Published online Oct 10, 2016. doi: 10.5306/wjco.v7.i5.395
Peer-review started: June 24, 2016
First decision: August 5, 2016
Revised: September 7, 2016
Accepted: September 21, 2016
Article in press: September 23, 2016
Published online: October 10, 2016
Processing time: 106 Days and 13.8 Hours
To analyze the association between oncohematological diseases and GSTT1/GSTM1/CYP1A1 polymorphisms, dietary habits and smoking, in an argentine hospital-based case-control study.
This hospital-based case-control study involved 125 patients with oncohematological diseases and 310 control subjects. A questionnaire was used to obtain sociodemographic data and information about habits. Blood samples were collected, and DNA was extracted using salting out methods. Deletions in GSTT1 and GSTM1 (null genotypes) were addressed by PCR. CYP1A1 MspI polymorphism was detected by PCR-RFLP. Odds ratio (OR) and 95%CI were calculated to estimate the association between each variable studied and oncohematological disease.
Women showed lower risk of disease compared to men (OR 0.52, 95%CI: 0.34-0.82, P = 0.003). Higher levels of education (> 12 years) were significantly associated with an increased risk, compared to complete primary school or less (OR 3.68, 95%CI: 1.82-7.40, P < 0.001 adjusted for age and sex). With respect to tobacco, none of the smoking categories showed association with oncohematological diseases. Regarding dietary habits, consumption of grilled/barbecued meat 3 or more times per month showed significant association with an increased risk of disease (OR 1.72, 95%CI: 1.08-2.75, P = 0.02). Daily consumption of coffee also was associated with an increased risk (OR 1.77, 95%CI: 1.03-3.03, P = 0.03). Results for GSTT1, GSTM1 and CYP1A1 polymorphisms showed no significant association with oncohematological diseases. When analyzing the interaction between polymorphisms and tobacco smoking or dietary habits, no statistically significant associations that modify disease risk were found.
We reported an increased risk of oncohematological diseases associated with meat and coffee intake. We did not find significant associations between genetic polymorphisms and blood cancer.
Core tip: Cancer is considered as a multi-factorial disease. Except certain genetic abnormalities, viruses, environmental exposures and chemotherapeutic agents, it is not well defined which are the risk factors for these diseases (leukemia, lymphoma, multiple myeloma, among others). Here, we analyzed lifestyle and genetic polymorphisms as risk factors for blood cancer. We reported an increased risk of disease associated with meat and coffee intake. No significant associations were found between metabolic gene polymorphisms and disease. Our study offers relevant insights into diverse aspects of oncohematological diseases etiology, particularly genes and environmental factors, in an Argentinean population.
- Citation: Cerliani MB, Pavicic W, Gili JA, Klein G, Saba S, Richard S. Cigarette smoking, dietary habits and genetic polymorphisms in GSTT1, GSTM1 and CYP1A1 metabolic genes: A case-control study in oncohematological diseases. World J Clin Oncol 2016; 7(5): 395-405
- URL: https://www.wjgnet.com/2218-4333/full/v7/i5/395.htm
- DOI: https://dx.doi.org/10.5306/wjco.v7.i5.395
Xenobiotic metabolizing enzymes (XME), coded by a family of xenobiotic metabolizing genes (XMG), transform endo and exogenous compounds in hydrophilic by-products, which are more easily excreted from the tissues[1]. It is well known since decades that genetic differences in the metabolism of drugs and environmental chemicals exist[2]. These differences are due to pharmacogenetic polymorphisms, which are allele variants that occur with a relatively high frequency in the population, and are generally associated with anomalies in gene expression or enzymatic function. Moreover, pharmacogenetic polymorphisms have been associated with an increased risk of some types of cancer, due to: (1) an impaired ability to inactivate endogenous or exogenous mutagenic molecules; or (2) the conversion of metabolites into highly reactive and toxic compounds. Both polymorphisms and the levels of exposure to their substrates, may impact on cancer susceptibility.
The cytochrome P450 family of enzymes is responsible for catalyzing phase I metabolism reactions. CYP1A1 is a member of the CYP family and plays an important role in the metabolism of estrogen and polycyclic aromatic hydrocarbons (PAHs), catalyzing the activation of pro-carcinogenic PAHs[3]. Dysfunction of CYPs enzymes can cause damage to DNA, lipids and proteins, leading to carcinogenesis[4,5]. A commonly studied single nucleotide polymorphism (SNP) in CYP1A1 gene is the T3801C (also named MspI polymorphism, *2A or m1), a T to C mutation in the 3’ flanking region of the gene. The C variant becomes more highly inducible than the T variant[6], which may cause enhanced enzymatic activity, thus modifying susceptibility to adduct formation and cancer risk[7]. In fact, T3801C polymorphism was associated with leukemia and cervical, hepatocellular, lung, prostate, and head and neck cancer[8].
Glutathione S-transferases (GSTs) constitute a superfamily of phase II detoxification enzymes which play a key role in cellular protection against environmental carcinogens, drugs, toxins and by-products of oxidative stress. GSTs catalyze the conjugation of reduced glutathione (GSH) to a wide variety of electrophilic compounds to facilitate their cellular excretion. In addition, as non-enzymatic proteins, GSTs can modulate signaling pathways that control cell proliferation, cell differentiation, apoptosis, anti- and pro-inflammatory functions and DNA damage processing, among other processes[9]. Genetic polymorphisms in GST genes are common in the human population. GSTM1 and GSTT1 exhibit variations in copy number due to complete gene deletion, resulting in the loss of enzymatic activity. The absence of enzyme has been associated with lung, breast and gastrointestinal cancer, among others[10], and also with adverse side effects and toxicity in chemotherapies[11].
Lifestyle and dietary habits are additional risk factors for cancer. Diet is known to modulate the immune system, and it may also influence cancer susceptibility through changes in the energy balance and in the levels of carcinogens and anticarcinogens[12]. Cigarette smoke contains more than 7000 chemicals and compounds, from which more than 70 are associated with cancer[13]. Benzene, present in tobacco smoke, is a strong carcinogen associated with leukemia and lymphoma development[14], and has long been recognized as hematotoxic[15].
It should not be forgotten that cancer susceptibility results from genetic and environmental factors, individually or in combination. According to this, it is expected that genetic, dietary and lifestyle factors interact with each others.
Several studies have inquired the epidemiologic risk factors associated with leukemia, lymphoma and/or myeloma. Except certain genetic abnormalities, viruses, environmental exposures and chemotherapeutic agents, little is known about risk factors that develop these onco-hematological diseases.
Argentina is within the range of countries with medium to high incidence of cancer, according to the International Agency for Research on Cancer (IARC) data for 2012. They estimated an incidence of 14.2 new cases/year/100000 persons for Hodgkin lymphoma (HL), leukemia, non-Hodgkin lymphoma (NHL) and multiple myeloma (MM) all together[16]. During 2012, nearly 3830 patients have died because of these diseases according to the Statistics and Health Information Office[17]. Between 2007 and 2011, oncohematological diseases account for the 6.5% of all cancer deaths[18].
The aim of this study was to analyze the association between oncohematological diseases and genetic polymorphisms in GSTT1, GSTM1 and CYP1A1, dietary habits and cigarette smoking, in an argentine hospital-based case-control study.
A hospital-based case-control study was performed, involving 125 patients with oncohematological diseases and 310 control subjects. Participants were recruited between June 2013 and March 2015 at the Unit of Diagnosis, Treatment and Support for Hematological Diseases of the Acute Care General Hospital “Prof. Dr. Rodolfo Rossi” (La Plata, Buenos Aires, Argentina). The study was approved by the hospital’s Ethics Committee. The International Classification of Diseases for Oncology information was not available.
Cases were patients diagnosed with acute lymphoblastic leukemia (ALL, n = 10), acute myeloblastic leukemia (AML, n = 18), chronic lymphoblastic leukemia (CLL, n = 10), chronic myeloblastic leukemia (CML, n = 20), MM (n = 29), HL (n = 18) and NHL (n = 20). Controls included patients frequently visiting the Unit for routine checks of disorders unrelated to cancer or preoperative blood analyses (i.e., for ophthalmic surgeries, pre-employment medical examinations, anemia, among others). All participants reside in Argentina. Cases and controls with previous history of cancer or pathologies closely related to oncohematological diseases were excluded from the study.
Patients were invited to participate in the study and signed an informed consent. A questionnaire was used to obtain sociodemographic data and information about habits, such as cigarette smoking (never, former and current smoker), and consumption of grilled/barbecued meat (times per month), canned food (times per week), alcohol (times per week) and coffee (cups per day). No other food or beverage items were asked. The survey also included weight, height, medication, working conditions, family history of cancer, history of disease (only in cases), and functionality data (quality of sleep, fatigue, changes in appetite and mood, etc.). The overall case and control response rate was higher than 90% for both groups.
All surveys were addressed by the same person. Blood samples were collected and kept in Vacutainer tubes with K2-EDTA (3.6 mg), and DNA was extracted from whole blood using salting out methods.
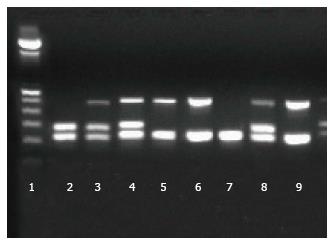
GSTT1 and GSTM1 gene deficiency resulted from the deletion of the loci (null alleles). The detection was performed by a multiplex PCR, using GSTT1 forward primer 5’-TTC CTT ACT ggT CCT CAC ATC TC-3’ and GSTT1 reverse primer 5’-TCA CCg gAT CAT ggC CAg CA-3’ (459 bp product), GSTM1 forward primer 5’-CTg CCC TAC TTg ATT gAT ggg-3’ and GSTM1 reverse primer 5’-CTg gAT TgT AgC AgA TCA TgC-3’ (273 bp product), and a third pair of primers, forward 5’-TCC AgC AgT TTC ATg AgA TgC-3’ and reverse 5’-gAg gTC ATT TCA Tag CTg AgC-3’ for a 221 bp product of the gene CLOCK, as an internal control of the reaction (Figure 1). PCR conditions were as follows, in a final volume of 15 μL: 1 × buffer, 50 ng DNA, 0.25 μmol/L each primer, 200 μmol/L dNTPs, 2 mmol/L MgCl2, 0.45 U Taq Platinum Polymerase (Invitrogen, Life Technologies) and H2O up to 15 μL. PCR cycling consisted in an initial denaturation at 94 °C for 5 min, followed by 35 cycles of 1 min at 94 °C, 1 min at 59 °C and 1 min at 72 °C, with a final extension at 72 °C for 5 min. Verification of PCR products, and subsequent identification of genotype, were performed using 2% agarose gels stained with GelRed (Biotium Inc., CA, United States). The absence of PCR product defines the null allele.
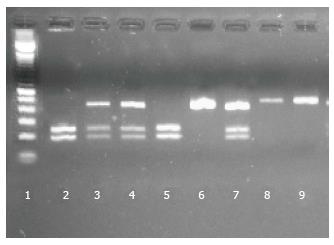
MspI polymorphism was detected by PCR-RFLP. A product of 420 bp was amplified by PCR in a final volume of 15 μL, containing buffer 1 ×, 2 mmol/L MgCl2, 250 nmol/L each primer, 200 μmol/L dNTPs, 0.4 U Taq DNA Polymerase Recombinant (Invitrogen), 30 ng DNA and H2O up to 15 μL. Primers were: forward 5’-ACC CCA TTC TgT gTT ggg TT-3’ and reverse 5’-TAg AgA ggg CgT AAg TCA gCA-3’. Cycling conditions were as follows: An initial denaturation at 94 °C for 5 min, followed by 35 cycles of 30 s at 94 °C, 30 s at 58 °C and 40 s at 72 °C, with a final extension at 72 °C for 5 min.
After checking amplification in 2% agarose gel stained with Gel Red, 7 μL of PCR product were digested with 5U of MspI enzyme (Thermo Scientific), buffer and H2O up to 15 μL. Incubation time was 5 h at 37 °C. Verification of digested products was carried out in 2% agarose gels stained with Gel Red. The C variant has the restriction site, generating 237 and 183 bp products (Figure 2).
Odds ratio (OR) and 95%CI were calculated to estimate the association between each variable studied and oncohematological disease. χ2 test was applied to obtain the statistical significance of the association. Analyses were performed with the softwares STATA 11.1[19] and Epidat 4.0[20]. Genotype and allele frequencies were calculated and tested for Hardy-Weinberg Equilibrium using the software GenAlEx 6.5[21]. The sample size of this survey achieved 80% power to detect an OR = 2. P-values ≤ 0.05 were considered statistically significant. The statistical review of the study was performed by a biomedical statistician (Gili JA, one of the authors).
In this association study, a total of 125 cases were compared to 310 controls, all of them patients from the Acute Care General Hospital “Prof. Dr. Rodolfo Rossi”. Demographic characteristics are listed in Table 1, and were already published by our group in Cerliani et al[22]. Missing data for each variable were not included in the analysis, nor are detailed in the tables. The maximum number of missing data in a variable was 18, representing 4.14% of the samples. Of all variables in the questionnaire, only height and weight were excluded due to > 10% missing data. There was no significant difference in the mean age of cases and controls. Women showed lower risk of disease compared to men (OR 0.52, 95%CI: 0.34-0.82, P = 0.003). Higher levels of education (> 12 years) were significantly associated with an increased risk, compared to complete primary school or less (OR 3.68, 95%CI: 1.82-7.40, P < 0.001 adjusted for age and sex). Marital status did not show association with the disease.
| Cases n = 125 | Controls n = 310 | OR (95%CI) | P | |
| Age mean (SD) | 48.5 (16.6) | 51 (18.5) | - | 0.507 |
| Sex | ||||
| Male | 74 (59.2) | 134 (43.2) | Ref. | 0.003 |
| Female | 51 (40.8) | 176 (56.8) | 0.52 (0.34-0.82) | |
| Education | ||||
| ≤ 7 yr (complete primary school or less) | 174 (56.31) | 55 (44) | Ref. | |
| 12 yr (complete secondary school) | 115 (37.22) | 48 (38.4) | 1.2 (0.73-1.95) | < 0.0011 |
| > 12 yr (complete college) | 20 (6.47) | 22 (17.6) | 3.68 (1.82-7.4) | |
| Marital status | ||||
| Single | 79 (25.48) | 27 (21.6) | Ref. | |
| In couple/married | 162 (52.26) | 86 (68.8) | 1.7 (0.99-2.95) | 0.051 |
| Divorced/separated | 27 (8.71) | 5 (4) | 0.64 (0.21-1.9) | 0.421 |
| Widowed | 42 (13.55) | 7 (5.6) | 0.75 (0.27-2.13) | 0.591 |
| Type of malignancy | ||||
| ALL | 10 (8) | |||
| AML | 18 (14.4) | |||
| CLL | 10 (8) | |||
| CML | 20 (16) | |||
| MM | 29 (23.2) | |||
| HL | 18 (14.4) | |||
| NHL | 20 (16) |
With respect to tobacco, none of the smoking categories showed association with oncohematological diseases. Regarding dietary habits, consumption of grilled/barbecued meat 3 or more times per month showed significant association with an increased risk of disease (OR 1.72, 95%CI: 1.08-2.75, P = 0.02). Daily consumption of coffee also was associated with an increased risk (OR 1.77, 95%CI: 1.03-3.03, P = 0.03). Since control patients with gastrointestinal problems could create a spurious OR (given that they may be abstaining from coffee), patients from the Gastroenterology Unit of the hospital were removed from the analysis, as well as control patients under treatment with gastric protectors or medication prescribed for other gastrointestinal issues. Therefore 19 controls were excluded from the analysis; nevertheless, coffee consumption still remains statistically correlated with disease, showing the same OR range (OR 1.01-3.02). No association was observed with consumption of canned food or alcohol. When assessing the risk associated with consumption of coffee and grilled/barbecued meat, within each of the pathologies, results showed no differences between groups for coffee consumption, but CML and MM cases exhibited a significant association with consumption of grilled/barbecued meat 3 or more times per month, adjusted for age, sex and educational level (data not shown). With regard to GSTT1, GSTM1 and CYP1A1 polymorphisms, results showed no significant association with oncohematological diseases. CYP1A1 MspI polymorphism was not in Hardy-Weinberg equilibrium. Allele and genotype frequencies are detailed in Table 2. Results from the association analysis between tobacco, dietary habits, XMG polymorphisms and oncohematological diseases are described in Table 3. When analyzing the interaction between XMG polymorphisms and tobacco smoking or dietary habits, no statistically significant associations that modify disease risk were found (data not shown).
| Cases n = 125 | Controls n = 310 | |
| Allele frequencies | ||
| CYP1A1 | ||
| T | 0.7 | 0.65 |
| C | 0.3 | 0.35 |
| Genotype frequencies (n) | ||
| GSTT1*null | 0.18 (22) | 0.18 (55) |
| GSTM1*null | 0.5 (61) | 0.47 (140) |
| CYP1A1 | ||
| TT | 0.53 (64) | 0.47 (140) |
| TC | 0.34 (42) | 0.37 (113) |
| CC | 0.13 (16) | 0.16 (47) |
| Cases n = 125 | Controls n =310 | OR (95%CI) | P | |
| Tobacco smoking status | ||||
| Never | 49 (39.8) | 142 (46.4) | Ref. | |
| Former | 47 (38.2) | 100 (32.7) | 1.36 (0.84-2.19) | 0.202 |
| Current | 27 (22) | 64 (20.9) | 1.22 (0.70-2.13) | 0.478 |
| Consumption of canned food | ||||
| 0-2 times/wk | 121 (96.8) | 292 (94.8) | Ref. | 0.370 |
| 3 or more times/wk | 4 (3.2) | 16 (5.2) | 0.60 (0.14-1.92) | |
| Consumption of grilled/barbecued meat | ||||
| 0-2 times/mo | 69 (55.2) | 217 (70.7) | Ref. | 0.0211 |
| 3 or more times/mo | 56 (44.8) | 90 (29.3) | 1.72 (1.08-2.75) | |
| Alcohol drinking | ||||
| 0-3 times/wk | 110 (88) | 268 (86.5) | Ref. | 0.665 |
| 4 or more times/wk | 15 (12) | 42 (13.5) | 0.87 (0.43-1.68) | |
| Consumption of coffee | ||||
| < 1 cup/d | 90 (72) | 259 (83.5) | Ref. | 0.0371 |
| 1 or more cups/d | 35 (28) | 51 (16.5) | 1.77 (1.03-3.03) | |
| GSTT1 | ||||
| Presence | 100 (82) | 245 (81.7) | Ref. | 0.942 |
| Null | 22 (18) | 55 (18.3) | 0.98 (0.54-1.74) | |
| GSTM1 | ||||
| Presence | 61 (50) | 160 (53.3) | Ref. | 0.534 |
| Null | 61 (50) | 140 (46.7) | 1.14 (0.73-1.78) | |
| CYP1A1 | ||||
| TT | 64 (52.5) | 140 (46.7) | Ref. | 0.280 |
| TC + CC | 58 (47.5) | 160 (53.3) | 0.79 (0.51-1.24) |
Carcinogenesis is considered as a multi-step and multi-factorial process that implied different genetic alterations and several biological pathways. Thus, it is expected that cancer risk factors interact with each others. Genetic polymorphisms may play different roles in cancer susceptibility according to the genetic background, environmental exposures and lifestyles[8]. Despite this, we did not find evidence of interaction between genetic and lifestyle factors, probably because the sample size is not big enough for this type of analysis.
Regarding tobacco use and dietary habits, we reported an increased risk of oncohematological diseases associated with grilled/barbecued meat intake 3 or more times per month, and with daily consumption of coffee, after adjustment for age, sex and educational level. Moreover, for both factors that showed an increased risk when analyzing all malignancies combined, we have also assessed the risk associated with each of the major groups within the case category. While for coffee consumption results showed no differences between groups, grilled/barbecued meat intake (3 times or more per month) has shown a significant correlation with CML and MM cases. However, it has to be considered that the sample size becomes small when divided by each pathology. Therefore, the real effect of each risk factor for a specific type of leukemia, lymphoma or myeloma might be determined in future studies by analyzing a larger set of samples.
Although cigarette smoke has been associated with mutagenic/carcinogenic effects, inflammation and immune suppression in animals and humans[23,24], previous studies on cigarette smoking and its association with blood cancers have generated inconsistent findings. Regarding NHL, studies reported little or no association with use of cigarettes/tobacco, or patterns related with duration or intensity of exposure (detailed in[25-27]). In a case-control study from Sweden, the effects of smoking on the risk of AML were weak and no significant[28]. However, a cohort study from the same country showed a 50% increased risk of AML for current smokers[29]; this study also indicated no association between current and former smokers with CML, ALL, CLL or MM. An Indian case-control study reported an increase of 2.1 fold in the risk of leukemia in the cigarette smokers, compared to non-smokers[30]. A US cohort study that evaluated risk factors for AML, showed hazard ratios of 1.79, 2.42 and 2.29 for former smokers of > 1 pack/d, current smokers of ≤ 1 pack/d, and current smokers of > 1 pack/d, respectively[31]. A meta-analysis done by Fircanis et al[32] including over 7500 cases of AML, found an increased risk of disease associated with smoking, regardless of sex, geographical region, study design and quality of studies. They also reported a higher risk with higher intensity and longer duration of smoking.
It is possible that the exposure to agents present in the smoke varies given the different usage patterns, exposure pathways and manufacturing processes.
A recent study done by Rubinstein et al[33] showed that 29.7% of the general population of Argentina, Chile and Uruguay (n = 7524) smoke cigarettes. The CARMELA study (Cardiovascular Risk Factor Multiple Evaluation in Latin America), performed between 2003 and 2005, reported that Buenos Aires (Argentina, n = 1482) and Santiago (Chile, n = 1655) have the highest smoking prevalence among the seven Latin American cities studied, with no gender differences (38.6% and 45.4% respectively)[34]. Our study reports that 20.9% of the controls and 22% of the cases are current smokers, with higher percentages for former smokers (32.7% for controls and 38.2% for cases). These results are probably due to that this study is based on hospital population, which strongly encourages quitting. Considering the high proportion of people exposed to tobacco in these countries, it would be interesting to continue assessing its impact on the development of blood cancer, given the limited information available.
Diet, probably among the most modifiable environmental factors, contributes to the development of 30%-35% of cancers[35]. Association studies in the field may be inconsistent due to differences in the frequency of food intake, varieties of available food, and different methods of preparation among populations.
According to a recent publication of the IARC Monograph Working Group, consumption of processed meat was classified as “carcinogenic to humans” (group 1), and consumption of red meat as “probably carcinogenic to humans” (group 2A)[36]. Meat processing can result in formation of N-nitroso compounds and PAH, while cooking it can produce heterocyclic aromatic amines and PAH. High-temperature cooking, such as grilling and barbecuing, produces the highest amounts of these carcinogens. Barbecued red meat is a frequent dish among the Argentinean population, where there is a high consumption of animal protein and fats obtained mainly from red meat[37,38]. Several epidemiologic studies from Córdoba, an Argentinean province, report significant associations between consumption of red meat, or dietary patterns that contains it, and breast, colorectal, prostate, and urinary tract cancers[37-42]. According to Navarro et al[38], all meats were associated with an increased risk for colorectal cancer when barbecued, a similar result to that observed in our study. In two other surveys, the Southern Cone dietary pattern (red meat, starchy vegetables and wine consumptions) was associated with higher risk of urinary tract tumors[39] and colorectal cancer[37]. In a case-control study from Uruguay, red meat, lamb, and boiled meat were associated with the risk of squamous cell carcinoma of the esophagus[43].
In relation to oncohematological diseases, there are no studies in Argentina concerning diet and lifestyle as risk factors. Several studies from other countries evaluated this possible association, with inconsistent results. Most of them reported no association between meat consumption and increased risk of NHL or CML[44-46]. A case-control study from US done by Li et al[47] reported a positive association between an increased risk of AML and beef intake among women. Our results are the first to report a significant association between oncohematological diseases and consumption of barbecued/grilled meat in our population. However, some bias could exist since portion size was not assessed, and the questionnaire does not differentiate between red and white meat. Despite this, our results are relevant given the fact of a high and frequent consumption of these foods in Argentina.
It has been suggested that light to moderate alcohol consumption has beneficial effects due to advantageous host cellular and humoral immune responses[48]. On the other hand, ethanol was classified as carcinogenic to humans by the IARC. Regarding hematologic malignancies, a pooled analysis from the International Lymphoma Epidemiology Consortium reported that ever or current drinking were associated with a lower risk of NHL, compared with non-drinkers[49]. However, they did not find a dose-response relation or a stronger trend with longer duration. A meta-analysis performed with 18 studies, including 5694 cases with MM and 7142 with leukemia, did not find any association between alcohol drinking and MM or leukemia risks[50,51]. As with beef consumption, Li et al[47] reported a positive association between beer and wine intake and AML, only among women. An Italian case-control study showed no clear association between leukemia or NHL and alcohol consumption[27]. In line with some of these reports, we found no association between alcohol consumption and oncohematological diseases in the Argentinean population under study.
Regarding coffee consumption, there is no consistent evidence suggesting protective or deleterious effects. On the one hand, coffee may decrease the risk of cancer through antioxidant, antihormonal, and anti-inflammatory mechanisms[52]. On the other hand, caffeine and Topo II inhibitors may elevate cancer risk. A United States cohort study reported no association between coffee intake and risk of all cancers combined, but they observed a decrease in the risk of endometrial cancer for women drinking 1 or more cups per day[53]. Although some studies on solid tumors have reported a protective effect (i.e., on liver, colorectal, breast and endometrial cancer[54-57]), studies on hematopoietic malignancies in adults are rare. In our study, daily consumption of coffee was associated with an increased risk of disease. An Indian case-control study showed a 40% reduction in the risk of leukemia for coffee drinkers[30], while an Italian one reported an increase in the risk of NHL[58]. Other studies found no significant associations between coffee consumption and hematologic cancer[44,59,60]. In 1991, the IARC Working Group classified coffee as possibly carcinogenic to humans (group 2B). Given the large number of studies published on the subject since that IARC publication, the IARC Advisory Group recommends a review of the evidence, giving to this exposure high priority for its inclusion in the monographs to be published between 2015 and 2019[61].
Genetic variations in XMG may be important factors in the etiology of onco-hematological diseases. Although they have low penetrance, they are highly prevalent in most populations, giving the chance to identify potential carcinogens and populations at higher risk of cancer[62]. These polymorphisms also interact with other polymorphisms and/or particular environmental factors, which vary between and within ethnic groups[8]. The increased activity/inducibility of CYP1A1*2A may contribute to the accumulation of genetic changes due to an increased production of mutagenic agents. In a similar way, the decreased activity of GSTs due to gene deletions may lead to a more intense cellular oxidative stress, increasing the level of DNA damage.
Regarding CYP1A1 MspI polymorphism, allele frequencies vary between ethnic groups, being 0.058, 0.149 and 0.218 for the C allele (*2A) in Caucasians, Asians and Africans, respectively[63]. Roco et al[64] reported a *2A allele frequency of 0.37 for a Chilean population. In this study, *2A allele frequencies were 0.34 for controls and 0.30 for cases, similar to that described for the Chilean population and away from that reported for Caucasians. Our genotypic data about this polymorphism deviate from Hardy-Weinberg equilibrium; this may be because the sample does not represent the entire population variability, or due to genotyping errors that create a bias towards increased homozygosity.
Our association analysis between MspI polymorphism and blood cancer showed an OR = 0.79 (95%CI: 0.51-1.24), with no statistical significance. Association studies between CYP1A1 MspI polymorphism and cancer have been inconsistent. A meta-analysis from 268 studies performed by He et al[8] showed that the variant *2A was associated with an increased risk of leukemia, cervical, hepatocellular, head and neck, lung and prostate cancer, but not with other cancer types. Another meta-analysis done by Han et al[65] reported a higher risk of leukemia associated with this variant, which remains significant for Caucasians when stratified by ethnicity. They also reported an increased risk for ALL and AML, especially in childhood ALL in Caucasians. Among Asians, Lu et al[66] showed that the presence of the MspI polymorphism increased the risk of AML. On the other hand, a meta-analysis performed by Zhuo et al[67] did not find significant associations between CYP1A1 variant and AML risk.
Many studies have analyzed the possible association between hematological cancer and the deletions of GSTT1 and GSTM1, with disparate results. In this study, frequencies for null genotypes were 0.18 in cases and controls for GSTT1, and 0.5 and 0.47 for GSTM1, in cases and controls respectively. Reported frequencies for GSTT1*null in controls were 0.13-0.26 for Caucasians[63], 0.10-0.12 for Chilean and Argentinean populations[64,68-70], and 0.09-0.24 for Native American Argentineans[71]. GSTM1*null has higher frequencies, with values of 0.42-0.60 for Caucasians[63], 0.36-0.46 for Chilean and Argentinean populations[64,68-70], and 0.29-0.49 for Native American Argentineans[71]. Our study showed an OR = 0.98 (95%CI: 0.54-1.74) for the GSTT1 deletion, and an OR = 1.14 (95%CI: 0.73-1.78) for GSTM1 deletion. He et al[62] carried out a meta-analysis, showing that GSTM1*null genotype significantly increased the risk of AML in East Asians, while GSTT1*null increased it in Caucasians. Double-null genotypes were associated with AML in both ethnic groups. Several case-control studies reported significant associations between GSTT1*null genotype and AML, CML, CLL and acute leukemia[72-78]. Conversely, other studies did not find such associations with all leukemia, acute leukemia, CML, AML or MM[79-82]. A similar scenario occurs with GSTM1*null genotype: many studies showed a significant increased risk of NHL, CLL, AML, MM, CML, and acute leukemia associated with the null genotype[72,73,76,78,83,84], while other ones reported no differences in cancer risk between controls and CML, AML, MM, acute leukemia and all leukemia cases[74,75,77,79-82]. In general, studies reported moderate OR, with values from 1 to 3; however, values up to 7 were reached in some assays, with OR values even greater when analyzing double null genotypes. It should be noted that these case-control studies are from different countries with different ethnic backgrounds, and with a variable number of participants.
Our study has strengths that are worth mentioning. Face-to-face interviews were performed by the same person, with all cases and controls, thus addressing reliable data about personal information, habits and lifestyle. Furthermore, although sample size is small, it has the power to detect an OR = 2, a value close to that reported in similar case-control studies. Even though cases and controls were not age- and gender-matched, statistical adjustment were used to minimize potential biases. It should be recall that hospital-based studies may have some bias, due to controls that might have benign diseases which are prone to turn malignant. This study design could reduce the generalization of the results to the general population. Additionally, hematological malignancies are heterogeneous illnesses, with potentially different causes. A larger number of samples will allow us to conduct studies of risk factors for each pathology independently.
There is a lack of studies for oncohematological diseases etiology in an argentinean population, particularly for genes and environmental factors. Taking that into account, our goal through the study was to address and offers relevant insight into diverse aspects for these pathologies in our population.
We thank the hospital’s technical staff for their help in attending the patients for sampling. Ignacio Miguel is thanked for helping in processing some DNA samples.
Risk factors for oncohematological diseases are not completely defined. As in other cancer pathologies, blood cancer susceptibility is related to both lifestyle and genetic factors. In regards to the Argentinean population, these pathologies are barely studied, even in relation to highly frequent risk factors for this population.
In the latest years, although specific diet patterns for Argentina have been evaluated as risk factors related to cancer development by many studies, hematological cancer was not included. Moreover, studies about tobacco, alcohol and coffee as risk factors for oncohematological diseases are still not conclusive; furthermore, the results can vary between different populations and study designs. On the other side, genetic variants in coding genes for enzymes associated to carcinogenic compounds metabolization are known genetics risk factors linked to cancer. Allelic frequencies for these enzymatic variants are different between populations; therefore, it becomes difficult to extrapolate an estimated risk from one population to another. For Argentina, genetic frequencies for metabolizing enzymes are already known; however, available data are limited to just a few cities and, mostly, for healthy population.
In that regards, in this work the authors reported an association between oncohematological diseases with coffee and meat intake. With respect to the latter risk factor, the result becomes extremely relevant given the fact of high meat consumption in Argentina. The results are the first one showing a possible association in the authors’ population. Moreover, Argentina has a medium-high cancer incidence; every research implying an evaluation of different risk factors frequently found in the population, could help in contributing with new information, in order to dilucidate which specific factors are really involved in the actual incidence of blood related cancer diseases.
The result could be joined with the whole set of studies looking to evaluate the impact of genetics and lifestyle factors in cancer development. The results reported for coffee and barbecued/grilled meat intake, could be taken into account to go deeply in future studies by evaluating specific kinds of meat, portion size, other cooking methods, etc. This could allow researchers to classify, more specifically, which particular characteristics related to nutrition patterns are associated with cancer development in a population. Additionally, each oncohematological pathology could be also analyzed individually from the main group, due to the fact that each specific genetic or lifestyle factor could be involved in different pathways and/or specific disease stages, independently.
Odds ratio (OR), in statistics, the OR is a way to quantify how strongly the presence or absence of property A is associated with the presence or absence of property B in a given population. Oncohematological diseases or hematology-oncology, the diagnosis, treatment and prevention of cancer developed in blood cells, and the research associated to them. Hematology-oncology includes diseases such as leukemias and lymphomas, as well as other blood disorders (i.e., iron deficiency anemia, hemophilia, sickle cell disease, and thalassemias).
The study indicates an increased risk of oncohematological diseases associated with meat and coffee intake. Overall the manuscript was written in a clear and concise manner.
Manuscript source: Invited manuscript
Specialty Type: Oncology
Country of Origin: Argentina
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Muscat JE, Song JJ S- Editor: Ji FF L- Editor: A E- Editor: Lui YJ
| 1. | Silbergeld EK. Toxicología. Herramientas y enfoques. Enciclopedia de Salud y Seguridad en el Trabajo. 1998;. |
| 2. | Kalow W. Pharmacogenetics of drug metabolism. New York: Pergamon Press 1992; . |
| 3. | Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41:89-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 548] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 4. | Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 684] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 5. | Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. BMC Cancer. 2009;9:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 6. | Shah PP, Saurabh K, Pant MC, Mathur N, Parmar D. Evidence for increased cytochrome P450 1A1 expression in blood lymphocytes of lung cancer patients. Mutat Res. 2009;670:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Rojas M, Cascorbi I, Alexandrov K, Kriek E, Auburtin G, Mayer L, Kopp-Schneider A, Roots I, Bartsch H. Modulation of benzo[a]pyrene diolepoxide-DNA adduct levels in human white blood cells by CYP1A1, GSTM1 and GSTT1 polymorphism. Carcinogenesis. 2000;21:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | He XF, Wei W, Liu ZZ, Shen XL, Yang XB, Wang SL, Xie DL. Association between the CYP1A1 T3801C polymorphism and risk of cancer: evidence from 268 case-control studies. Gene. 2014;534:324-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Lo HW, Ali-Osman F. Genetic polymorphism and function of glutathione S-transferases in tumor drug resistance. Curr Opin Pharmacol. 2007;7:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Singh MS, Michael M. Role of xenobiotic metabolic enzymes in cancer epidemiology. Methods Mol Biol. 2009;472:243-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Gonzalez FJ, Tukey RH. Drug Metabolism: How Humans Cope with Exposure to Xenobiotics. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. New York: McGraw-Hill 2012; 71-91. |
| 12. | Calder PC, Kew S. The immune system: a target for functional foods? Br J Nutr. 2002;88 Suppl 2:S165-S177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 192] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | US Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. Atlanta, GA: A Report of the Surgeon General 2010; . |
| 14. | IARC Monograph Working Group. List of classifications by cancer sites, volumes 1 to 114 [Internet]. Available from: https://monographs.iarc.fr/ENG/Classification/Table4.pdf. |
| 15. | Schnatter AR, Rosamilia K, Wojcik NC. Review of the literature on benzene exposure and leukemia subtypes. Chem Biol Interact. 2005;153-154:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F. Incidence/mortality data [Internet]. GLOBOCAN. 2012;IARC, 2013 Available from: http://globocan.iarc.fr. |
| 17. | Ministerio de Salud de la Nación, Secretaría de Políticas Regulación e Institutos, Dirección de Estadísticas e Información de Salud. Estadísticas Vitales. Información básica. Buenos Aires. 2013;. |
| 18. | Ministerio de Salud, Instituto Nacional del Cáncer. Argentina: Atlas de Mortalidad por Cáncer, 2007-2011. |
| 19. | StataCorp. Stata Statistical Software: Release 11, 2009. |
| 20. | Epidat: programa para análisis epidemiológico de datos. Version 4.0 [Internet]. Available from: http://dxsp.sergas.es. |
| 21. | Peakall R, Smouse PE. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research--an update. Bioinformatics. 2012;28:2537-2539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6878] [Cited by in RCA: 7106] [Article Influence: 546.6] [Reference Citation Analysis (0)] |
| 22. | Cerliani MB, Gili JA, Pavicic WH, Klein G, Saba S, Richard SM. Association between PER3 length polymorphism and onco-hematological diseases and its influence on patients’ functionality. Adv Mod Oncol Res. 2015;1:132-140. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Sopori ML, Kozak W. Immunomodulatory effects of cigarette smoke. J Neuroimmunol. 1998;83:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 224] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1039] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 25. | Bracci PM, Holly EA. Tobacco use and non-Hodgkin lymphoma: results from a population-based case-control study in the San Francisco Bay Area, California. Cancer Causes Control. 2005;16:333-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Schöllkopf C, Smedby KE, Hjalgrim H, Rostgaard K, Gadeberg O, Roos G, Porwit-Macdonald A, Glimelius B, Adami HO, Melbye M. Cigarette smoking and risk of non-Hodgkin’s lymphoma--a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14:1791-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Parodi S, Santi I, Marani E, Casella C, Puppo A, Garrone E, Fontana V, Stagnaro E. Lifestyle factors and risk of leukemia and non-Hodgkin’s lymphoma: a case-control study. Cancer Causes Control. 2016;27:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Björk J, Johansson B, Broberg K, Albin M. Smoking as a risk factor for myelodysplastic syndromes and acute myeloid leukemia and its relation to cytogenetic findings: a case-control study. Leuk Res. 2009;33:788-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Fernberg P, Odenbro A, Bellocco R, Boffetta P, Pawitan Y, Zendehdel K, Adami J. Tobacco use, body mass index, and the risk of leukemia and multiple myeloma: a nationwide cohort study in Sweden. Cancer Res. 2007;67:5983-5986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Balasubramaniam G, Saoba SL, Sarhade MN, Kolekar SA. Lifestyle factors including diet and leukemia development: a case-control study from Mumbai, India. Asian Pac J Cancer Prev. 2013;14:5657-5661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Ma X, Park Y, Mayne ST, Wang R, Sinha R, Hollenbeck AR, Schatzkin A, Cross AJ. Diet, lifestyle, and acute myeloid leukemia in the NIH-AARP cohort. Am J Epidemiol. 2010;171:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Fircanis S, Merriam P, Khan N, Castillo JJ. The relation between cigarette smoking and risk of acute myeloid leukemia: an updated meta-analysis of epidemiological studies. Am J Hematol. 2014;89:E125-E132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Rubinstein AL, Irazola VE, Calandrelli M, Elorriaga N, Gutierrez L, Lanas F, Manfredi JA, Mores N, Olivera H, Poggio R. Multiple cardiometabolic risk factors in the Southern Cone of Latin America: a population-based study in Argentina, Chile, and Uruguay. Int J Cardiol. 2015;183:82-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Champagne BM, Sebrié EM, Schargrodsky H, Pramparo P, Boissonnet C, Wilson E. Tobacco smoking in seven Latin American cities: the CARMELA study. Tob Control. 2010;19:457-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Fitzgibbon M, Stolley M, Tussing-Humphreys L. Diet and Cancer. Psycho-Oncology. Oxford: Oxford University Press 2015; 864. |
| 36. | Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16:1599-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1085] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 37. | Pou SA, Díaz Mdel P, Osella AR. Applying multilevel model to the relationship of dietary patterns and colorectal cancer: an ongoing case-control study in Córdoba, Argentina. Eur J Nutr. 2012;51:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Navarro A, Muñoz SE, Lantieri MJ, del Pilar Diaz M, Cristaldo PE, de Fabro SP, Eynard AR. Meat cooking habits and risk of colorectal cancer in Córdoba, Argentina. Nutrition. 2004;20:873-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Pou SA, Niclis C, Eynard AR, Díaz Mdel P. Dietary patterns and risk of urinary tract tumors: a multilevel analysis of individuals in rural and urban contexts. Eur J Nutr. 2014;53:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Niclis C, Román MD, Osella AR, Eynard AR, Díaz Mdel P. Traditional Dietary Pattern Increases Risk of Prostate Cancer in Argentina: Results of a Multilevel Modeling and Bias Analysis from a Case-Control Study. J Cancer Epidemiol. 2015;2015:179562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Román MD, Niclis C, Tumas N, Díaz Mdel P, Osella AR, Muñoz SE. Tobacco smoking patterns and differential food effects on prostate and breast cancers among smokers and nonsmokers in Córdoba, Argentina. Eur J Cancer Prev. 2014;23:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Tumas N, Niclis C, Aballay LR, Osella AR, Díaz Mdel P. Traditional dietary pattern of South America is linked to breast cancer: an ongoing case-control study in Argentina. Eur J Nutr. 2014;53:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | De Stefani E, Deneo-Pellegrini H, Ronco AL, Boffetta P, Correa P, Aune D, Mendilaharsu M, Acosta G, Silva C, Landó G. Meat consumption, cooking methods, mutagens, and risk of squamous cell carcinoma of the esophagus: a case-control study in Uruguay. Nutr Cancer. 2012;64:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Chang ET, Smedby KE, Zhang SM, Hjalgrim H, Melbye M, Ost A, Glimelius B, Wolk A, Adami HO. Dietary factors and risk of non-hodgkin lymphoma in men and women. Cancer Epidemiol Biomarkers Prev. 2005;14:512-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Kabat GC, Wu JW, Moore SC, Morton LM, Park Y, Hollenbeck AR, Rohan TE. Lifestyle and dietary factors in relation to risk of chronic myeloid leukemia in the NIH-AARP Diet and Health Study. Cancer Epidemiol Biomarkers Prev. 2013;22:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Talamini R, Polesel J, Montella M, Dal Maso L, Crovatto M, Crispo A, Spina M, Canzonieri V, La Vecchia C, Franceschi S. Food groups and risk of non-Hodgkin lymphoma: a multicenter, case-control study in Italy. Int J Cancer. 2006;118:2871-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Li Y, Moysich KB, Baer MR, Weiss JR, Brasure J, Graham S, McCann SE. Intakes of selected food groups and beverages and adult acute myeloid leukemia. Leuk Res. 2006;30:1507-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Díaz LE, Montero A, González-Gross M, Vallejo AI, Romeo J, Marcos A. Influence of alcohol consumption on immunological status: a review. Eur J Clin Nutr. 2002;56 Suppl 3:S50-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 49. | Cross AJ, Lim U. The role of dietary factors in the epidemiology of non-Hodgkin’s lymphoma. Leuk Lymphoma. 2006;47:2477-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Rota M, Porta L, Pelucchi C, Negri E, Bagnardi V, Bellocco R, Corrao G, Boffetta P, La Vecchia C. Alcohol drinking and multiple myeloma risk--a systematic review and meta-analysis of the dose-risk relationship. Eur J Cancer Prev. 2014;23:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Rota M, Porta L, Pelucchi C, Negri E, Bagnardi V, Bellocco R, Corrao G, Boffetta P, La Vecchia C. Alcohol drinking and risk of leukemia-a systematic review and meta-analysis of the dose-risk relation. Cancer Epidemiol. 2014;38:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1854] [Cited by in RCA: 1654] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 53. | Hashibe M, Galeone C, Buys SS, Gren L, Boffetta P, Zhang ZF, La Vecchia C. Coffee, tea, caffeine intake, and the risk of cancer in the PLCO cohort. Br J Cancer. 2015;113:809-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 54. | Larsson SC, Wolk A. Coffee consumption and risk of liver cancer: a meta-analysis. Gastroenterology. 2007;132:1740-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 55. | Je Y, Liu W, Giovannucci E. Coffee consumption and risk of colorectal cancer: a systematic review and meta-analysis of prospective cohort studies. Int J Cancer. 2009;124:1662-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Tang N, Zhou B, Wang B, Yu R. Coffee consumption and risk of breast cancer: a metaanalysis. Am J Obstet Gynecol. 2009;200:290.e1-290.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Bravi F, Scotti L, Bosetti C, Gallus S, Negri E, La Vecchia C, Tavani A. Coffee drinking and endometrial cancer risk: a metaanalysis of observational studies. Am J Obstet Gynecol. 2009;200:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Franceschi S, Serraino D, Carbone A, Talamini R, La Vecchia C. Dietary factors and non-Hodgkin’s lymphoma: a case-control study in the northeastern part of Italy. Nutr Cancer. 1989;12:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Tavani A, Negri E, Franceschi S, Talamini R, La Vecchia C. Coffee consumption and risk of non-Hodgkin’s lymphoma. Eur J Cancer Prev. 1994;3:351-356. [PubMed] |
| 60. | Matsuo K, Hamajima N, Hirose K, Inoue M, Takezaki T, Kuroishi T, Tajima K. Alcohol, smoking, and dietary status and susceptibility to malignant lymphoma in Japan: results of a hospital-based case-control study at Aichi Cancer Center. Jpn J Cancer Res. 2001;92:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Report of the Advisory Group to Recommend Priorities for IARC Monographs during 2015-2019; 25 June 2014. |
| 62. | He HR, You HS, Sun JY, Hu SS, Ma Y, Dong YL, Lu J. Glutathione S-transferase gene polymorphisms and susceptibility to acute myeloid leukemia: meta-analyses. Jpn J Clin Oncol. 2014;44:1070-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL, Baranova H, Bathum L, Benhamou S, Boffetta P. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10:1239-1248. [PubMed] |
| 64. | Roco A, Quiñones L, Agúndez JA, García-Martín E, Squicciarini V, Miranda C, Garay J, Farfán N, Saavedra I, Cáceres D. Frequencies of 23 functionally significant variant alleles related with metabolism of antineoplastic drugs in the chilean population: comparison with caucasian and asian populations. Front Genet. 2012;3:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 65. | Han F, Tan Y, Cui W, Dong L, Li W. Novel insights into etiologies of leukemia: a HuGE review and meta-analysis of CYP1A1 polymorphisms and leukemia risk. Am J Epidemiol. 2013;178:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Lu J, Zhao Q, Zhai YJ, He HR, Yang LH, Gao F, Zhou RS, Zheng J, Ma XC. Genetic polymorphisms of CYP1A1 and risk of leukemia: a meta-analysis. Onco Targets Ther. 2015;8:2883-2902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Zhuo W, Zhang L, Wang Y, Zhu B, Chen Z. CYP1A1 MspI polymorphism and acute myeloid leukemia risk: meta-analyses based on 5018 subjects. J Exp Clin Cancer Res. 2012;31:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Moore LE, Wiencke JK, Bates MN, Zheng S, Rey OA, Smith AH. Investigation of genetic polymorphisms and smoking in a bladder cancer case-control study in Argentina. Cancer Lett. 2004;211:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Weich N, Nuñez MC, Galimberti G, Elena G, Acevedo S, Larripa I, Fundia AF. Polymorphic variants of GSTM1, GSTT1, and GSTP1 genes in childhood acute leukemias: A preliminary study in Argentina. Hematology. 2015;20:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Porcel de Peralta M, Scagnetti J, Grigolato R, Sylvestre J, Kleinsorge E, Simoniello M. Evaluación del daño oxidativo al ADN y efecto de la susceptibilidad genética en una población laboral y ambientalmente expuesta a mezclas de plaguicidas. Rev FABICIB. 2011;15:119-129. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 71. | Bailliet G, Santos MR, Alfaro EL, Dipierri JE, Demarchi DA, Carnese FR, Bianchi NO. Allele and genotype frequencies of metabolic genes in Native Americans from Argentina and Paraguay. Mutat Res. 2007;627:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Lima C, Lourenço G, Angelo S, Honma H, Silva E, Nascimento H, Cardoso-Filho C, Ortega M, AF S, Costa F. An analysis of the GST genetic polymorphism in cancer risk in Southeastern Brazil. J Clin Oncol. 2008;26 Suppl:22053. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Al-Achkar W, Azeiz G, Moassass F, Wafa A. Influence of CYP1A1, GST polymorphisms and susceptibility risk of chronic myeloid leukemia in Syrian population. Med Oncol. 2014;31:889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 74. | Bajpai P, Tripathi AK, Agrawal D. Increased frequencies of glutathione-S-transferase (GSTM1 and GSTT1) null genotypes in Indian patients with chronic myeloid leukemia. Leuk Res. 2007;31:1359-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Kassogue Y, Dehbi H, Quachouh M, Quessar A, Benchekroun S, Nadifi S. Association of glutathione S-transferase (GSTM1 and GSTT1) genes with chronic myeloid leukemia. Springerplus. 2015;4:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 76. | Tsabouri S, Georgiou I, Katsaraki A, Bourantas KL. Glutathione sulfur transferase M1 and T1 genotypes in chronic lymphoblastic leukemia. Hematol J. 2004;5:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 77. | Zhou L, Zhu YY, Zhang XD, Li Y, Liu ZG. Risk effects of GST gene polymorphisms in patients with acute myeloid leukemia: a prospective study. Asian Pac J Cancer Prev. 2013;14:3861-3864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Zi Y, Wu S, Ma D, Yang C, Yang M, Huang Y, Yang SJ. Association of GSTTI and GSTM1 variants with acute myeloid leukemia risk. Genet Mol Res. 2014;13:3681-3685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 79. | Chauhan PS, Ihsan R, Yadav DS, Mishra AK, Bhushan B, Soni A, Kaushal M, Devi TR, Saluja S, Gupta DK. Association of glutathione S-transferase, EPHX, and p53 codon 72 gene polymorphisms with adult acute myeloid leukemia. DNA Cell Biol. 2011;30:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Ortega MM, Honma HN, Zambon L, Lorand-Metze I, Costa FF, De Souza CA, Lima CS. GSTM1 and codon 72 P53 polymorphism in multiple myeloma. Ann Hematol. 2007;86:815-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 81. | Chen HC, Hu WX, Liu QX, Li WK, Chen FZ, Rao ZZ, Liu XF, Luo YP, Cao YF. Genetic polymorphisms of metabolic enzymes CYP1A1, CYP2D6, GSTM1 and GSTT1 and leukemia susceptibility. Eur J Cancer Prev. 2008;17:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 82. | Bănescu C, Trifa AP, Voidăzan S, Moldovan VG, Macarie I, Benedek Lazar E, Dima D, Duicu C, Dobreanu M. CAT, GPX1, MnSOD, GSTM1, GSTT1, and GSTP1 genetic polymorphisms in chronic myeloid leukemia: a case-control study. Oxid Med Cell Longev. 2014;2014:875861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Gra OA, Glotov AS, Nikitin EA, Glotov OS, Kuznetsova VE, Chudinov AV, Sudarikov AB, Nasedkina TV. Polymorphisms in xenobiotic-metabolizing genes and the risk of chronic lymphocytic leukemia and non-Hodgkin’s lymphoma in adult Russian patients. Am J Hematol. 2008;83:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Aydin-Sayitoglu M, Hatirnaz O, Erensoy N, Ozbek U. Role of CYP2D6, CYP1A1, CYP2E1, GSTT1, and GSTM1 genes in the susceptibility to acute leukemias. Am J Hematol. 2006;81:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |