Published online Jun 24, 2025. doi: 10.5306/wjco.v16.i6.106228
Revised: April 8, 2025
Accepted: April 27, 2025
Published online: June 24, 2025
Processing time: 121 Days and 0.7 Hours
Gastric cancer (GC) is frequently diagnosed at advanced stages, often with lymph node metastasis (LNM), which complicates prognosis. Swollen LNM (SLNM) in GC has been linked to poor outcomes, yet its prognostic value requires validation.
To evaluate the prognostic significance of SLNM in GC patients undergoing curative-intent gastrectomy.
A retrospective analysis included 507 GC patients with LNM, categorized by SLNM status into positive (SLNM present) and negative (SLNM absent) groups. Survival outcomes were compared between groups, including propensity score matching and multivariate analysis to assess the role of SLNM as an independent prognostic factor.
One hundred and thirty-nine (27.4%) patients exhibited SLNM, associated with significantly lower 5-year overall survival (OS) compared to non-SLNM patients (13.6% vs 35.8%, P < 0.001). After matching, SLNM-positive patients maintained worse OS rates (13.4% vs 21.2%, P = 0.006). Multivariate analysis confirmed SLNM as an independent prognostic factor (hazard ratio = 1.318, P = 0.031). Additionally, T4 stage, N3 stage, and neoadjuvant chemotherapy independently influenced survival outcomes for SLNM-positive patients. Those who received neoadjuvant chemotherapy demonstrated better prognosis.
SLNM is an independent predictor of poor prognosis in GC. Neoadjuvant chemotherapy followed by D2 gastrectomy and adjuvant chemotherapy may offer survival benefits for patients with SLNM.
Core Tip: This multi-center retrospective study of 1532 gastric cancer patients demonstrates that swollen lymph node (LN) metastasis (SLNM) is an independent prognostic factor, particularly in N0-1 stage patients. Using propensity score matching and Cox regression, SLNM was associated with a 48% increased mortality risk (hazard ratio = 1.48). Findings suggest integrating SLNM into tumor–node–metastasis staging could refine prognosis prediction and guide adjuvant therapy decisions, emphasizing the clinical value of meticulous LN dissection and pathological evaluation.
- Citation: Cui JL, Zhao K, Li XL, Wang F, Yang YS, Zheng X. Swollen lymph node metastasis and survival in gastric cancer: Multi-institutional post-resection analysis. World J Clin Oncol 2025; 16(6): 106228
- URL: https://www.wjgnet.com/2218-4333/full/v16/i6/106228.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i6.106228
Gastric cancer (GC) is the fifth most common malignancy and the fourth leading cause of cancer-related death worldwide[1]. The majority of GC in China is at advanced stage at diagnosis and the disease burden is high[2]. Despite progress in treatment, the prognosis of GC remains unsatisfactory[3-5]. While curative gastrectomy with lymph node (LN) dissection (LND) remains the primary curative approach for GC, tumors with LN metastasis (LNM) confined to regional stations are still considered locoregional and potentially curable. However, achieving R0 resection becomes particularly challenging in cases involving swollen LNM (SLNM) along the celiac artery and its branches, where anatomical complexity increases surgical difficulty[6]. GC patients with SLNM have poor prognosis even after curative resection[7,8]. Advanced GC, especially with extensive LNM, can undergo downstaging and further radical resection with neoadjuvant chemotherapy[9-11]. Several studies have shown that neoadjuvant chemotherapy contributes to improved survival of advanced GC[12-14].
Researchers have explored the therapeutic strategy for patients with GC with SLNM[9,10]. Two phase 2 trials (JCOG0001 and JCOG0405) in Japan focused on this population. These patients received two or three cycles of neoadjuvant chemotherapy with S-1 and cisplatin or irinotecan and cisplatin regimens followed by surgical resection, and demonstrated good 3-year overall survival (OS) of 27.0% in JCOG0001 and 58.1% in JCOG0405[9,10]. Based on these results, S-1 plus cisplatin has become the standard neoadjuvant chemotherapy regimen for advanced GC[15]. At present, SLNM is regarded as an indication for neoadjuvant chemotherapy of GC. Several small studies have reported the treatment and prognosis of GC patients with SLNM[16-18]. Few patients with SLNM survive > 3 years after nonradical resection[6]. In the case of radical resection, the clinical significance of SLNM has never been clarified.
In the present study, we retrospectively analyzed GC patients with SLNM who underwent curative resection. The aims were to evaluate the potential impact of SLNM on the long-term outcomes of GC patients, and to provide a reference for the treatment of GC with SLNM.
This study was approved by the Ethics Committees of the Tai’an City Central Hospital, Weifang People’ Hospital, and The Second Hospital of Jilin University. All methods were performed in accordance with relevant guidelines and regulations, including the Declaration of Helsinki. Given the retrospective design and use of anonymized patient data, informed consent was waived.
All patients who underwent gastrectomy with curative intent for gastric adenocarcinoma between January 2010 and December 2016 were considered for inclusion. Standard D2 LND was routinely performed. All patients were pathologically confirmed to have LNM. Patients who received limited LN dissection or had < 16 LNs retrieved were excluded. Patients with distant metastasis, who died due to postoperative complications or did not complete follow up were also excluded. Patients with a history of gastrectomy or other malignancy were not included.
Clinicopathological data including sex, age, preoperative tumor markers, tumor location, tumor size, Borrmann type, histology, depth of invasion, LNM, tumor deposits, lymphovascular invasion, type of gastrectomy and SLNM were collected. The levels of serum tumor markers including carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9 and CA242, were detected within 1 week before surgery. The normal upper limits of serum tumor markers were adopted as follows: (1) CEA (5.0 ng/mL); (2) CA19-9 (37 U/mL); and (3) CA242 (20 IU/mL). Elevated serum tumor markers indicated that the level of at least one of the three markers was above the upper limit of normal (ULN), while normal serum tumor markers indicated that the levels of all three tumor markers were below ULN.
SLNM was defined as LNs with diameter ≥ 3 cm or at least two LNs ≥ 1.5 cm confirmed by contrast-enhanced computed tomography (CT). Swollen LNs (SLNs) were located along the celiac artery and its branches, including 8a, 9, 11p, 11d and 12a stations. All patients were categorized into two groups according to the status of SLNM: (1) The positive group, in which SLNM was present; and (2) The negative group, in which SLNM was absent.
Pathological diagnosis was established by two dedicated pathologists. Tumors were staged according to the eighth edition of the Union for International Cancer Control tumor–node–metastasis (TNM) classification system, whereas lymphadenectomy and LN stations were defined according to the third English edition of the Japanese Classification of Gastric Carcinoma and the fifth English edition of the Japanese Gastric Cancer Treatment Guidelines[15,19]. Tumor deposits were defined as the presence of cancer cells in soft tissue that were discontinuous with the primary lesion or in perigastric soft tissue distinct from the LNs or in the area of locoregional LN stations[20].
Patients were followed up every 3 months for up to 2 years after surgery, every 6 months for up to 5 years, and every year until the end of follow-up or until death. Physical examination, laboratory tests and abdominal ultrasound were performed at each visit, whereas chest and abdominal CT and endoscopy were routinely performed every 6 months. OS was calculated from the day of surgery until death or last follow-up. Date of the last follow-up was December 30, 2021.
Categorical variables were compared using the χ² or Fisher's exact test. Logistic regression analysis was used to determine the independent risk factors for SLNM. OS curves were calculated by the Kaplan–Meier method. The log-rank test was used to assess significant differences between curves. Independent prognostic factors were identified by the Cox proportional hazards regression model. To overcome bias due to the different distribution of covariates in the two groups, the propensity score analysis according to the nearest-neighbor matching method was used. A caliper width of 0.25 of the standard deviation of the logit of the propensity score was set. Variables including patients’ demographic, clinical and tumor characteristics were entered in the propensity model. P < 0.050 with two tails was considered statistically significant. The statistical analysis was performed using Statistical Package for the Social Sciences 22.0 (SPSS, Chicago, IL, United States).
A total of 507 patients with GC were enrolled, including 127 from The Second Hospital of Jilin University, 171 from Weifang People’ Hospital, and 209 from Tai’an City Central Hospital. There were 148 females (29.9%), and median age was 63 (range: 26–89) years. Most patients (64.1%) underwent subtotal gastrectomy. Four hundred and eighty-seven (96.1%) patients received postoperative adjuvant chemotherapy with 5-fluorouracil, leucovorin and oxaliplatin; capecitabine and oxaliplatin (XELOX); S-1 and oxaliplatin (SOX); S-1; or capecitabine. Fifty-seven patients with SLNM received neoadjuvant chemotherapy with 5-fluorouracil, docetaxel and oxaliplatin (FLOT); docetaxel, oxaliplatin and capecitabine or S-1 (DOX/ DOS); XELOX or SOX.
Clinicopathological variables in the SLNM positive and negative groups before and after propensity score matching are shown in Table 1. The incidence of SLNM was significantly higher in patients with elevated preoperative tumor marker (40.8% vs 23.3%, P < 0.001); tumor occupation of two thirds or more of the stomach (P < 0.001); large tumor size (31.5% vs 20.4%, P = 0.007); Borrmann type III/IV (33.2% vs 17.6%, P < 0.001); histologically undifferentiated type (30.6% vs 19.7%, P < 0.001); tumor deposits (34.4% vs 25.0%, P = 0.039); lymphovascular invasion (43.1% vs 22.0%, P < 0.001); deeper invasion (P < 0.001); and advanced N stage (P < 0.001). Patients in the SLNM positive group were more likely to receive total gastrectomy than those in the SLNM negative group. In multivariate logistic analysis, presence of SLNM was independently associated with Borrmann type III/IV [hazard ratio (HR) = 1.665, 95%CI: 1.017–2.723, P = 0.042]; lym
| Characteristics | Whole study series | Matched pairs (case-control method) | ||||
| SLNM positive (n = 368) | SLNM negative (n = 139) | P value | SLNM positive (n = 133) | SLNM negative (n = 133) | P value | |
| Sex | 0.900 | 0.683 | ||||
| Male | 99 (71.2) | 260 (70.7) | 97 (72.9) | 94 (70.7) | ||
| Female | 40 (28.8) | 108 (29.3) | 36 (27.1) | 39 (29.3) | ||
| Age (years) | 0.132 | 0.496 | ||||
| < 70 | 104 (74.8) | 250 (67.9) | 98 (73.7) | 93 (69.9) | ||
| ≥ 70 | 35 (25.2) | 118 (32.1) | 35 (26.3) | 40 (30.1) | ||
| Preoperative tumor marker | < 0.001 | 0.695 | ||||
| Normal | 90 (64.7) | 297 (80.7) | 88 (66.2) | 91 (68.4) | ||
| Elevated | 49 (35.3) | 71 (19.3) | 45 (33.8) | 42 (31.6) | ||
| Tumor location | < 0.001 | 0.130 | ||||
| Lower one-third | 46 (33.1) | 126 (34.2) | 44 (33.1) | 38 (28.6) | ||
| Middle one-third | 21 (15.1) | 53 (14.4) | 19 (14.3) | 23 (17.3) | ||
| Upper one-third | 27 (19.4) | 140 (38.0) | 27 (20.3) | 41 (30.8) | ||
| 2/3 or more | 45 (32.4) | 49 (13.3) | 43 (32.3) | 31 (23.3) | ||
| Tumor size | 0.007 | 0.891 | ||||
| < 5 cm | 38 (27.3) | 148 (40.2) | 36 (27.1) | 37 (27.8) | ||
| ≥ 5 cm | 101 (72.7) | 220 (59.8) | 97 (72.9) | 96 (72.2) | ||
| Borrmann type | < 0.001 | 0.061 | ||||
| I, II | 33 (23.7) | 155 (42.1) | 33 (24.8) | 47 (35.3) | ||
| III, IV | 106 (76.3) | 213 (57.9) | 100 (75.2) | 86 (64.7) | ||
| Histology | 0.013 | 0.769 | ||||
| Differentiated | 29 (20.9) | 118 (32.1) | 29 (21.8) | 31 (23.3) | ||
| Undifferentiated | 110 (79.1) | 250 (67.9) | 104 (78.2) | 102 (76.7) | ||
| T stage | < 0.001 | 0.290 | ||||
| T1 | 2 (1.4) | 16 (4.3) | 2 (1.5) | 0 (0.0) | ||
| T2 | 2 (1.4) | 29 (7.9) | 2 (1.5) | 4 (3.0) | ||
| T3 | 7 (5.0) | 53 (14.4) | 7 (5.3) | 12 (9.0) | ||
| T4a | 115 (82.7) | 252 (68.5) | 111 (83.5) | 102 (76.7) | ||
| T4b | 13 (9.4) | 18 (4.9) | 11 (8.3) | 15 (11.3) | ||
| N stage | < 0.001 | 0.977 | ||||
| N1 | 13 (9.4) | 136 (37.0) | 13 (9.8) | 12 (9.0) | ||
| N2 | 49 (35.3) | 125 (34.0) | 49 (36.8) | 49 (36.8) | ||
| N3 | 77 (55.4) | 107 (29.1) | 71 (53.4) | 72 (54.1) | ||
| Tumor deposits | 0.039 | 0.689 | ||||
| Negative | 94 (67.6) | 282 (76.6) | 91 (68.4) | 94 (70.7) | ||
| Positive | 45 (32.4) | 86 (23.4) | 42 (31.6) | 39 (29.3) | ||
| Lymphovascular invasion | < 0.001 | 0.122 | ||||
| Negative | 83 (59.7) | 294 (79.9) | 81 (60.9) | 93 (69.9) | ||
| Positive | 56 (40.3) | 74 (20.1) | 52 (39.1) | 40 (30.1) | ||
| Type of gastrectomy | 0.036 | 0.454 | ||||
| Subtotal | 79 (56.8) | 246 (66.8) | 76 (57.1) | 82 (61.7) | ||
| Total | 60 (43.2) | 122 (33.2) | 57 (42.9) | 51 (38.3) | ||
After adjusting for sex, age, preoperative tumor marker, tumor location, tumor size, Borrmann type, histology, T stage, N stage, lymphovascular involvement, tumor deposits, and type of gastrectomy, 133 patients in the SLNM negative group were matched with an equal number of patients in the SLNM positive group. All covariates were equally distributed over the two matched groups.
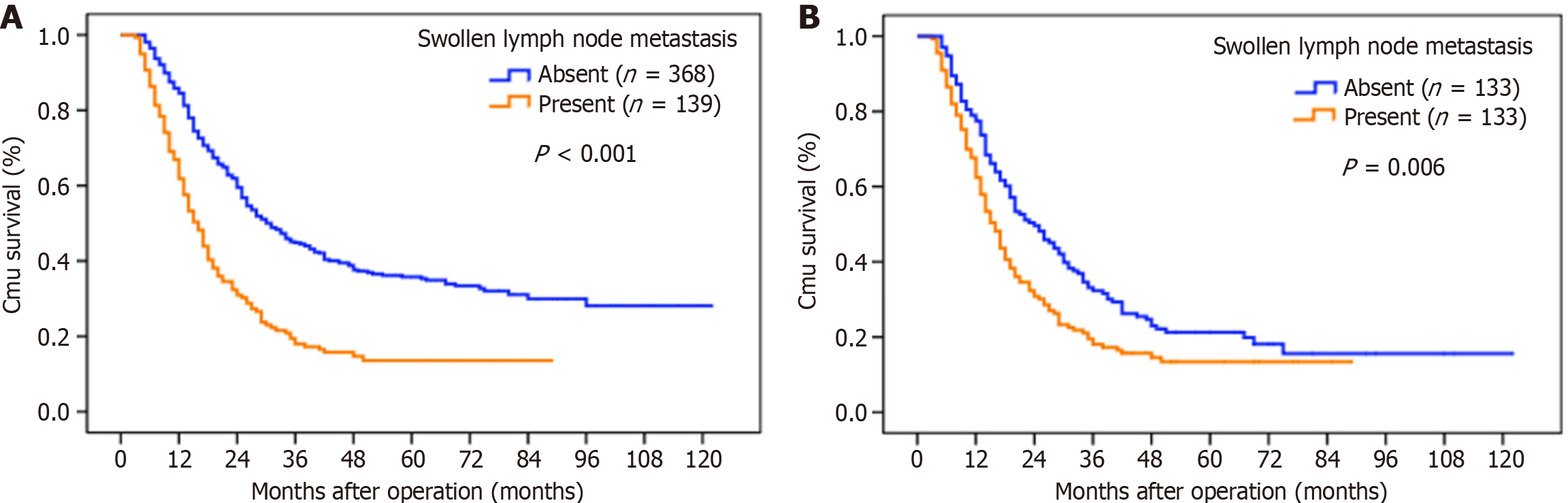
The median OS was 25 months, and the 5-year OS rate was 29.7% for the whole study series. The results of the univariate and multivariate survival analyses are shown in Table 2. The following factors evaluated in the univariate analysis had a significant effect on survival: (1) Age at surgery; (2) Preoperative serum tumor marker; (3) Tumor location; (4) Tumor size; (5) Borrmann type; (6) Histology; (7) Depth of invasion; (8) N stage; (9) Tumor deposits; (10) Lymphovascular invasion; (11) Type of gastrectomy; and (12) SLNM. Patients with SLNM had a significantly worse OS than those without SLNM (5-year OS rates: 13.6% vs 35.8%, P < 0.001) (Figure 1A). Even after matching, patients with SLNM still had a decreased OS rate (5-year OS: 13.4% vs 21.2%, P = 0.006) (Figure 1B) than those without SLNM. In the multivariate analysis, SLNM (HR was 1.318 for patients with SLNM, P = 0.031), age at surgery, elevated serum tumor marker, Borrmann type III and IV, depth of invasion, N stage and lymphovascular invasion were independently associated with OS.
| Variables | n | 5-year overall survival (%) | Univariate analysis | Multivariate analysis | ||
| χ² value | P value | Hazard ratio (95%CI) | P value | |||
| Sex | 2.743 | 0.098 | ||||
| Male | 359 | 27.1 | ||||
| Female | 148 | 36.1 | ||||
| Age (years) | 10.663 | 0.001 | ||||
| < 70 | 354 | 33.7 | 1 (Reference) | |||
| ≥ 70 | 153 | 20.4 | 1.478 (1.178-1.856) | 0.001 | ||
| Preoperative tumor marker | 13.180 | < 0.001 | ||||
| Normal | 387 | 33.5 | 1 (Reference) | |||
| Elevated | 120 | 17.4 | 1.339 (1.046-1.713) | 0.020 | ||
| Tumor location | 31.899 | < 0.001 | ||||
| Lower one-third | 172 | 37.6 | 1 (Reference) | |||
| Middle one-third | 74 | 30.6 | 1.412 (0.977-2.040) | 0.066 | ||
| Upper one-third | 167 | 29.0 | 1.443 (1.091-1.909) | 0.010 | ||
| 2/3 or more | 94 | 16.7 | 1.543 (1.090-2.182) | 0.014 | ||
| Tumor size | 17.164 | < 0.001 | ||||
| < 5 cm | 186 | 40.0 | 1 (Reference) | |||
| ≥ 5 cm | 321 | 23.7 | 1.248 (0.992-1.569) | 0.058 | ||
| Borrmann type | 17.506 | < 0.001 | ||||
| I, II | 188 | 38.3 | 1 (Reference) | |||
| III, IV | 319 | 24.5 | 1.352 (1.070-1.707) | 0.011 | ||
| Histology | 7.512 | 0.006 | ||||
| Differentiated | 147 | 38.4 | 1 (Reference) | |||
| Undifferentiated | 360 | 26.2 | 1.156 (0.904-1.480) | 0.248 | ||
| Depth of invasion | ||||||
| T1 | 18 | 88.5 | 81.327 | < 0.001 | 1 (Reference) | |
| T2 | 31 | 79.5 | 1.480 (0.377-5.815) | 0.575 | ||
| T3 | 60 | 45.7 | 4.344 (1.313-14.367) | 0.016 | ||
| T4a | 367 | 21.4 | 7.788 (2.440-24.855) | 0.001 | ||
| T4b | 31 | 12.1 | 9.456 (2.779-32.171) | < 0.001 | ||
| N stage | 77.324 | < 0.001 | ||||
| N1 | 149 | 48.2 | 1 (Reference) | |||
| N2 | 174 | 32.5 | 1.448 (1.061-1.976) | 0.020 | ||
| N3 | 184 | 11.9 | 1.624 (1.206-2.187) | 0.001 | ||
| Tumor deposits | 9.634 | 0.002 | ||||
| Negative | 376 | 31.7 | 1 (Reference) | |||
| Positive | 131 | 24.1 | 1.279 (0.999-1.637) | 0.051 | ||
| Lymphovascular invasion | 12.039 | < 0.001 | ||||
| Negative | 377 | 34.2 | 1 (Reference) | |||
| Positive | 130 | 16.4 | 1.356 (10.68-1.722) | 0.012 | ||
| Type of gastrectomy | 7.213 | 0.007 | ||||
| Subtotal | 325 | 32.4 | 1 (Reference) | |||
| Total | 182 | 25.0 | 1.018 (0.789-1.314) | 0.890 | ||
| Swollen lymph node metastasis | 45.585 | < 0.001 | ||||
| Absent | 368 | 35.8 | 1 (Reference) | |||
| Present | 139 | 13.6 | 1.318 (1.026-1.695) | 0.031 | ||
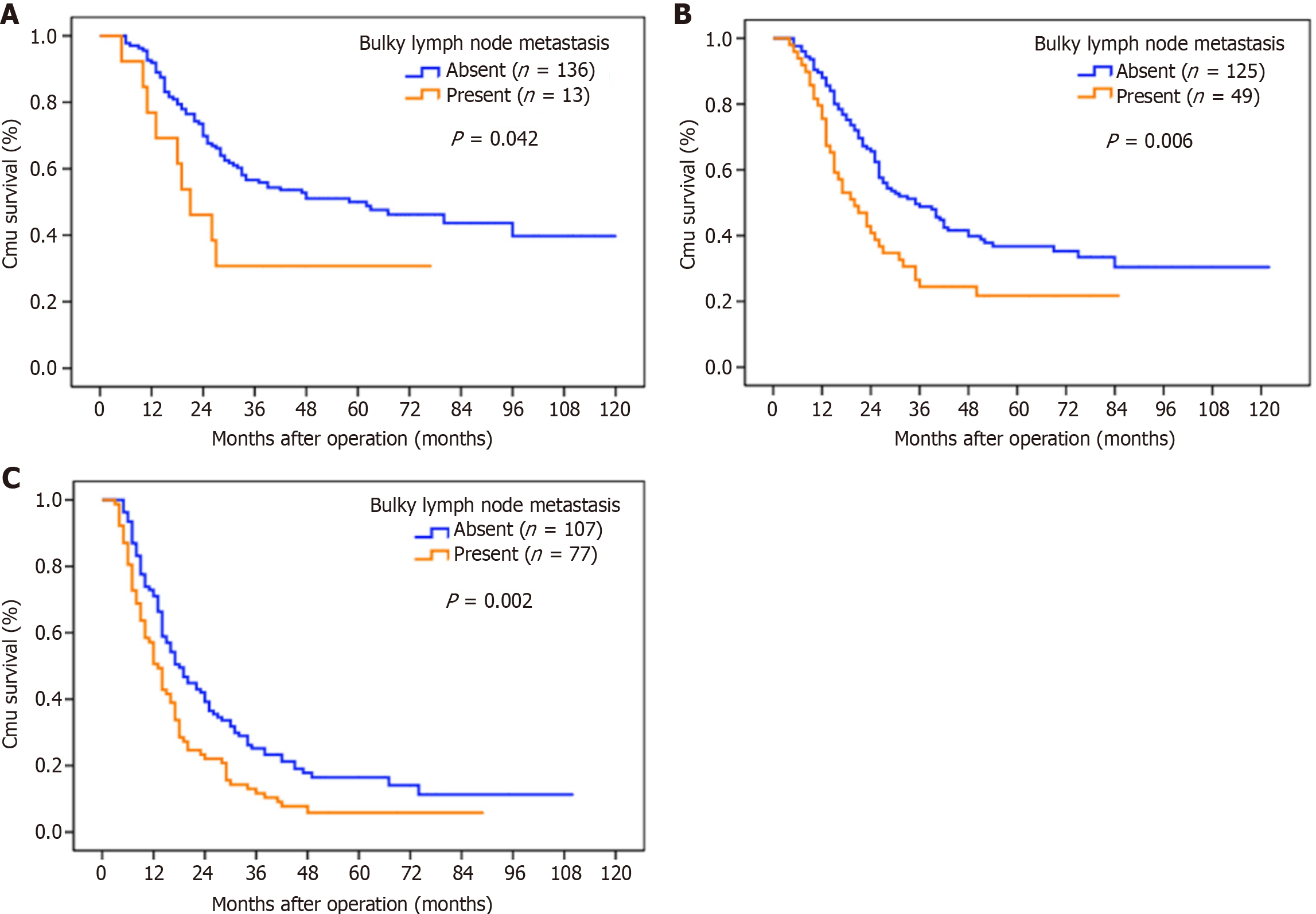
With the strata analysis, the significant prognostic differences between the two groups were still observed in patients at the N1–N3 stages (Table 3, Figure 2).
| N stage | SLNM positive | SLNM negative | χ² value | P value | ||
| n | 5-year OS (%) | n | 5-year OS (%) | |||
| N1 | 136 | 50.0 | 13 | 30.8 | 4.131 | 0.042 |
| N2 | 125 | 36.7 | 59 | 21.8 | 7.535 | 0.006 |
| N3 | 107 | 16.5 | 77 | 5.8 | 9.376 | 0.002 |
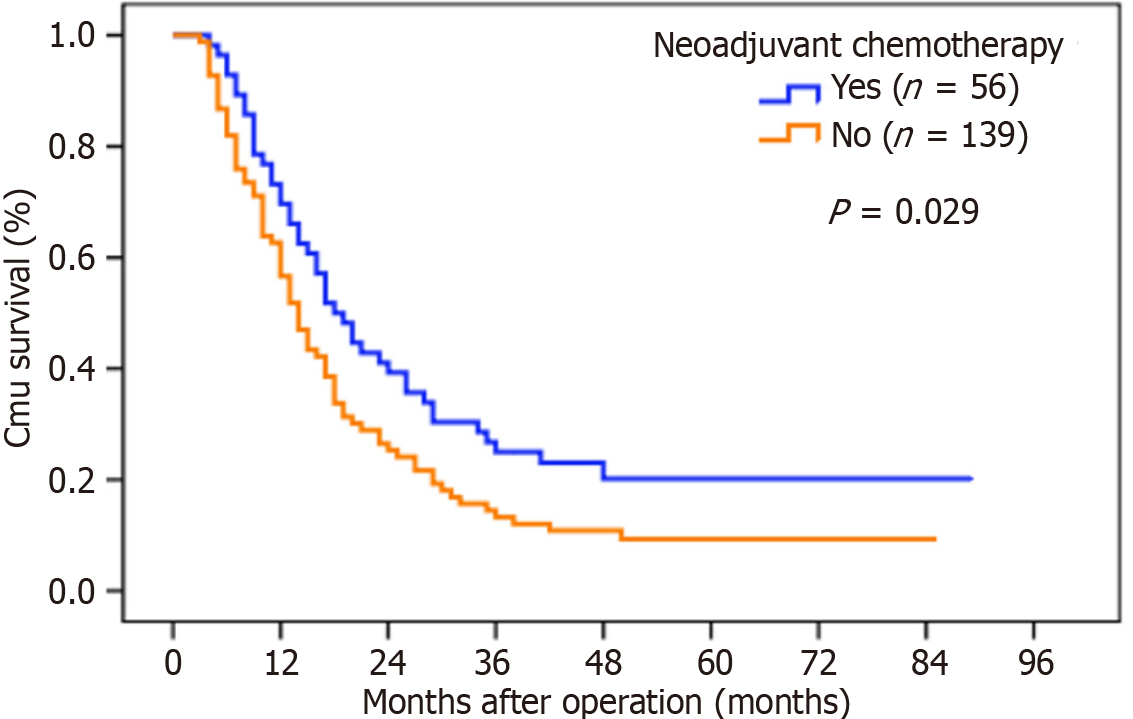
Potential prognostic factors for patients with SLNM were also evaluated. In the univariate analysis, T4 stage, N3 stage, tumor deposits and neoadjuvant chemotherapy were significant prognostic factors. Patients who received neoadjuvant chemotherapy had a significantly higher OS rate (5-year OS rates: 20.2% vs 9.3%, P = 0.029) and longer median survival time (18.0 months vs 14.0 months, P = 0.029) than those who did not (Figure 3). In the multivariate analyses, only neoadjuvant chemotherapy, T4 stage and N3 stage were found to be independent prognostic factors for OS (Table 4).
| Variables | Hazard ratio | 95%CI | P value |
| T stage (T4 vs T1-3) | 2.526 | 2.098-5.813 | 0.029 |
| N stage (N3 vs N1-2) | 1.722 | 1.176-2.522 | 0.005 |
| Tumor deposits (positive vs negative) | 1.394 | 0.948-2.048 | 0.091 |
| Neoadjuvant chemotherapy (yes vs no) | 0.626 | 0.429-0.916 | 0.016 |
In recent years, with the development of neoadjuvant and conversion therapy[21-23], more studies have focused on GC with extensive LNM, including SLNM and para-aortic LNM (PALNM)[9,10,16,18]. Several trials in Japan concluded that preoperative chemotherapy followed by D2 + PALND and postoperative S-1 was the standard treatment for GC patients, regardless of PALNM or SLNM[9,10]. However, SLNs along the celiac artery and its branches were locoregional nodes, which was different from distant PALNs[19]. Whether the addition of PALND to D2 dissection could bring survival benefits for patients with SLNM alone remains unclear, as does the prognostic value of SLNM in patients with positive LNs. GC with SLNM is not uncommon in clinical practice. Therefore, it is necessary to study the clinicopathological features and prognosis of this population. In the present study, we investigated SLNM in GC patients who underwent curative resection. Presence of SLNM was confirmed as an independent prognostic factor for GC with LNM. Although patients with SLNM had poor prognosis, neoadjuvant chemotherapy followed by D2 gastrectomy and adjuvant chemotherapy might bring survival benefits for these patients. This study was limited by its retrospective design, which may have introduced selection bias. To address this, we rigorously applied propensity score matching to balance baseline characteristics (e.g., T/N stage, tumor size) between SLNM-positive and negative groups (Table 1). However, prospective validation is warranted to confirm our findings.
Previous studies have focused on the treatment of GC with SLNM, and few have investigated its clinicopathological features[9,10,16,18]. Our results revealed that SLNM was correlated with advanced stage and aggressive characteristics of GC, such as lymphovascular invasion and Borrmann type III/IV. In western countries, GC with SLNM is generally considered unresectable. Chemotherapy is administered as palliative care, and patients with SLNM rarely survive > 3 years[6,24,25]. In East Asia, GC patients with SLNM usually receive neoadjuvant chemotherapy first, and those with downstaging of tumors are then scheduled for surgical resection[5,26]. SLNM is often used as a criterion for unresectability or neoadjuvant chemotherapy. As an indication of high aggressiveness of GC, the prognostic impact of SLNM is still uncertain. Xu et al[6] demonstrated that GC patients with swollen N2 disease alone had better OS than those with PALNM alone; however, the prognosis of patients with both swollen N2 and PALN metastasis was poorer than those with PALNM alone. Tsuburaya et al[10] found that OS of patients with SLNM alone was similar to that in patients with PALN involvement alone, but better than in patients with both SLNM and PALNM. These results suggested that SLNM had a negative impact on prognosis, even for GC with PALNM. Does SLNM affect the prognosis of GC patients with regional LNM alone? To answer this question, we focused on GC patients with regional LNM but without PALNM. It was confirmed that patients with SLNM had a significantly lower 5-year OS than those without, and the survival differences at the same N stage still existed after N stage-stratified analysis. To overcome bias, a one-to-one propensity score matching method was used. Even after matching, patients with SLNM still had significantly worse OS those without SLNM. In the multivariate analyses, SLNM was identified as an independent prognostic factor for patients with GC. SLNM can affect survival of GC patients independently of N stage. GC patients with SLNM have a poor prognosis; however, those without PALNM should still be regarded as curable. Radical surgical resection should be attempted and close follow-up is necessary. Although we performed N-stage-stratified analyses (Table 3, Figure 2), further subgroup analyses (e.g., by chemotherapy regimens or tumor location) were limited by sample size. Larger cohorts are needed to identify patients who would maximally benefit from specific therapies.
To identify appropriate treatment for patients with SLNM, survival of GC patients with SLNM alone was also analyzed. Previous studies have found that neoadjuvant chemotherapy followed by curative resection could contribute to satisfactory prognosis for GC patients with SLNM[9,10,16,18]. Our results were consistent with these studies. We found that the OS of GC patients with SLNM was independently associated with T4, N3 stage and neoadjuvant chemotherapy. Patients who received neoadjuvant chemotherapy had a better survival than those who did not. Recently, a phase 3 randomized controlled trial from China showed that perioperative SOX significantly improved 3-year disease-free survival in patients with locally advanced GC treated with D2 gastrectomy, compared with the adjuvant CapOx[12]. However, there is still no international consensus on the indications of neoadjuvant chemotherapy. Oh et al[27] assessed patients with Borrmann type IV or large type III GC. According to the clinical guidelines of the Chinese Society of Clinical Oncology, neoadjuvant chemotherapy is recommended for patients with cT3-4aN+M0 or cT4bNanyM0 GC[12]. In Japan, neoadjuvant chemotherapy is conditionally recommended for patients with SLNs that are borderline resectable around branches of the celiac artery[15]. Even so, neoadjuvant chemotherapy can downstage the tumor, increase the rate of curative resection, and prolong survival. Based on these results, we believe that neoadjuvant chemotherapy should be administered first for GC patients with SLNM, then D2 gastrectomy should be performed for those with tumor regression. Clinically, our findings suggest that SLNM status should be integrated into preoperative imaging assessments. For SLNM-positive patients, intensified neoadjuvant regimens (e.g., FLOT) followed by D2 resection and adjuvant therapy may optimize outcomes, as demonstrated by the survival benefit in our cohort (Figure 3). Future TNM staging revisions should consider incorporating SLNM as a stratification factor.
GC with SLNM is not uncommon. SLNM indicates high aggressiveness and advanced stage of GC. GC with SLNM alone is still a localized disease, and radical resection should be attempted. SLNM is an independent prognostic factor for GC. Although GC patients with SLNM have poor prognosis, neoadjuvant chemotherapy can bring survival benefits. For GC with SLNM, the ideal therapeutic strategy should be neoadjuvant chemotherapy first followed by D2 gastrectomy and adjuvant chemotherapy.
| 1. | Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2411] [Cited by in RCA: 2968] [Article Influence: 742.0] [Reference Citation Analysis (7)] |
| 2. | He Y, Wang Y, Luan F, Yu Z, Feng H, Chen B, Chen W. Chinese and global burdens of gastric cancer from 1990 to 2019. Cancer Med. 2021;10:3461-3473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Kim YH, Ji J, Yeh TS, Button P, Sirzén F, Noh SH; CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1291] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 4. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5326] [Article Influence: 355.1] [Reference Citation Analysis (3)] |
| 5. | Pietrantonio F, Randon G, Di Bartolomeo M, Luciani A, Chao J, Smyth EC, Petrelli F. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO Open. 2021;6:100036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 6. | Xu W, Liu W, Wang L, He C, Lu S, Ni Z, Hua Z, Zhu Z, Sah BK, Yang Z, Zheng Y, Feng R, Li C, Yao X, Chen M, Yan C, Yan M, Zhu Z. Is D2 Lymphadenectomy Alone Suitable for Gastric Cancer With Bulky N2 and/or Para-Aortic Lymph Node Metastases After Preoperative Chemotherapy? Front Oncol. 2021;11:709617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Park IH, Kim SY, Kim YW, Ryu KW, Lee JH, Lee JS, Park YI, Kim NK, Park SR. Clinical characteristics and treatment outcomes of gastric cancer patients with isolated para-aortic lymph node involvement. Cancer Chemother Pharmacol. 2011;67:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Cheong O, Oh ST, Kim BS, Yook JH, Kim JH, Im JT, Park GC. Large metastatic lymph node size, especially more than 2 cm: independent predictor of poor prognosis in node-positive gastric carcinoma. World J Surg. 2008;32:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, Oshita H, Ito S, Kawashima Y, Fukushima N. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009;96:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 242] [Article Influence: 22.0] [Reference Citation Analysis (1)] |
| 11. | Ito S, Sano T, Mizusawa J, Takahari D, Katayama H, Katai H, Kawashima Y, Kinoshita T, Terashima M, Nashimoto A, Nakamori M, Onaya H, Sasako M. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer. 2017;20:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, Yu J, Bu Z, Chen L, Du Y, Wang X, Wu A, Li G, Su X, Xiao G, Cui M, Wu D, Chen L, Wu X, Zhou Y, Zhang L, Dang C, He Y, Zhang Z, Sun Y, Li Y, Chen H, Bai Y, Qi C, Yu P, Zhu G, Suo J, Jia B, Li L, Huang C, Li F, Ye Y, Xu H, Wang X, Yuan Y, E JY, Ying X, Yao C, Shen L, Ji J; RESOLVE study group. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 261] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 13. | Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, Schmalenberg H, Luley KB, Prasnikar N, Egger M, Probst S, Messmann H, Moehler M, Fischbach W, Hartmann JT, Mayer F, Höffkes HG, Koenigsmann M, Arnold D, Kraus TW, Grimm K, Berkhoff S, Post S, Jäger E, Bechstein W, Ronellenfitsch U, Mönig S, Hofheinz RD. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017;3:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 332] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 14. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1648] [Article Influence: 274.7] [Reference Citation Analysis (0)] |
| 15. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 608] [Article Influence: 304.0] [Reference Citation Analysis (2)] |
| 16. | Takahari D, Ito S, Mizusawa J, Katayama H, Terashima M, Sasako M, Morita S, Nomura T, Yamada M, Fujiwara Y, Kimura Y, Ikeda A, Kadokawa Y, Sano T; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Long-term outcomes of preoperative docetaxel with cisplatin plus S-1 therapy for gastric cancer with extensive nodal metastasis (JCOG1002). Gastric Cancer. 2020;23:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Zhang N, Deng J, Sun Y, Xiao J, Li H, Liang H. An unresectable gastric cancer with bulky lymph node metastases treated with SOX chemotherapy plus apatinib followed by D3 radical gastrectomy: a case report. Transl Cancer Res. 2021;10:537-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Katayama H, Tsuburaya A, Mizusawa J, Nakamura K, Katai H, Imamura H, Nashimoto A, Fukushima N, Sano T, Sasako M. An integrated analysis of two phase II trials (JCOG0001 and JCOG0405) of preoperative chemotherapy followed by D3 gastrectomy for gastric cancer with extensive lymph node metastasis. Gastric Cancer. 2019;22:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2873] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 20. | Liang Y, Wu L, Liu L, Ding X, Wang X, Liu H, Meng J, Xu R, He D, Liang H. Impact of extranodal tumor deposits on prognosis and N stage in gastric cancer. Surgery. 2019;166:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Yang H, Ji K, Ji J. Current status and perspectives of conversion therapy for advanced gastric cancer. Chin J Cancer Res. 2022;34:109-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | Morgagni P, Solaini L, Framarini M, Vittimberga G, Gardini A, Tringali D, Valgiusti M, Monti M, Ercolani G. Conversion surgery for gastric cancer: A cohort study from a western center. Int J Surg. 2018;53:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Sato Y, Ohnuma H, Nobuoka T, Hirakawa M, Sagawa T, Fujikawa K, Takahashi Y, Shinya M, Katsuki S, Takahashi M, Maeda M, Okagawa Y, Naoki U, Kikuch S, Okamoto K, Miyamoto H, Shimada M, Takemasa I, Kato J, Takayama T. Conversion therapy for inoperable advanced gastric cancer patients by docetaxel, cisplatin, and S-1 (DCS) chemotherapy: a multi-institutional retrospective study. Gastric Cancer. 2017;20:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-v49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1118] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 25. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 960] [Article Influence: 320.0] [Reference Citation Analysis (0)] |
| 26. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 463] [Article Influence: 115.8] [Reference Citation Analysis (1)] |
| 27. | Oh SY, Kwon HC, Jeong SH, Joo YT, Lee YJ, Cho Sh, Kang MH, Go SI, Lee GW, Kim Hg, Kang JH. A phase II study of S-1 and oxaliplatin (SOx) combination chemotherapy as a first-line therapy for patients with advanced gastric cancer. Invest New Drugs. 2012;30:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |