, indicating conjugated LPrA2, and approximately 3 kD indicating unbound LPrA2. Unconjugated LPrA2 and LPrA2-Sc were used as positive and negative controls, respectively (Figure 1B). To further characterize IONP-LPrA2, 1:10000 dilutions of the conjugated and unconjugated IONPs were measured by NanoSight nanoparticle tracking analysis (Malvern); in which the left and right Y-axes show particle number and percent distribution, and the X-axis displays particle size. The size of the unconjugated IONP was found to be 14 nm while conjugated IONP-LPrA2 measured 20 nm. This data suggests that the conjugation of LPrA2 to IONPs was successful.
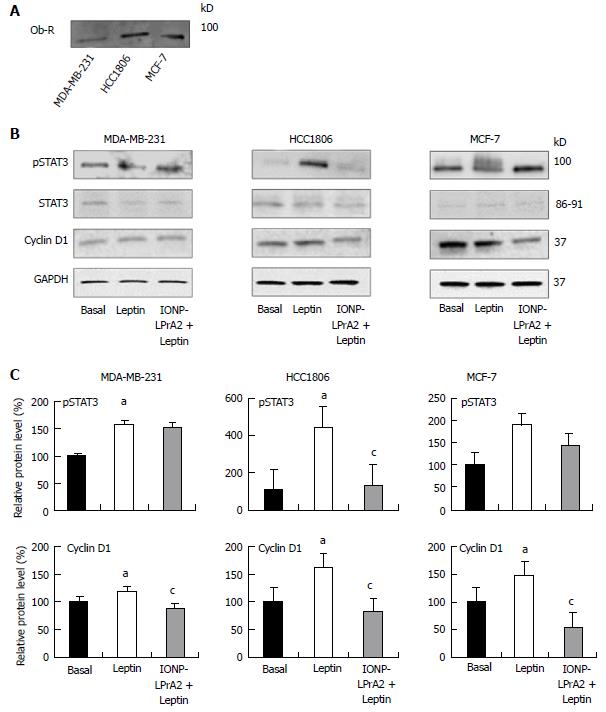
In order to determine the effects of IONP-LPrA2 on leptin signaling inhibition, we first had to confirm expression of the leptin receptor, Ob-R, in the human breast cancer cell lines. Immunoprecipitation and subsequent Western blot analysis showed Ob-R expression in MDA-MB-231, HCC1806, and MCF-7 cells (Figure 2A). Leptin signaling activates the JAK2/STAT3, MAPK/ERK, and PI3/Akt signaling pathways, which are implicated in its anti-apoptotic activity[9]. For this reason, we aimed to determine the effect of IONP-LPrA2 treatment on active/phosphorylated, pSTAT3. Leptin significantly increased the level of pSTAT3 in MDA-MB-231 and HCC1806 cells. IONP-LPrA2 significantly inhibited the effect of leptin on pSTAT3 levels in HCC1806 cells. No significant changes occurred in pSTAT3 levels in MCF-7 cells treated with leptin and IONP-LPrA2 (Figure 2B and C). Because leptin has been shown to increase cyclin D1 levels in breast cancer cells[14,15], we sought to determine the effect of IONP-LPrA2 treatment on cyclin D1 expression in MDA-MB-231, HCC1806, and MCF-7 breast cancer cells. Leptin significantly induced cyclin D1 expression in all cell lines (Figure 2B and C). The addition of IONP-LPrA2 significantly inhibited the effect of leptin on cyclin D1 expression in all cell lines (Figure 2B and C). These results suggest that IONP-LPrA2 abrogates the effect of leptin on leptin-induced signaling pathways.
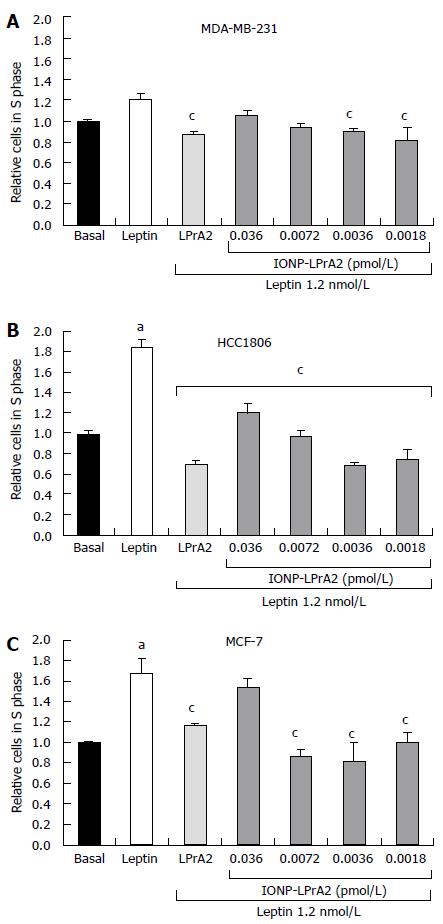
Leptin has been shown to increase expression of the cell cycle associated protein, cyclin D1[14,15]. To illustrate the effect of leptin on cell cycle progression, the number of cells in the S phase was determined by cell cycle analysis with the Cellometer Vision (Nexcelom). MDA-MB-231, HCC1806, and MCF-7 human breast cancer cells lines were treated with leptin (1.2 nmol/L) and IONP-LPrA2 plus leptin in order to determine its antagonistic effect. The cells were treated with IONP-LPrA2 concentrations ranging from 0.0018-0.036 pmol/L. MDA-MB-231 and HCC1806 TNBC cell lines were treated for 24 h while the ER+ MCF-7 cells were treated for 48 h to produce an effect. Treatment with leptin caused a significant increase in cell cycle progression in HCC1806 (Figure 3B) and MCF-7 (Figure 3C), but had no significant effect on MDA-MB-231 cells (Figure 3A). Treatment with IONP-LPrA2 plus leptin abrogated leptin-induced cell cycle progression at 0.0018-0.0036 pmol/L in MDA-MB-231, at 0.0018-0.036 pmol/L in HCC1806, and at 0.0018-0.0072 in MCF-7 cells (Figure 3). This data elucidated the effective dilution of IONP-LPrA2 for abrogation of leptin-induced cell cycle progression in each of the cell lines.
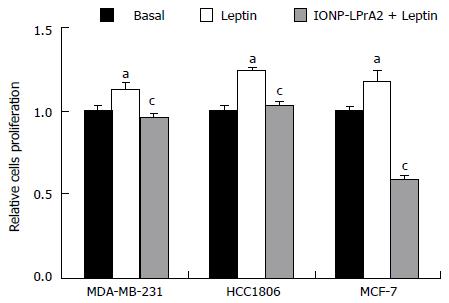
Leptin signaling stimulates breast cancer cell survival and proliferation[8]. To ascertain the manner in which IONP-LPrA2 affects cell proliferation, an MTT assay was performed. MDA-MB-231, HCC1806, and MCF-7 cell were treated with leptin (1.2 nmol/L) and IONP-LPrA2 (0.0036) plus leptin (1.2 nmol/L). Leptin treatment significantly increased cell proliferation and IONP-LPrA2 significantly diminished the effect of leptin in all of the cell lines (Figure 4). This data indicates that IONP-LPrA2 prevents leptin induction of cell proliferation.
Self-renewal is a hallmark of cancer. Leptin has been shown to increase self-renewal and breast cancer stem cell (BCSC) growth[24]. To learn how IONP-LPrA2 affects BCSC growth, tumorsphere formation was assessed. MDA-MB-231 TNBC cells were treated with leptin (1.2 nmol/L) and IONP-LPrA2 (0.0036 pmol/L) plus leptin (1.2 nmol/L). Untreated, basal, MDA-MB-231 cells developed few small and medium tumorspheres (100-200 μm), cells treated with leptin showed a significant increase in the development of medium (200 μm) and large tumorspheres (> 200 μm) in comparison to basal. Cells treated with IONP-LPrA2 plus leptin displayed a significant decrease in medium tumorsphere growth relative to the leptin treated (Figure 5). This data shows that IONP-LPrA2 treatment may decrease BCSC growth.
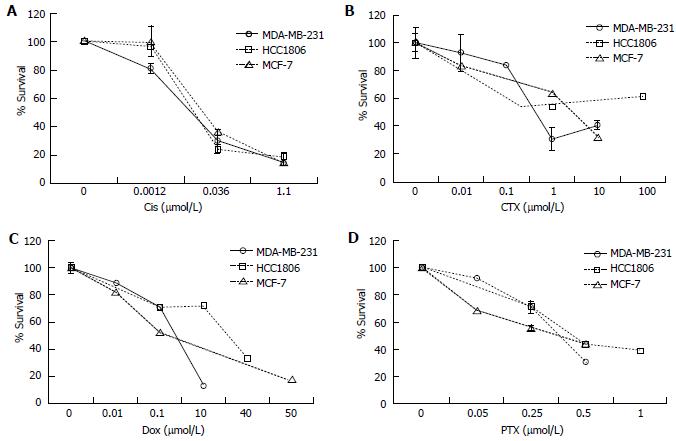
Chemotherapy is among the most common treatments for breast cancer in addition to radiation and surgery[25]. To determine the effective dose of chemotherapeutics, cells were treated with a panel of anti-cancer drugs and viability was tested by the Annexin V FITC/PI Assay (Nexcelom). MDA-MB-231, HCC1806, and MCF-7 cells were treated with Cis (0.001-1.1 μmol/L), CTX (0.01-100 μmol/L), Dox (0.01-50 μmol/L), and PTX (0.05-1.0 μmol/L) for time periods ranging from 1-6 d to determine an effective dose to reduce cell viability (Figure 6). Cis and Dox reduced cell viability in 24 h while CTX and PTX treated cells required up to 6 d to produce an effect. All cell lines displayed a similar response to Cis and PTX (Figure 6A and D). MDA-MB-231 cells appeared to be more sensitive to CTX and Dox (Figure 6B and C).
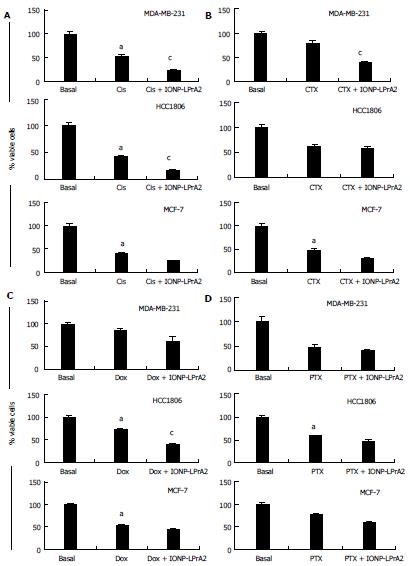
Chemotherapy has many detrimental side effects; because of this it is advantageous to utilize adjuvant therapies in order to reduce the effective dose. To determine the adjuvant potential of IONP-LPrA2, cells were treated with chemotherapeutics combined with IONP-LPrA2 and analyzed for viability by the Annexin V FITC/PI Assay (Nexcelom). MDA-MB-231, HCC1806, and MCF-7 cells were treated with chemotherapeutics at concentrations determined in Figure 6 in media containing 5% FBS to mimic physiological leptin levels, in addition to IONP-LPrA2 (0.0036 pmol/L) for time periods ranging from 1-6 d. The treatment concentrations were MDA-MB-231 (Cis 0.001 μmol/L, CTX 0.5 μmol/L, Dox 0.4 μmol/L, PTX 0.5 μmol/L); HCC1806 (Cis 0.036 μmol/L, CTX 1 μmol/L, Dox 10 μmol/L, PTX 0.5 μmol/L); and MCF-7 (Cis 0.036 μmol/L, CTX 5 μmol/L, Dox 0.01 μmol/L, PTX 1 μmol/L). MDA-MB-231 TNBC cells treated with IONP-LPrA2 displayed a significant decrease in viable cells when dosed with Cis and CTX (Figure 7A and B). HCC1806 TNBC cells treated with IONP-LPrA2 showed a significant reduction in viable cells when dosed with Cis and Dox (Figure 7A and C). ER+ MCF-7 cells treated with IONP-LPrA2 did not show a significant decrease in viable cells when treated with chemotherapeutics (Figure 7). Although cells were treated with PTX for up to 6 d to reduce cell viability, IONP-LPrA2 showed no additional decrease in viability when combined with PTX (Figure 7D). PTX is an anti-microtubule agent which acts on the M phase of the cell cycle while the other chemotherapeutics act on DNA which affects the S phase[2]. This data suggests that IONP-LPrA2 increases the potency of chemotherapeutics on TNBC cells, particularly anti-cancer drugs which target DNA.
In spite of methods for early detection of breast cancer, it remains the second leading cause of cancer deaths in women in the United States[1]. TNBC is a subtype of breast cancer characterized by the lack of hormone receptor expression. The absence of hormone receptors makes this more aggressive form of breast cancer even more difficult to treat. Obesity is often associated with poorer outcomes in individuals with breast cancer, particularly those with TNBC[25]. Obesity is characterized by an excess of the inflammatory cytokine, leptin. Elevated leptin levels display a significant correlation with metastasis and lower breast cancer patient survival[26]. The leptin antagonist, LPrA2 has been shown to inhibit leptin signaling in breast and other cancer types, but the actions of LPrA2 are restricted by its low MW of < 3 kD, short half-life, and insolubility in water[8,27]. IONP-LPrA2 was developed to circumvent these limitations. IONPs conjugated to other peptides, such as the amino terminal fragment of urokinase type plasminogen activator (ATF-uPA) are stable for more than 48 h in in vivo imaging experiments[20]. IONPs are amphiphilic, small (10 nm core size), and uniformly sized to facilitate delivery which prevents phagocytosis[28]. The characteristics of IONPs make them an ideal delivery system for LPrA2 to target and treat breast cancer. In the present study, IONP-LPrA2 was used to evaluate its ability to inhibit leptin signaling in human breast cancer cells. The data indicates that IONP-LPrA2 abrogates cell cycle progression and acts as an adjuvant when administered with chemotherapeutics.
Decreased levels of pSTAT3 and cyclin D1 with IONP-LPrA2 treatment were shown by Western blot. Cyclin D1 is a cell cycle regulatory gene. STAT3 is a transcription factor responsible for the regulation of cyclin D1[10]. Decreased levels of pSTAT3 with IONP-LPrA2 treatment were seen at time points as early as 5-15 min post treatment. Previous studies have shown that leptin is mitogenic and increases cyclin D1 in ER+ MCF-7 breast cancer cells[14,15]. Because leptin increases cyclin D1 and IONP-LPrA2 inhibits the effect of leptin, utilizing agents that target cyclin D1 may be a plausible method to treat breast cancer. In this study, we have shown that IONP-LPrA2 decreases pSTAT3 and cyclin D1. The decreased levels of these leptin-induced targets may inhibit cell cycle progression in ER+ MCF-7 cells as well as MDA-MB-231 and HCC1806 TNBC cells.
Inhibition of cell cycle progression by IONP-LPrA2 was displayed by image based cytometry. Leptin has been shown to increase levels of cyclin D1[14,15]. In this study, we show that IONP-LPrA2 decreases cyclin D1 expression, but the effect on cell cycle progression was yet to be determined. Here we show that IONP-LPrA2 treatment decreases the percentage of cells in the S phase of the cell cycle, where DNA is synthesized, as or more effectively than LPrA2 alone. Interestingly, the greatest decrease in the percentage of cells in S phase with IONP-LPrA2 treatment was seen in HCC1806 TNBC cells derived from a non-metastatic squamous cell carcinoma in contrast to MCF-7 and MDA-MB-231 cells derived from metastatic adenocarcinomas. This data suggests that IONP-LPrA2 inhibition of cell cycle progression may reduce the advancement of breast cancer, and may be particularly beneficial in the treatment of non-metastatic and squamous cell carcinomas.
Chemotherapy is the first line of treatment for TNBC. Although TNBC is generally more responsive to chemotherapy than other forms of breast cancer, there is an increased risk of developing drug resistance[29]. BCSC growth and self-renewal play an important role in breast cancer drug resistance and leptin increases the risk[24]. These cells express molecular markers for breast cancer, CD44+CD24-/ALDH+[10]. We have demonstrated that leptin induces in vitro BCSC, tumorsphere, formation and treatment with IONP-LPrA2 attenuates the effect of leptin in MDA-MB-231 TNBC cells. These results indicate that IONP-LPrA2 prevents BCSC formation and may decrease chemoresistance in TNBC.
Chemotherapeutic treatment of breast cancer is plagued with high toxicity. Toxic side effects and the development of drug resistance are cause for the development of adjuvant therapies. The need for adjuvant therapies is exacerbated in TNBC patients who often experience relapse and develop resistance to chemotherapy[29]. TNBC is commonly treated with combination chemotherapy[25]. Here, we treated breast cancer cells with a panel of commonly used chemotherapeutics (Cis, CTX, Dox and PTX) in addition to IONP-LPrA2 to test its ability to decrease cell viability more than the drugs alone. We demonstrated that TNBC cells, MDA-MB-231 displayed a significant decrease in viability with Cis and CTX plus IONP-LPrA2; and HCC1806 showed a significant reduction in live cells when treated with Cis and Dox plus IONP-LPrA2. ER+ MCF-7 cells treated with chemotherapeutics plus IONP-LPrA2 did not show a significant decrease in viable cells. Also, there was no significant decrease in viability in the cells treated with PTX plus IONP-LPrA2. This may be due, in part, to PTX’s anti-microtubule action, which affects the M phase of the cell cycle[25]. Cis, CTX, and Dox act on DNA which affects the S phase[25]. These drugs may work synergistically with IONP-LPrA2, which also appears to act on the S phase. These data indicate that IONP-LPrA2 may act as a chemotherapeutic adjuvant by decreasing viability, thereby decreasing the effective dose in TNBC.
In conclusion, IONP-LPrA2 was found to decrease the level of leptin-induced targets pSTAT3 and cyclin D1. IONP-LPrA2 decreased DNA synthesis during the S phase of the cell cycle and reduced proliferation in both ER+ and TNBC cells. When combined with chemotherapeutics, particularly drugs targeting the S phase, IONP-LPrA2 showed an additive effect on the reduction of live breast cancer cells. These findings indicate that IONP-LPrA2 may be useful in the prevention of tumor cell growth and proliferation in breast cancer. Further, treatment with IONP-LPrA2 may allow for lower chemotherapeutic dosing. These results are potentially beneficial for obese patients with elevated leptin levels, whom have a higher incidence and thus poorer outcome of TNBC. Taken together, the present data provides confirmation of our hypothesis that IONP-LPrA2 treatment may be useful in impairing tumor growth and when given in combination with the indicated chemotherapeutics has the potential to increase drug effectiveness. These data indicate that there is a synergistic effect with IONP-LPrA2 and chemotherapeutics which affect the S phase of the cell cycle in vitro.
The authors warmly thank Dr. Ming Bo Huang for facilitating the nanoparticle tracking analysis. We also thank Mr. Patrick Abramson and Ms. Aria Armstrong for aesthetic assistance with diagrams and figures.
Obesity and high leptin levels are strongly associated with breast cancer relapse, drug resistance, and poorer patient outcomes. Overexpression of leptin and its receptor, Ob-R, induce breast cancer cell growth and proliferation. Triple negative breast cancer (TNBC) is a subtype of breast cancer which comprises approximately 15% of cases and is an aggressive form of the disease with no targeted therapy. TNBC chemotherapeutic treatment often leads to chemoresistance and shows several undesirable side effects. Leptin is proliferative and is a survival factor for breast cancer treated with chemotherapeutics. Therefore, the authors have developed a leptin peptide receptor antagonist coupled to IONP-LPrA2, which successfully inhibits leptin signaling as well as increases chemotherapeutic effectiveness in breast cancer and is particularly promising for TNBC treatment.
IONP-LPrA2 could be a new and effective biological for blocking pro-oncogenic and drug resistance effects of leptin in breast cancer, especially in obese patients suffering from TNBC that are treated with chemotherapeutics.
This study describes for the first time the production and characterization of a new biological bound to nanoparticles that can effectively block leptin signaling inducing proliferation and survival in breast cancer cells treated with chemotherapeutics.
In recent years, IONPs have become an important tool for biomedical applications. The use IONPs has been employed in vaccinations, drug delivery, MRI, and molecular imaging. The authors’ data suggests combining IONPs with the leptin antagonist, LPrA2, prevents the growth of breast cancer cells and acts as a chemotherapeutic adjuvant by reducing the effective dose.
Leptin signaling occurs when the hormone is secreted by the adipose tissue and binds to its receptor, Ob-R. Breast cancer, particularly in obese individuals, is associated with high levels of leptin. Leptin signaling leads to increased breast cancer cell growth, proliferation and drug resistance. The inhibition of leptin signaling with the nanoparticle-linked leptin antagonist, IONP-LPrA2, provides a promising new way to improve breast cancer chemotherapy.
This manuscript provides useful information to the medical students, clinicians, and researchers in this field, therefore, is acceptable for publication.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sonoda K, Zhang XQ S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | 1 American Cancer Society. 2015-2016. Available from: http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/breast-cancer-key-statistics. |
| 2. | Chavez KJ, Garimella SV, Lipkowitz S. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010;32:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 512] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 3. | Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23 Suppl 6:vi7-v12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 413] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 4. | Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16 Suppl 1:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 585] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 5. | Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 705] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 6. | Rock CL, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol. 2002;20:3302-3316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 275] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Guo S, Colbert LS, McGlothen TZ, Gonzalez-Perez RR. Regulation of Angiogenesis in Human Cancer via Vascular Endothelial Growth Factor Receptor -2 (VEGFR-2) (In Tumor Angiogenesis, 2011). Available from: http: //www.intechweb.org/. |
| 8. | Gonzalez RR, Leavis PC. A peptide derived from the human leptin molecule is a potent inhibitor of the leptin receptor function in rabbit endometrial cells. Endocrine. 2003;21:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Gonzalez RR, Leavis PC. Leptin Peptide Antagonists, USSN 7612043, 2009. |
| 10. | Guo S, Liu M, Wang G, Torroella-Kouri M, Gonzalez-Perez RR. Oncogenic role and therapeutic target of leptin signaling in breast cancer and cancer stem cells. Biochim Biophys Acta. 2012;1825:207-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim Biophys Acta. 2011;1815:197-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab. 2008;294:E817-E826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Bassi M, Furuya WI, Zoccal DB, Menani JV, Colombari E, Hall JE, da Silva AA, do Carmo JM, Colombari DS. Control of respiratory and cardiovascular functions by leptin. Life Sci. 2015;125:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, Sullivan BT, Sakamoto H, Olawaiye A, Serikawa T. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2). J Biol Chem. 2006;281:26320-26328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, Penichet ML. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Gillespie C, Quarshie A, Penichet M, Gonzalez-Perez RR. Potential Role of Leptin Signaling in DMBA-induced Mammary Tumors by Non-Responsive C57BL/6J Mice Fed a High-Fat Diet. J Carcinogene Mutagene. 2012;3: 132. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Crujeiras AB, Carreira MC, Cabia B, Andrade S, Amil M, Casanueva FF. Leptin resistance in obesity: An epigenetic landscape. Life Sci. 2015;140:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 18. | James FR, Wootton S, Jackson A, Wiseman M, Copson ER, Cutress RI. Obesity in breast cancer--what is the risk factor? Eur J Cancer. 2015;51:705-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Gonzalez RR, Leavis PC. Leptin Peptide Antagonists. 2004;Patent US. 7407929, Application No. 10/841, 218; International application No. PCT/US 05/15198. |
| 20. | Yang L, Peng XH, Wang YA, Wang X, Cao Z, Ni C, Karna P, Zhang X, Wood WC, Gao X. Receptor-targeted nanoparticles for in vivo imaging of breast cancer. Clin Cancer Res. 2009;15:4722-4732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Battle M, Gillespie C, Quarshie A, Lanier V, Harmon T, Wilson K, Torroella-Kouri M, Gonzalez-Perez RR. Obesity induced a leptin-Notch signaling axis in breast cancer. Int J Cancer. 2014;134:1605-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:R25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 755] [Cited by in RCA: 805] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 23. | Cell Signaling Technology. CST Guide: Pathways & Protocols, 2015. |
| 24. | Cioce M, Gherardi S, Viglietto G, Strano S, Blandino G, Muti P, Ciliberto G. Mammosphere-forming cells from breast cancer cell lines as a tool for the identification of CSC-like- and early progenitor-targeting drugs. Cell Cycle. 2010;9:2878-2887. [PubMed] |
| 25. | Aysola K, Desai A, Welch C, Xu J, Qin Y, Reddy V, Matthews R, Owens C, Okoli J, Beech DJ. Triple Negative Breast Cancer - An Overview. Hereditary Genet. 2013;2013:pii: 001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Scolaro L, Cassone M, Kolaczynski JW, Otvos L Jr, Surmacz E. Leptin-based therapeutics. Expert Rev Endocrinol Metab. 2010;5:875-889. |
| 27. | Otvos L, Kovalszky I, Riolfi M, Ferla R, Olah J, Sztodola A, Nama K, Molino A, Piubello Q, Wade JD. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur J Cancer. 2011;47:1578-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Yang E, Qian W, Cao Z, Wang L, Bozeman EN, Ward C, Yang B, Selvaraj P, Lipowska M, Wang YA. Theranostic nanoparticles carrying doxorubicin attenuate targeting ligand specific antibody responses following systemic delivery. Theranostics. 2015;5:43-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Rodler E, Korde L, Gralow J. Current treatment options in triple negative breast cancer. Breast Dis. 2010;32:99-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |