Copyright
©The Author(s) 2025.
World J Clin Oncol. Aug 24, 2025; 16(8): 109934
Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.109934
Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.109934
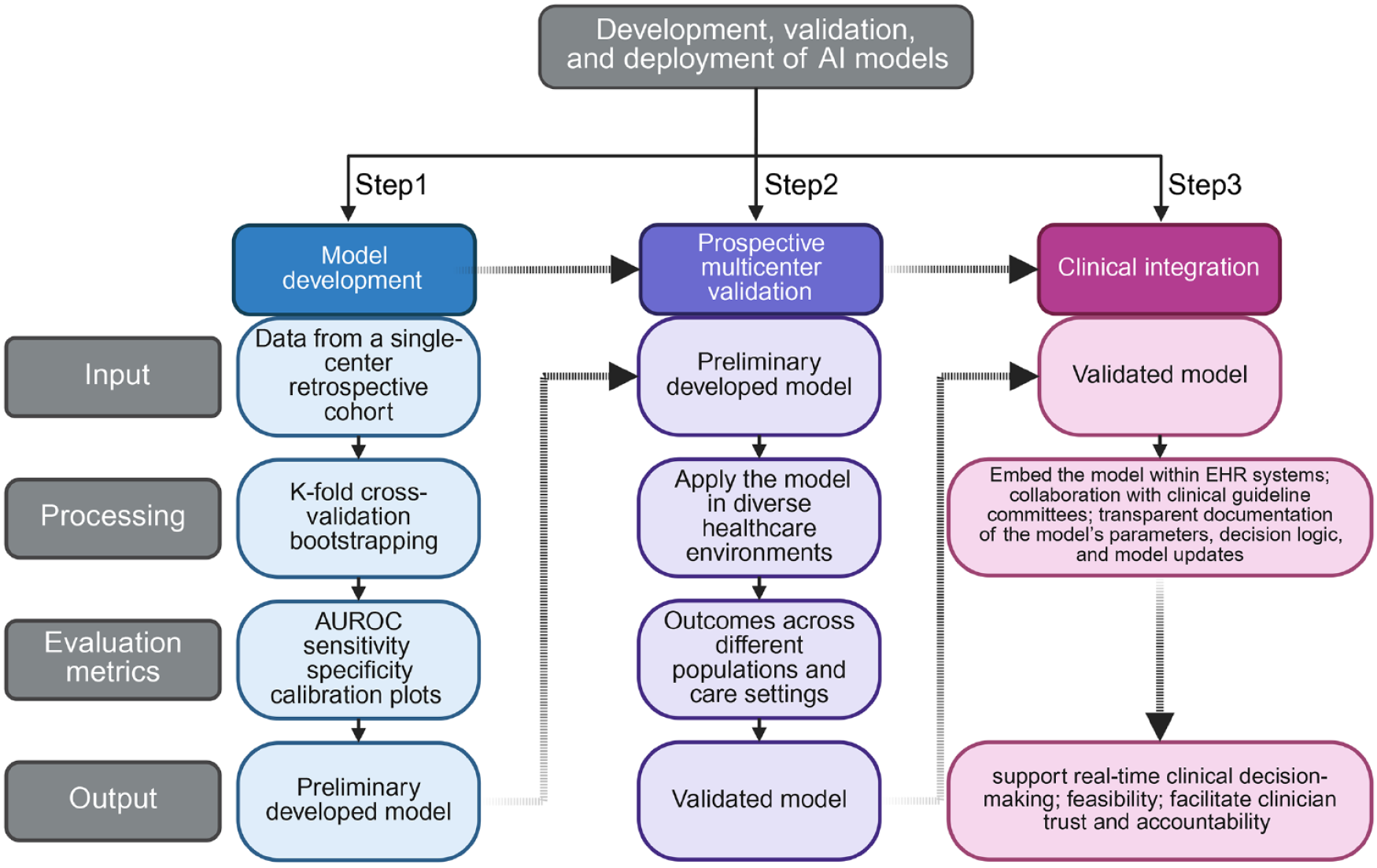
Figure 2 Standardized pipeline for development, validation, and clinical deployment of artificial intelligence models in clinical settings.
The schematic illustrates a three-step framework: (1) Model development involves training using single-center retrospective data, with internal validation via k-fold cross-validation and bootstrapping, and performance assessed by area under the receiver operating characteristic curve, sensitivity, specificity, and calibration plots; (2) Prospective multicenter validation applies the preliminary model across heterogeneous clinical settings to evaluate generalizability and establish external validity. This is crucial to ensure the model performs equally good across external populations; and (3) Clinical integration incorporates the validated model into Electronic Health Record systems, with guideline committee collaboration and transparent documentation of parameters, decision logic, and updates to enable real-time clinical use and support provider confidence. AUROC: Area under the receiver operating characteristic.
- Citation: Wang C, Chen MY, Wang YG, Shi M. Integrating tumor location into artificial intelligence-based prognostic models in cancer. World J Clin Oncol 2025; 16(8): 109934
- URL: https://www.wjgnet.com/2218-4333/full/v16/i8/109934.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i8.109934