Published online Jun 24, 2025. doi: 10.5306/wjco.v16.i6.105910
Revised: April 11, 2025
Accepted: May 23, 2025
Published online: June 24, 2025
Processing time: 122 Days and 18.2 Hours
Gallbladder cancer is a highly malignant and aggressive tumor, often diagnosed at an advanced stage. The prognosis for advanced gallbladder cancer remains poor, with limited options for effective treatment.
A 65-year-old male patient presented with a soft tissue mass in the gallbladder. Following laparoscopic exploration, he underwent radical surgery for gallbladder cancer, with the tumor staged as pT2N1M0 (stage III). Post-surgery, the patient received four cycles of adjuvant chemotherapy. However, one month later, over 60 metastatic lesions were detected in the liver, lungs, and lymph nodes. In response to the widespread metastasis, he underwent six cycles of combination therapy consisting of sintilimab, albumin-bound paclitaxel, and cisplatin. After two cycles, a partial response was achieved. Maintenance therapy was initiated with a combination of sintilimab and albumin-bound paclitaxel for four cycles. Monotherapy with sintilimab was continued thereafter until October 2023. Complete remission was confirmed in September 2021. The patient sustained this complete response for more than three years, including an eleven-month period without disease progression following the discontinuation of sintilimab. Genetic analysis revealed a tumor mutational burden of 15.4 mutations/megabase, which indicates a potentially favorable response to immunotherapy.
This case demonstrates the potential of combining immunotherapy (sintilimab) with chemotherapy to achieve durable remission in metastatic gallbladder cancer, even in a patient with extensive metastasis. In addition, it indicated the impor
Core Tip: We present a rare case of metastatic gallbladder cancer with over sixty liver, lung, and lymph node metastases achieving durable complete remission via sintilimab combined with albumin-bound paclitaxel and cisplatin. Following six cycles, complete response was maintained for over three years, including eleven months post-treatment. High tumor mutational burden (15.4 mutations/megabase) predicted immunotherapy efficacy. This highlights the potential of programmed death-1 inhibitors with chemotherapy in advanced disease and emphasizes tumor mutational burden’s role as a predictive biomarker.
- Citation: Wang F, Gong XL, Chen XN, Yang ZH, Chu XY. Durable complete response to sintilimab plus chemotherapy in gallbladder cancer with extensive metastasis: A case report. World J Clin Oncol 2025; 16(6): 105910
- URL: https://www.wjgnet.com/2218-4333/full/v16/i6/105910.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i6.105910
Gallbladder cancer is a highly malignant and insidiously progressing tumor, with early diagnosis often being cha
In October 2019, a 65-year-old male underwent a routine ultrasound during a local health check-up, which revealed the presence of a “gallbladder mass” (Figure 1).
Subsequently, on October 17, 2019, he was admitted to the Department of Surgical Oncology of Affiliated Jinling Hospital for further evaluation and management.
The patient has a medical history of cerebral infarction diagnosed in 2000, with no residual sequelae. He has been on long-term aspirin therapy since the diagnosis. He had no history of hepatitis.
His lifestyle history included a ten-year smoking habit (ten cigarettes per day, quit two years ago) and twenty years of alcohol consumption (100 mL/day, quit two years ago). There was no family history of liver cancer or other malignancies.
The patient exhibited no fever and had stable vital signs.
Blood tests for tumor markers such as carbohydrate antigen 19-9 (CA19-9), CA125, carcinoembryonic antigen, and alpha-fetoprotein were all within normal ranges.
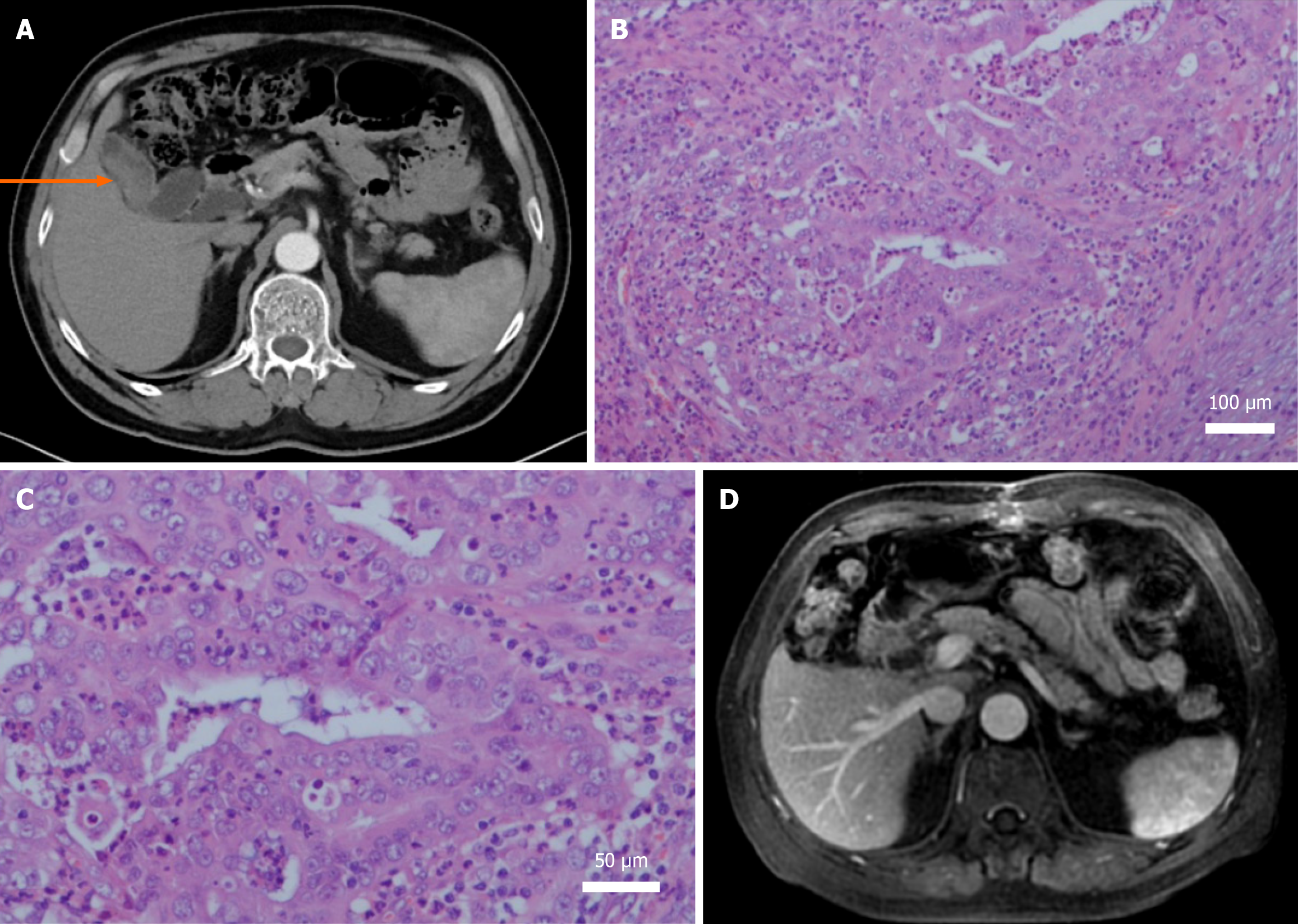
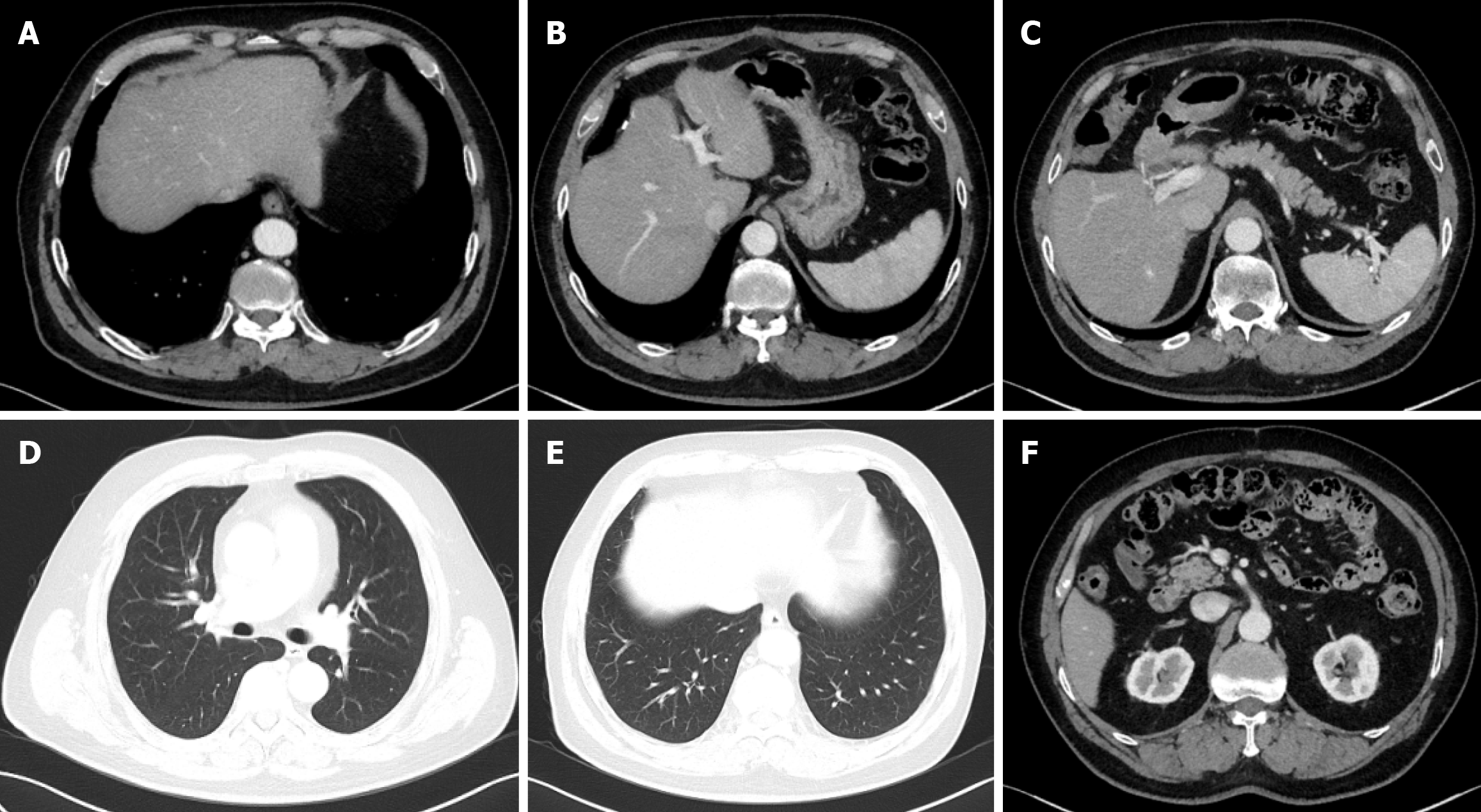
Abdominal computer tomography (CT) imaging on October 22, 2019, revealed a soft tissue mass in the gallbladder, highly suggestive of gallbladder cancer, with mild dilation of the common bile duct (Figure 2A). Chest imaging showed no abnormalities.
On October 23, 2019, the patient underwent laparoscopic exploration and radical gallbladder cancer surgery. Postoperative pathology revealed moderately to poorly differentiated adenocarcinoma of the gallbladder with necrosis (Figure 2B and C). The cancer infiltrated the full thickness of the gallbladder wall, but no residual cancer tissue was found at the gallbladder stump. R0 resection was achieved. Metastasis was detected in one of five lymph nodes from the “group 12” lymph node station. Based on the American Joint Committee on Cancer staging criteria, the tumor was classified as pT2N1M0, which corresponds to stage IIIB. A magnetic resonance imaging conducted on November 19, 2019, revealed no signs of tumor recurrence or metastasis (Figure 2D).
Between November 20, 2019 and February 22, 2020, the patient completed four cycles of adjuvant chemotherapy consisting of gemcitabine (1.4 g administered intravenously on days 1 and 8) and capecitabine (1000 mg/m2 taken orally twice daily on days 1-14), repeated every three weeks for a total of four cycles. During chemotherapy, the patient experienced grade 2-3 leukopenia and neutropenia, which resolved following the administration of granulocyte colony-stimulating factor.
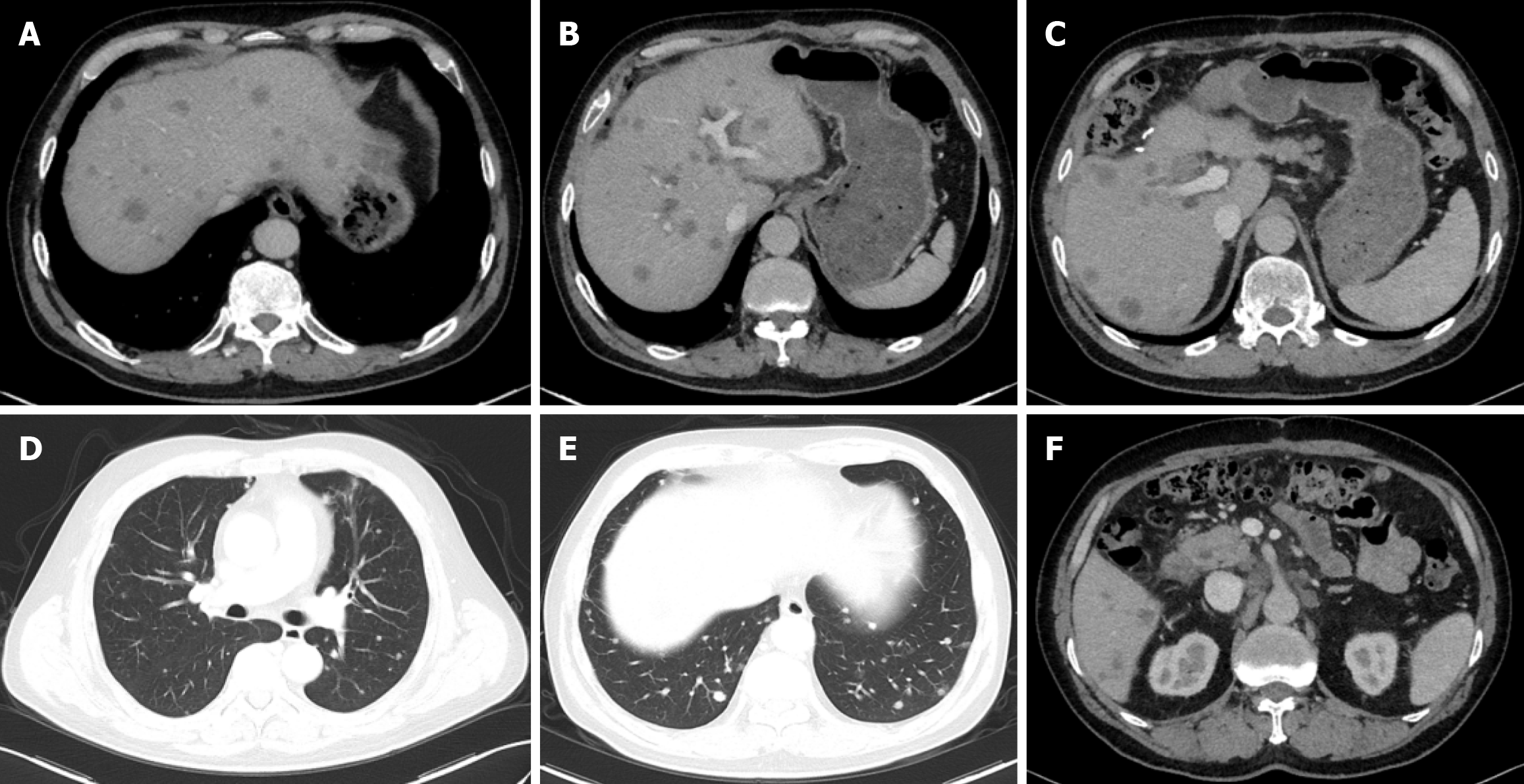
On March 25, 2020, a CT scan revealed extensive metastatic disease, including more than sixty lesions in the liver, as well as metastatic deposits in the lungs and multiple lymph nodes located in both supraclavicular fossae, the right cardiophrenic angle, the anterior margin of the lower thoracic spine, the hepatogastric region, the hepatic hilum, and the retroperitoneal area (Figure 3). Subsequent tumor marker testing showed a markedly elevated CA125 level (330.9 U/mL), while carcinoembryonic antigen, CA19-9, and alpha-fetoprotein remained within normal ranges. These findings confirmed a stage IVB (T2N1M1) gallbladder adenocarcinoma with liver, lymph node, and lung metastases. At the time of his disease relapse, the patient’s Eastern Cooperative Oncology Group performance status was 0, and the Child-Pugh score was 5 (Class A).
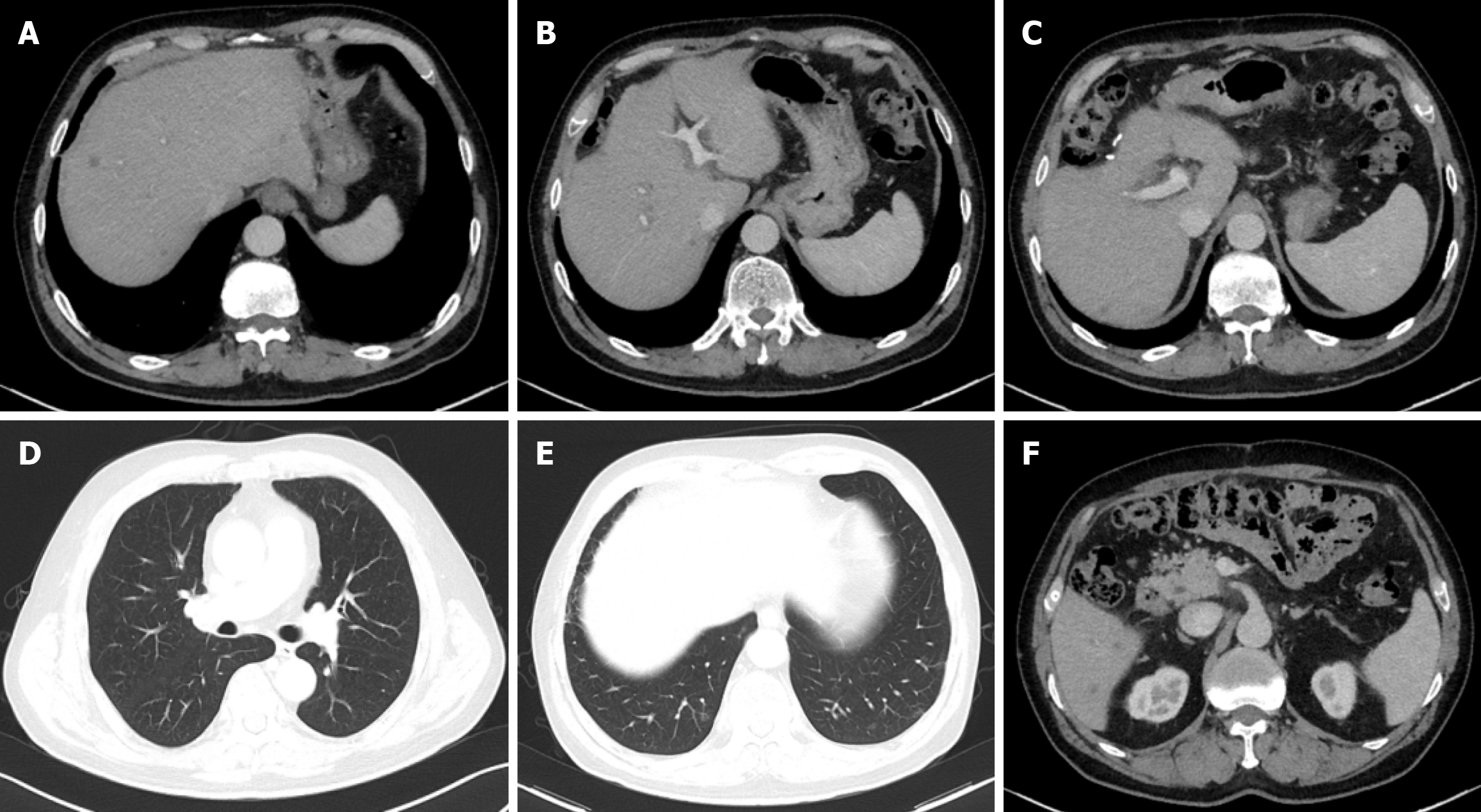
Starting March 27, 2020, the patient received sintilimab, 200 mg intravenous on day 5; albumin-bound paclitaxel, 0.2 g intravenous on day 1 and 0.1 g intravenous on day 8; and cisplatin, 20 mg intravenous on days 2-6. Every three weeks as his first-line systemic treatment. After two treatment cycles, the patient’s CA125 levels normalized, and a follow-up CT scan on May 18, 2020, revealed significant shrinkage of metastases in the liver, lymph nodes, and lungs, with some lesions disappearing entirely. The patient was evaluated as having achieved partial response (Figure 4). After six cycles of treatment, the regimen was adjusted to maintenance therapy with sintilimab and albumin-bound paclitaxel for four cycles. However, due to peripheral neurotoxicity (numbness in hands and feet) and bone marrow suppression, albumin-bound paclitaxel was discontinued, and maintenance therapy was continued with sintilimab monotherapy. During monotherapy, the patient occasionally developed rashes or pruritus, which improved with oral loratadine.
On September 16, 2021, the patient was evaluated as having achieved complete response (Figure 5). The patient gradually extended the dosing interval of sintilimab after achieving the first complete remission, administering it once every 4-8 weeks, for a total of thirty-eight doses. On October 23, 2023, sintilimab was discontinued. Subsequent follow-up imaging assessments, including the latest on September 27, 2024, confirmed that the patient maintained complete response status. Genetic testing was performed on the original gallbladder tumor surgical specimen in March 2024. The results showed a PD-L1 tumour proportion score of < 1%, combined positive score of 3, and a tumor mutational burden (TMB) of 15.4 mutations/megabase.
On July 14, 2020, laboratory tests revealed elevated thyroid-stimulating hormone (TSH) at 89.50 IU/mL, reduced free triiodothyronine at 2.32 pmol/L, and free thyroxine levels below 5.15 pmol/L. Despite the absence of related symptoms, the patient was clinically diagnosed with subclinical hypothyroidism. Starting July 14, 2020, the patient was prescribed levothyroxine sodium tablets (Euthyrox) at an initial dose of 25 μg per day, gradually increased to 100 μg per day. By September 2020, TSH levels had normalized, and the patient continued oral Euthyrox therapy. Following the discontinuation of sintilimab on October 2023, no further episodes of rashes or skin itching occurred. In August 2024, the dose of Euthyrox was reduced to 50 μg per day. However, in September 2024, repeat testing showed elevated TSH levels at 48.48 mIU/L, prompting the resumption of Euthyrox at 100 μg per day.
This case report discusses a rare and clinically significant instance of long-term remission in a patient with metastatic gallbladder cancer treated with a combination of the PD-1 inhibitor sintilimab and chemotherapy. In this case, posto
Systemic therapy is the primary treatment modality for advanced gallbladder cancer. The ABC-02 study established the combination of gemcitabine and cisplatin as the first-line treatment standard for advanced biliary tract cancers[3]. Results from the phase III clinical trial TOPAZ-1 demonstrated that adding the PD-L1 inhibitor durvalumab to ge
Based on these two studies, immune checkpoint inhibitors (ICIs) combined with the gemcitabine-cisplatin regimen have become the first-line treatment recommendation in domestic and international guidelines. In recent years, triplet or quadruplet regimens for biliary tract cancer have shown promising results. A phase II study reported that the com
Currently, the approach and duration of maintenance therapy following first-line treatment for biliary tract tumors remain undefined. In the TOPAZ-1 study, the experimental group received a maximum of eight cycles of gemcitabine and cisplatin combined with durvalumab, followed by maintenance therapy with durvalumab monotherapy until disease progression. In the KEYNOTE-966 study, pembrolizumab monotherapy was used for maintenance, with a total treatment duration of thirty-five cycles. In clinical practice, given the highly aggressive biological behavior of biliary tract cancer, the efficacy of single-agent ICIs for maintenance may be suboptimal. Therefore, some clinicians may opt for a main
In this case, after six cycles of albumin-bound paclitaxel, cisplatin, and sintilimab, the patient underwent four cycles of maintenance therapy with albumin-bound paclitaxel and sintilimab. Due to bone marrow suppression and neurotoxicity caused by albumin-bound paclitaxel, maintenance was switched to sintilimab monotherapy. The total duration of immunotherapy reached forty-three months, after which the treatment was discontinued. Subsequent imaging evaluations repeatedly confirmed sustained complete remission, with a survival period of five years since the initial diagnosis of gallbladder cancer.
With the widespread use of ICIs, immune-related adverse events have garnered significant attention. Endocrine and skin toxicities are among the most common immune-related adverse events and may be associated with improved survival outcomes[8]. In this patient, immune-related hypothyroidism developed four months after initiating sintilimab. Thyroid function normalized with levothyroxine (Euthyrox) treatment. Following discontinuation of sintilimab, the levothyroxine dose was briefly reduced, but TSH levels subsequently increased again, indicating that some patients may experience long-term or even permanent endocrine toxicities. During immune-chemotherapy, the patient also experienced recurrent reductions in white blood cells and platelets, which were successfully managed with standard treatments such as granulocyte-colony stimulating factor and thyroid peroxidase. Neurotoxicity (numbness in hands and feet) caused by albumin-bound paclitaxel resolved within six months after discontinuing the drug.
Accurately identifying patient populations that benefit most from immunotherapy remains a significant challenge. The results of the KEYNOTE-158 study showed that tumors with high microsatellite instability or deficient mismatch repair had a response rate of 30.8% to pembrolizumab, with a median duration of response of 47.5 months[9]. Whether combining immunotherapy with chemotherapy or targeted therapy could achieve better outcomes than immunotherapy alone in this subset of patients still requires clinical validation.
This case of gallbladder cancer is accompanied by diffuse liver metastasis, bilateral lung metastasis, and multiple lymph node metastasis. After treatment with combination therapy of sintilimab and chemotherapy, complete remission was achieved, and the efficacy lasted for more than four years, which is rare in clinical practice. Genetic testing of the tumor tissue revealed high TMB expression (15.4 mutations/megabase). At 15.4 mutations/megabase, the tumor’s mutational burden qualifies as high, exceeding the commonly used cutoff (≥ 10 mutations/megabase) for “TMB-high” status. High TMB has emerged as a predictive biomarker for response to ICIs. For example, TMB-high tumors showed significantly greater response rates to PD-1/PD-L1 blockade (pembrolizumab), leading to a tumor-agnostic approval of pembrolizumab for TMB-high solid tumors. Mechanistically, tumors with very high mutation loads generate a larger pool of neoantigens that can be presented on major histocompatibility complex molecules, enhancing tumor immunogenicity and making them more recognizable to T cells under checkpoint inhibition. In biliary tract malignancies such as gallbladder cancer, TMB-high cases are relatively rare but when present this biomarker may identify patients more likely to benefit from immunotherapy, as illustrated by the favorable response in this case. It should be noted, however, that TMB is an imperfect biomarker: Its predictive value varies across tumor types (not all TMB-high tumors respond, and some lower-TMB tumors can still respond).
In recent years, significant breakthroughs have been made in the precision-targeted therapy of biliary tract tumors. Approved targeted therapies now address mutations such as IDH1, FGFR fusions, HER-2 overexpression/amplification, BRAF V600E mutations, and NTRK fusions[10], which are often used as second-line or later treatment options. The patient’s genetic testing did not detect any abnormalities in these targets. Targeted therapy is often used as a second-line or above treatment option. Whether targeted drugs can be used in first-line treatment and whether they have synergistic effects with immune drugs or chemotherapy requires further investigation through related clinical studies.
In conclusion, our report highlights the potential of combining immunotherapy with traditional chemotherapy to achieve long-term remission, even in patients with advanced and metastatic disease. Furthermore, we emphasize the importance TMB as a predictive biomarker for immunotherapy efficacy, providing valuable insights for future treatment strategies in gallbladder cancer.
| 1. | Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J; BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 810] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 2. | Nakachi K, Ikeda M, Konishi M, Nomura S, Katayama H, Kataoka T, Todaka A, Yanagimoto H, Morinaga S, Kobayashi S, Shimada K, Takahashi Y, Nakagohri T, Gotoh K, Kamata K, Shimizu Y, Ueno M, Ishii H, Okusaka T, Furuse J; Hepatobiliary and Pancreatic Oncology Group of the Japan Clinical Oncology Group (JCOG-HBPOG). Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet. 2023;401:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 130] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 3. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3149] [Article Influence: 209.9] [Reference Citation Analysis (1)] |
| 4. | Oh DY, Ruth He A, Qin S, Chen LT, Okusaka T, Vogel A, Kim JW, Suksombooncharoen T, Ah Lee M, Kitano M, Burris H, Bouattour M, Tanasanvimon S, McNamara MG, Zaucha R, Avallone A, Tan B, Cundom J, Lee CK, Takahashi H, Ikeda M, Chen JS, Wang J, Makowsky M, Rokutanda N, He P, Kurland JF, Cohen G, Valle JW. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022;1:EVIDoa2200015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 524] [Article Influence: 174.7] [Reference Citation Analysis (1)] |
| 5. | Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, Verslype C, Bouattour M, Park JO, Barajas O, Pelzer U, Valle JW, Yu L, Malhotra U, Siegel AB, Edeline J, Vogel A; KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1853-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 451] [Article Influence: 225.5] [Reference Citation Analysis (0)] |
| 6. | Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, Raghav KPS, Iwasaki M, Masci P, Ramanathan RK, Ahn DH, Bekaii-Saab TS, Borad MJ. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 341] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 7. | Cheon J, Lee CK, Sang YB, Choi HJ, Kim MH, Ji JH, Ko KH, Kwon CI, Kim DJ, Choi SH, Kim C, Kang B, Chon HJ. Real-world efficacy and safety of nab-paclitaxel plus gemcitabine-cisplatin in patients with advanced biliary tract cancers: a multicenter retrospective analysis. Ther Adv Med Oncol. 2021;13:17588359211035983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Shi GM, Huang XY, Wu D, Sun HC, Liang F, Ji Y, Chen Y, Yang GH, Lu JC, Meng XL, Wang XY, Sun L, Ge NL, Huang XW, Qiu SJ, Yang XR, Gao Q, He YF, Xu Y, Sun J, Ren ZG, Fan J, Zhou J. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct Target Ther. 2023;8:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 9. | Wan G, Chen W, Khattab S, Roster K, Nguyen N, Yan B, Rajeh A, Seo J, Rashdan H, Zubiri L, Hadfield MJ, Demehri S, Yu KH, Lotter W, Gusev A, LeBoeuf NR, Reynolds KL, Kwatra SG, Semenov YR. Multi-organ immune-related adverse events from immune checkpoint inhibitors and their downstream implications: a retrospective multicohort study. Lancet Oncol. 2024;25:1053-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 10. | Lamarca A, Edeline J, Goyal L. How I treat biliary tract cancer. ESMO Open. 2022;7:100378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |