Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.109934
Revised: June 20, 2025
Accepted: July 17, 2025
Published online: August 24, 2025
Processing time: 86 Days and 17.9 Hours
This letter is a commentary on the findings of Huang et al, who emphasize the prognostic value of tumor location in gastric cancer. Analyzing data from 3287 patients using Kaplan-Meier and multivariate Cox models, the authors found that the tumor location correlated with patient prognosis following surgery. Patients with tumors situated nearer to the stomach’s proximal end were associated with shorter survival periods and poorer outcomes. Notably, gender-based differences in tumor markers, particularly carbohydrate antigen 72-4, further highlight the need for sex-specific influence on the tumor location. Despite increasing reco
Core Tip: This study highlights the prognostic significance of tumor location in gastric cancer, showing that proximal tumors are associated with worse survival outcomes. Gender differences, particularly in carbohydrate antigen 72-4 expression, further influence prognosis. The letter proposes integrating tumor location into artificial intelligence-based clinical prediction models to improve prognostic accuracy. It outlines a stepwise framework for model development, multicenter validation, and clinical implementation, while addressing critical technical, ethical, and interoperability challenges for real-world application.
- Citation: Wang C, Chen MY, Wang YG, Shi M. Integrating tumor location into artificial intelligence-based prognostic models in cancer. World J Clin Oncol 2025; 16(8): 109934
- URL: https://www.wjgnet.com/2218-4333/full/v16/i8/109934.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i8.109934
The article by Huang et al[1] highlights the clinical significance of tumor location in gastric cancer (GC) outcomes. Methodologically, the study used a retrospective design, analyzing data from a total of 3287 gastric adenocarcinoma cases who underwent gastrectomy from 2008-2019. Kaplan-Meier survival analysis and multivariate Cox regression analyses were utilized to evaluate overall survival and adjusting for covariates. The main finding is that tumor location is an independent prognostic factor for GC. Tumors in the proximal stomach are significantly linked to worse outcomes than distal tumors (log-rank test, P < 0.001). This may be due to delayed diagnosis, more aggressive pathology, and anatomical challenges. Furthermore, clinicopathological indices such as gender, age, serum levels of carcinoembryonic antigen, carbohydrate antigen 19-9 (CA19-9) and CA72-4 exhibited various influences on tumor location. Although statistically robust, the study has limitations. First, the study did not consider the potential influence of Helicobacter pylori infection on tumor location[2]. Similarly, GC cases are significantly affected by geographic region, ethnicity, and dietary habits, all of which can influence tumor localization[3,4]. These important variables were not included in the current study. This letter goes beyond the current finding by underscoring the potential value of integrating tumor location into artificial intelligence (AI)-driven prognostic tools. It outlines a stepwise approach from model development to clinical application, and discusses key technical, ethical, and interoperability considerations necessary for effective real-world clinical implementation.
An intriguing discovery in the study is the gender-based variation in prognostic indicators. Huang et al[1] found that CA72-4 could serve as a tumor marker in female patients, particularly for assessing the incidence of GC subsites. However, in male patients, CA72-4 was not a useful prognostic marker for GC subsites. This difference may be attributed to estrogen signaling, hormone receptor expression, and sex-specific environmental exposures. Accumulated evidence has shown that sex differences influence tumor development, immune responses, and treatment efficacy[5-7]. For example, in hepatocellular carcinoma, estrogen is believed to confer a protective effect in females by modulating inflammatory and proliferative signaling pathways[8]. Similarly, in lung cancer, different mutational spectra and treatment responses have been observed between men and women[9-11]. Such disparities emphasize the need for sex-stratified analyses in both prognostic model development and decision-making.
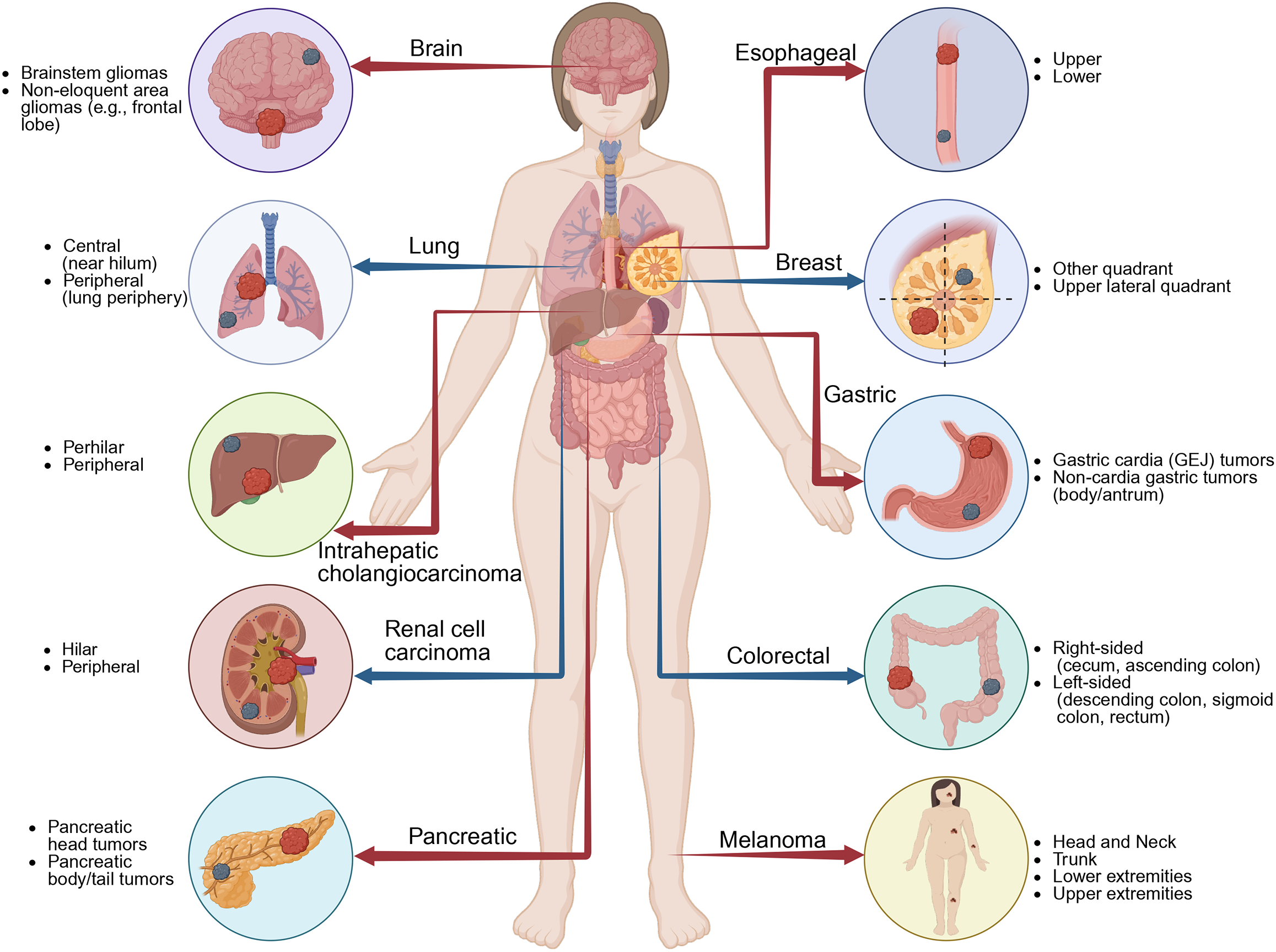
In addition to GC, tumor location[12] affects prognosis in many other types of cancers (Figure 1)[13-17]. For example, in colorectal cancer, a well-established survival discrepancy exists between right-sided and left-sided tumors[18]. This could be due to proximal colon tumors often present with microsatellite instability and BRAF mutations, leading to poor prognoses[19]. Similarly, in pancreatic cancer, tumors in the head of the pancreas are generally diagnosed earlier due to bile duct obstruction. However, pancreatic tail tumors are often diagnosed later and worse[20]. Furthermore, in breast cancer, tumor location has been linked to recurrence patterns due to varying lymphatic spread[21]. These studies reinforce the anatomical and biological significance of tumor location across multiple cancer types. Through incor
The advancement of AI-based models has significantly enhanced the development of clinical prognostic tools. Tumor location is not only linked to disease progression and survival outcomes but also reflects underlying molecular, anatomical, and therapeutic differences[22]. Given the multifaceted implications of tumor location, there is a pressing need to incorporate this factor into AI-driven prognostic models. These models can integrate both structured data such as age, sex, laboratory values, histologic subtype, TNM staging and tumor location, and unstructured data including imaging findings, and pathology reports[23]. In addition, advanced algorithmic approaches should be considered when building these models. These approaches may include ensemble learning such as gradient boosting and random forest, as well as deep learning techniques such as convolutional neural networks and recurrent neural networks[24,25]. Moreover, hybrid algorithms combining multiple modalities may be utilized to enhance model performance[26]. In this way, it would better capture the prognostic impact of tumor location across various cancer patients.
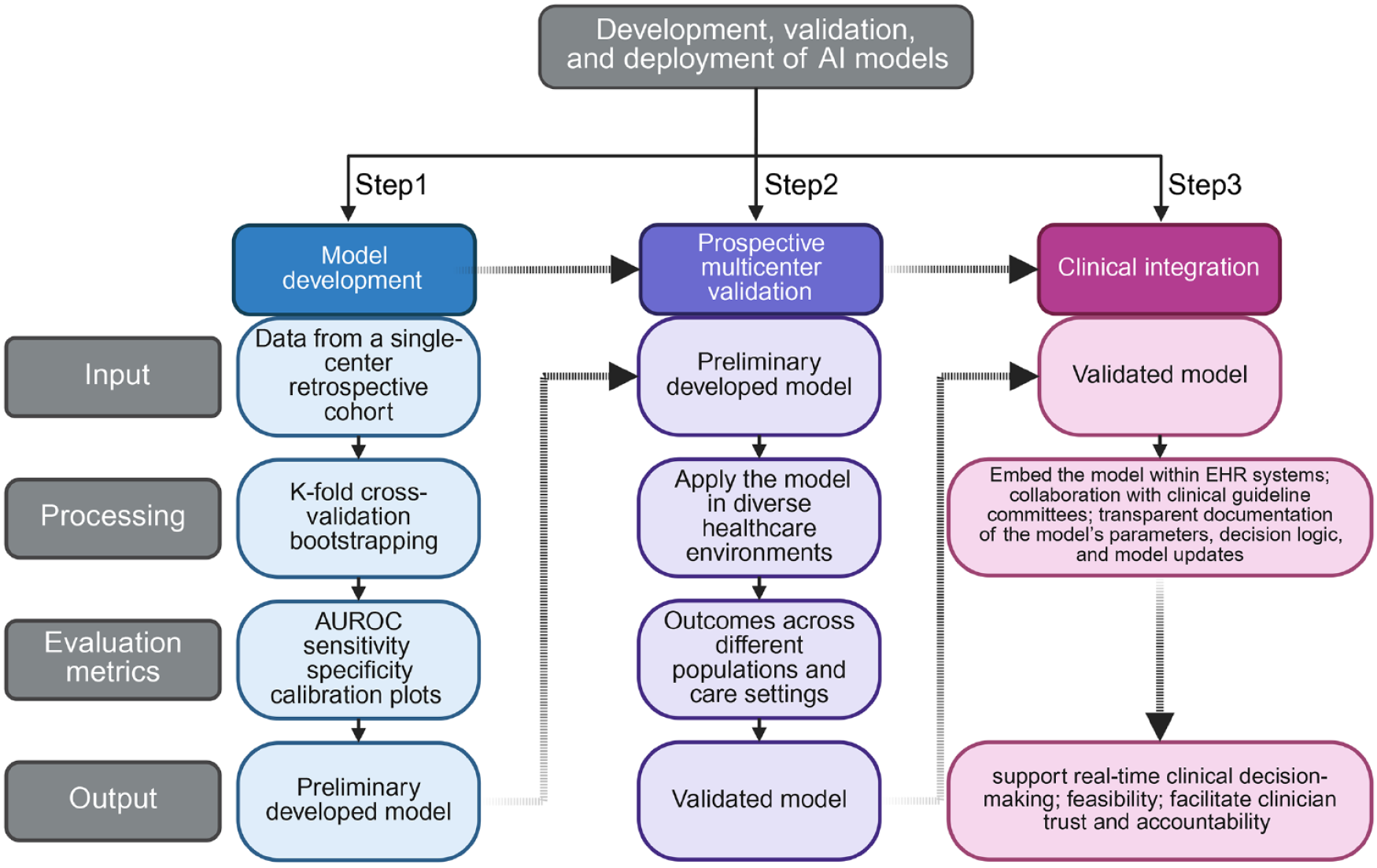
A rigorous, step-by-step strategy is essential for the development, validation, and deployment of AI models in clinical settings. In step 1, the algorithm should be developed using data from a single-center retrospective cohort. Internal validation can be achieved through k-fold cross-validation and bootstrapping[27]. Generally, the performance could be evaluated by area under the receiver operating characteristic curve, sensitivity, specificity, and calibration plots[28]. Step 2 would involve prospective multicenter validation. The model should be applied in diverse healthcare environments, including urban academic centers, community hospitals, and rural clinics. This aims to test its generalizability. By evaluating outcomes across different populations and care settings, external validity can be confirmed[29]. Notably, standardization of data input and processing pipelines is essential to minimize variability across sites, particularly when deploying across different Electronic Health Record (EHR) systems or institutions[30]. Step 3 would focus on clinical integration. This includes embedding the model within EHR systems to support real-time clinical decision-making[31]. Furthermore, a collaboration with clinical guideline committees to evaluate the feasibility of AI-derived risk scores is urgently needed. In addition, transparent documentation of the model’s parameters, decision logic, and model updates will facilitate clinician trust and accountability (Figure 2)[32]. For example, Choi et al[33] developed a deep learning-based model using multi-classification DNN to predict overall survival in 1020 patients with laryngeal squamous cell carcinoma. The model incorporated multiple clinical variables, including tumor location (glottis, supraglottis, subglottis), and demonstrated improved performance over traditional TNM-based models (C-index = 0.859 vs 0.504). This highlights the prognostic relevance of tumor location and supports its inclusion in AI-based prediction models.
Several key challenges must be addressed to ensure successful clinical translation. First, interoperability is crucial as AI systems must be compatible with existing hospital information technology infrastructure and data standards[34]. Second, ethical and regulatory frameworks must be established appropriately. For example, Patient privacy must be protected in accordance with applicable national laws. Informed consent must be completed for AI-driven decision making[35]. Furthermore, models should be explainable to gain clinician trust and facilitate adoption. Explainable AI methods, like Shapley Additive exPlanations values, which allocate importance values to model features, help make complex model decisions more transparent and interpretable to end users[36].
Another technical challenge in AI-based clinical applications is data imbalance, especially in rare tumor sites, which may lead to biased predictions and poor generalization. Recent studies have shown that ensemble learning methods, such as Balanced Random Forest or Easy Ensemble, combined with advanced resampling techniques like SMOTEENN (a hybrid technique combining SMOTE and Edited Nearest Neighbors), can effectively mitigate class imbalance and enhance model robustness across diverse cancer datasets[37]. Incorporating these approaches during model development is crucial for improving the reliability of predictions, particularly for underrepresented patient groups.
To further enhance cross-institutional model development, federated learning has emerged as a promising approach enabling collaborative AI training without sharing raw patient data. This is especially relevant in global oncology, where privacy laws and institutional barriers often hinder data sharing. For example, Pati et al[38] demonstrated that federated learning frameworks can match the performance of centrally trained models in multi-institutional cancer imaging tasks, while ensuring patient data remains within each institution’s local environment. Although anatomical location offers valuable clinical context, relying on it alone may miss important biological differences. Tumors at the same location can behave differently depending on their molecular subtypes or tumor microenvironment. Combining these features may lead to more accurate and comprehensive AI models. In summary, the manuscript by Huang et al[1] significantly advances our understanding of the prognostic relevance of tumor location in GC. This study can guide personalized care and highlights a broader pattern across other malignancies, which further emphasizes the relevance of tumor location in cancer prognosis. Integrating tumor location into AI-based predictive models represents a logical and clinically impactful strategy. Through a well-established model development framework, robust validation, and careful attention to ethical and technical considerations, AI-based prognostic scores have the potential to revolutionize prognosis and improve patient outcomes in cancer patients.
| 1. | Huang YX, He HY, Chen K, Liu HD, Zu D, Liang C, Bao QM, Hu YC, Liu GX, Zhong YK, Zhang CK, Deng MC, He YH, Jing J, Shi Y, Xu SF, Teng YS, Ye Z, Cheng XD. Prognostic impact and reasons for variability by tumor location in gastric cancer. World J Gastroenterol. 2024;30:4709-4724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 2. | Duan Y, Xu Y, Dou Y, Xu D. Helicobacter pylori and gastric cancer: mechanisms and new perspectives. J Hematol Oncol. 2025;18:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 3. | Zhang L, Dong Q, Wang Y, Li X, Li C, Li F, Zhang J. Global trends and risk factors in gastric cancer: a comprehensive analysis of the Global Burden of Disease Study 2021 and multi-omics data. Int J Med Sci. 2025;22:341-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Abengozar R, Sharma A, Sharma R. Gastric cancer: lessons learned from high-incidence geographic regions. J Gastrointest Oncol. 2021;12:S350-S360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Ye Y, Jing Y, Li L, Mills GB, Diao L, Liu H, Han L. Sex-associated molecular differences for cancer immunotherapy. Nat Commun. 2020;11:1779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 6. | Canzian J, Conforti F, Jacobs F, Benvenuti C, Gaudio M, Gerosa R, De Sanctis R, Zambelli A. Sex-Related Differences in Immunotherapy Toxicities: Insights into Dimorphic Responses. Cancers (Basel). 2025;17:1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Wang S, Cowley LA, Liu XS. Sex Differences in Cancer Immunotherapy Efficacy, Biomarkers, and Therapeutic Strategy. Molecules. 2019;24:3214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Guo Y, Wu G, Yi J, Yang Q, Jiang W, Lin S, Yang X, Cai X, Mao L. Anti-Hepatocellular Carcinoma Effect and Molecular Mechanism of the Estrogen Signaling Pathway. Front Oncol. 2021;11:763539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Mosleh B, Sarova P, Zehetmayer S, Oberndorfer F, Widder J, Prosch H, Idzko M, Aigner C, Hoda MA, Gompelmann D. Sex-based differences in lung cancer susceptibility and molecular genetics in the 2020s. Heliyon. 2025;11:e42089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Stabellini N, Bruno DS, Dmukauskas M, Barda AJ, Cao L, Shanahan J, Waite K, Montero AJ, Barnholtz-Sloan JS. Sex Differences in Lung Cancer Treatment and Outcomes at a Large Hybrid Academic-Community Practice. JTO Clin Res Rep. 2022;3:100307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Ragavan M, Patel MI. The evolving landscape of sex-based differences in lung cancer: a distinct disease in women. Eur Respir Rev. 2022;31:210100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 12. | Hossain MJ, Xiao W, Tayeb M, Khan S. Epidemiology and prognostic factors of pediatric brain tumor survival in the US: Evidence from four decades of population data. Cancer Epidemiol. 2021;72:101942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Ito M, Yamashita Y, Miyata Y, Ohara M, Tsutani Y, Ikeda T, Misumi K, Harada H, Omori K. Prognostic impact of the primary tumor location based on the hilar structures in non-small cell lung cancer with mediastinal lymph node metastasis. Lung Cancer. 2012;76:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Wei T, Lu J, Xiao XL, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Koerkamp BG, Itaru E, Lv Y, Zhang XF, Pawlik TM; International Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma Study Groups; U. S. Extrahepatic Biliary Malignancy Consortium. Classification of Intrahepatic Cholangiocarcinoma into Perihilar Versus Peripheral Subtype. Ann Surg Oncol. 2024;31:1232-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Semko SL, Voylenko OA, Pikul MV, Stakhovskyi OE, Kononenko OA, Vitruk IV, Stakhovsky EO, Hrechko B. Comparison of aggressiveness in central versus peripheral T1a clear-cell renal cell carcinoma. Urol Oncol. 2024;42:31.e9-31.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Deng HY, Zheng X, Alai G, Li G, Luo J, Zhuo ZG, Lin YD. Tumor location is an independent prognostic factor of esophageal adenocarcinoma based on the eighth edition of TNM staging system in Chinese patients. Ann Transl Med. 2019;7:365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Shannon CM, Mehta NK, Li H, Nguyen SA, Koochakzadeh S, Elston DM, Kaczmar JM, Day TA. Anatomic Region of Cutaneous Melanoma Impacts Survival and Clinical Outcomes: A Population-Based Analysis. Cancers (Basel). 2023;15:1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 18. | Hodges N, Mackenzie H, D'Souza N, Brown G, Miskovic D. Survival outcomes for right-versus left-sided colon cancer and rectal cancer in England: A propensity-score matched population-based cohort study. Eur J Surg Oncol. 2022;48:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Asghari-Jafarabadi M, Wilkins S, Plazzer JP, Yap R, McMurrick PJ. Prognostic factors and survival disparities in right-sided versus left-sided colon cancer. Sci Rep. 2024;14:12306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Sun K, Mylavarapu C, Crenshaw A, Zhang Y, Hsu E, Xu J, Niravath M, Jones SL, Ordonez A, Abdelrahim M. Pancreatic head vs pancreatic body/tail cancer: Are they different? World J Gastrointest Oncol. 2022;14:716-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Xiong X, Zheng LW, Ding Y, Chen YF, Cai YW, Wang LP, Huang L, Liu CC, Shao ZM, Yu KD. Breast cancer: pathogenesis and treatments. Signal Transduct Target Ther. 2025;10:49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 22. | Ciepiela I, Szczepaniak M, Ciepiela P, Hińcza-Nowak K, Kopczyński J, Macek P, Kubicka K, Chrapek M, Tyka M, Góźdź S, Kowalik A. Tumor location matters, next generation sequencing mutation profiling of left-sided, rectal, and right-sided colorectal tumors in 552 patients. Sci Rep. 2024;14:4619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 23. | Schutte K, Brulport F, Harguem-Zayani S, Schiratti JB, Ghermi R, Jehanno P, Jaeger A, Alamri T, Naccache R, Haddag-Miliani L, Orsi T, Lamarque JP, Hoferer I, Lawrance L, Benatsou B, Bousaid I, Azoulay M, Verdon A, Bidault F, Balleyguier C, Aubert V, Bendjebbar E, Maussion C, Loiseau N, Schmauch B, Sefta M, Wainrib G, Clozel T, Ammari S, Lassau N. An artificial intelligence model predicts the survival of solid tumour patients from imaging and clinical data. Eur J Cancer. 2022;174:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Zhu J, Zhao Z, Yin B, Wu C, Yin C, Chen R, Ding Y. An integrated approach of feature selection and machine learning for early detection of breast cancer. Sci Rep. 2025;15:13015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Kumar Y, Shrivastav S, Garg K, Modi N, Wiltos K, Woźniak M, Ijaz MF. Automating cancer diagnosis using advanced deep learning techniques for multi-cancer image classification. Sci Rep. 2024;14:25006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Lotter W, Hassett MJ, Schultz N, Kehl KL, Van Allen EM, Cerami E. Artificial Intelligence in Oncology: Current Landscape, Challenges, and Future Directions. Cancer Discov. 2024;14:711-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 39] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 27. | Reps JM, Ryan P, Rijnbeek PR. Investigating the impact of development and internal validation design when training prognostic models using a retrospective cohort in big US observational healthcare data. BMJ Open. 2021;11:e050146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Ogliari FR, Traverso A, Barbieri S, Montagna M, Chiabrando F, Versino E, Bosco A, Lin A, Ferrara R, Oresti S, Damiano G, Viganò MG, Ferrara M, Riva ST, Nuccio A, Venanzi FM, Vignale D, Cicala G, Palmisano A, Cascinu S, Gregorc V, Bulotta A, Esposito A, Tacchetti C, Reni M. Exploring machine learning tools in a retrospective case-study of patients with metastatic non-small cell lung cancer treated with first-line immunotherapy: A feasibility single-centre experience. Lung Cancer. 2025;199:108075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Ozaki Y, Broughton P, Abdollahi H, Valafar H, Blenda AV. Integrating Omics Data and AI for Cancer Diagnosis and Prognosis. Cancers (Basel). 2024;16:2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 30. | Jeong J, Kim S, Pan L, Hwang D, Kim D, Choi J, Kwon Y, Yi P, Jeong J, Yoo SJ. Reducing the workload of medical diagnosis through artificial intelligence: A narrative review. Medicine (Baltimore). 2025;104:e41470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Ye J, Woods D, Jordan N, Starren J. The role of artificial intelligence for the application of integrating electronic health records and patient-generated data in clinical decision support. AMIA Jt Summits Transl Sci Proc. 2024;2024:459-467. [PubMed] [DOI] [Full Text] |
| 32. | Lund B, Orhan Z, Mannuru NR, Bevara RVK, Porter B, Vinaih MK, Bhaskara P. Standards, frameworks, and legislation for artificial intelligence (AI) transparency. In: MacIntyre J, Medsker L, editors. AI and Ethics. Berlin: Springer, 2025. [DOI] [Full Text] |
| 33. | Choi N, Kim J, Yi H, Kim H, Kim TH, Chung MJ, Ji M, Kim Z, Son YI. The use of artificial intelligence models to predict survival in patients with laryngeal squamous cell carcinoma. Sci Rep. 2023;13:9734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Maleki Varnosfaderani S, Forouzanfar M. The Role of AI in Hospitals and Clinics: Transforming Healthcare in the 21st Century. Bioengineering (Basel). 2024;11:337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 104] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 35. | Waters M. AI meets informed consent: a new era for clinical trial communication. JNCI Cancer Spectr. 2025;9:pkaf028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Rosenbacke R, Melhus Å, McKee M, Stuckler D. How Explainable Artificial Intelligence Can Increase or Decrease Clinicians' Trust in AI Applications in Health Care: Systematic Review. JMIR AI. 2024;3:e53207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 37. | Gurcan F, Soylu A. Learning from Imbalanced Data: Integration of Advanced Resampling Techniques and Machine Learning Models for Enhanced Cancer Diagnosis and Prognosis. Cancers (Basel). 2024;16:3417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 38. | Pati S, Baid U, Edwards B, Sheller M, Wang SH, Reina GA, Foley P, Gruzdev A, Karkada D, Davatzikos C, Sako C, Ghodasara S, Bilello M, Mohan S, Vollmuth P, Brugnara G, Preetha CJ, Sahm F, Maier-Hein K, Zenk M, Bendszus M, Wick W, Calabrese E, Rudie J, Villanueva-Meyer J, Cha S, Ingalhalikar M, Jadhav M, Pandey U, Saini J, Garrett J, Larson M, Jeraj R, Currie S, Frood R, Fatania K, Huang RY, Chang K, Balaña C, Capellades J, Puig J, Trenkler J, Pichler J, Necker G, Haunschmidt A, Meckel S, Shukla G, Liem S, Alexander GS, Lombardo J, Palmer JD, Flanders AE, Dicker AP, Sair HI, Jones CK, Venkataraman A, Jiang M, So TY, Chen C, Heng PA, Dou Q, Kozubek M, Lux F, Michálek J, Matula P, Keřkovský M, Kopřivová T, Dostál M, Vybíhal V, Vogelbaum MA, Mitchell JR, Farinhas J, Maldjian JA, Yogananda CGB, Pinho MC, Reddy D, Holcomb J, Wagner BC, Ellingson BM, Cloughesy TF, Raymond C, Oughourlian T, Hagiwara A, Wang C, To MS, Bhardwaj S, Chong C, Agzarian M, Falcão AX, Martins SB, Teixeira BCA, Sprenger F, Menotti D, Lucio DR, LaMontagne P, Marcus D, Wiestler B, Kofler F, Ezhov I, Metz M, Jain R, Lee M, Lui YW, McKinley R, Slotboom J, Radojewski P, Meier R, Wiest R, Murcia D, Fu E, Haas R, Thompson J, Ormond DR, Badve C, Sloan AE, Vadmal V, Waite K, Colen RR, Pei L, Ak M, Srinivasan A, Bapuraj JR, Rao A, Wang N, Yoshiaki O, Moritani T, Turk S, Lee J, Prabhudesai S, Morón F, Mandel J, Kamnitsas K, Glocker B, Dixon LVM, Williams M, Zampakis P, Panagiotopoulos V, Tsiganos P, Alexiou S, Haliassos I, Zacharaki EI, Moustakas K, Kalogeropoulou C, Kardamakis DM, Choi YS, Lee SK, Chang JH, Ahn SS, Luo B, Poisson L, Wen N, Tiwari P, Verma R, Bareja R, Yadav I, Chen J, Kumar N, Smits M, van der Voort SR, Alafandi A, Incekara F, Wijnenga MMJ, Kapsas G, Gahrmann R, Schouten JW, Dubbink HJ, Vincent AJPE, van den Bent MJ, French PJ, Klein S, Yuan Y, Sharma S, Tseng TC, Adabi S, Niclou SP, Keunen O, Hau AC, Vallières M, Fortin D, Lepage M, Landman B, Ramadass K, Xu K, Chotai S, Chambless LB, Mistry A, Thompson RC, Gusev Y, Bhuvaneshwar K, Sayah A, Bencheqroun C, Belouali A, Madhavan S, Booth TC, Chelliah A, Modat M, Shuaib H, Dragos C, Abayazeed A, Kolodziej K, Hill M, Abbassy A, Gamal S, Mekhaimar M, Qayati M, Reyes M, Park JE, Yun J, Kim HS, Mahajan A, Muzi M, Benson S, Beets-Tan RGH, Teuwen J, Herrera-Trujillo A, Trujillo M, Escobar W, Abello A, Bernal J, Gómez J, Choi J, Baek S, Kim Y, Ismael H, Allen B, Buatti JM, Kotrotsou A, Li H, Weiss T, Weller M, Bink A, Pouymayou B, Shaykh HF, Saltz J, Prasanna P, Shrestha S, Mani KM, Payne D, Kurc T, Pelaez E, Franco-Maldonado H, Loayza F, Quevedo S, Guevara P, Torche E, Mendoza C, Vera F, Ríos E, López E, Velastin SA, Ogbole G, Soneye M, Oyekunle D, Odafe-Oyibotha O, Osobu B, Shu'aibu M, Dorcas A, Dako F, Simpson AL, Hamghalam M, Peoples JJ, Hu R, Tran A, Cutler D, Moraes FY, Boss MA, Gimpel J, Veettil DK, Schmidt K, Bialecki B, Marella S, Price C, Cimino L, Apgar C, Shah P, Menze B, Barnholtz-Sloan JS, Martin J, Bakas S. Federated learning enables big data for rare cancer boundary detection. Nat Commun. 2022;13:7346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 41.0] [Reference Citation Analysis (0)] |