Published online Jun 24, 2025. doi: 10.5306/wjco.v16.i6.105175
Revised: April 6, 2025
Accepted: May 15, 2025
Published online: June 24, 2025
Processing time: 157 Days and 18.8 Hours
There is a lack of integrated Chinese and Western medicine treatment regimens supported by high-level evidence-based medicine in the maintenance therapy phase of metastatic colorectal cancer (mCRC). Based on the traditional Chinese medicine theory of “Yin tumor”, we believe that “Yang does not transform Yin, and it is blocked in the intestines” is the core pathogenesis of mCRC. Based on the basic treatment principle of “warming Yang and dredging intestines”, we deve
To confirm and clinically validate that the combination of “warming Yang and dredging intestines method” prescription with Western medicine standard regi
The study has a prospective, open-label, randomized, controlled study design. Patients have been recruited beginning November 2023 from Xiyuan Hospital of China Academy of Chinese Medical Sciences, Guang’anmen Hospital of China Academy of Chinese Medical Sciences, Dongfang Hospital of Beijing University of Chinese Medicine, and Xinjiang Uygur Autonomous Region Hospital of Traditional Chinese Medicine. The study period is from March 2024 to March 2026. After screening in outpatient clinics or wards, subjects who met the inclusion criteria are randomized into the treatment or control group in a 2:1 ratio. The treatment group receives the “warming Yang and dredging intestines method” formula combined with Western standard maintenance therapy. The control group receives Western standard maintenance therapy formulated by the investigators based on the Chinese Society of Clinical Oncology guidelines for colorectal cancer. All participants receive treatment until the occurrence of disease progression, death, or unmanageable adverse effects, with post-treatment monitoring continued until mortality. An independent panel of chief physicians with extensive clinical experience evaluates the progression of the disease.
This study aims to clarify whether the combination of warming Yang and dredging intestines method formula with standard Western medicine regimens can prolong PFS during maintenance therapy for mCRC and whether the treatment has a favorable safety profile. The goal is to provide a combined Chinese and Western medicine treat
This study aims to clarify whether the combination of warming Yang and dredging intestines method formula with standard Western medicine regimens can prolong PFS during maintenance therapy for mCRC and whether the treatment has a favorable safety profile.
Core Tip: The current Chinese medicine guideline for metastatic colorectal cancer. lacks a combined Chinese and Western medicine treatment plan for metastatic colorectal cancer maintenance therapy. On the basis of long-term clinical practice and experimental research, this project focuses on the weak link in the maintenance treatment stage, and verifies that the combination of traditional Chinese medicine and Western standard maintenance protocols based on the principle of “warming Yang and dredging intestines” can further improve the clinical efficacy.
- Citation: Sun YX, Zhang T, Bian JY, He WT, Wang XQ, Liu CB. Efficacy and safety of warming Yang and dredging intestines method in metastatic colon cancer maintenance. World J Clin Oncol 2025; 16(6): 105175
- URL: https://www.wjgnet.com/2218-4333/full/v16/i6/105175.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i6.105175
After Western medicine entered the era of precision treatment, overall survival (OS) of metastatic colorectal cancer (mCRC) continued to increase. The OS of mCRC treated with current Western standard protocols is up to 3 years[1]. Patients with improved prognosis are characterized by wild-type RAS and BRAF genes[2-4]. However, in the real world, results from a registry study based on the United States Surveillance, Epidemiology, and End Results database showed that the average median survival period of 77490 mCRC patients was only 14 months[5]. In China, the proportion of mCRC patients who can receive standard Western medicine regimens from first- to third-line treatment is very low. Studies have revealed an increase of over 40% in the proportion of mCRC patients with RAS gene mutation, affected right half of the colon, advanced age, poor health, poor economic status, and low treatment willingness who cannot benefit from standard or maintenance therapy in Western medicine[6,7]. These factors hinder the treatment of mCRC. Studies have shown that integrated Chinese and Western medicine treatment can prolong the survival of mCRC patients[8]. Therefore, we believe that the complementary advantages of combined Chinese and Western medicine could overcome the bottleneck of Western medicine in the maintenance treatment stage of mCRC. Based on the traditional Chinese medicine (TCM) theory of “Yin tumors”, we believe that the core pathogenesis of mCRC is “Yang does not transform Yin, and it is blocked in the intestines”. Accordingly, based on the basic treatment principle of “warming Yang and dredging intestines” method, we developed the Quxie Capsule (QX). A previous randomized controlled clinical trial verified that QX can significantly prolong the OS of patients with mCRC[9]. Sixty mCRC patients are randomized to conventional treatment combined with QX and conventional treatment combined with placebo group. The results showed that OS was prolonged by 9.6 months in the treatment group compared with the control group (23.9 vs 14.3, P = 0.032). The current mCRC guideline lacks combination of Chinese and Western medicine for maintenance therapy. Based on the previous research, this study focuses on the weak link in the maintenance therapy stage, aiming to clarify whether the combination of the “warming Yang and dredging intestines method” prescription with Western medicine standard regimen can prolong progression-free survival during the maintenance therapy of mCRC and to observe the safety of the combination.
This study intends to confirm and clinically validate that the combination of “warming Yang and dredging intestines method” prescription with Western medicine standard regimen can prolong progression-free survival (PFS) during maintenance treatment of mCRC. The safety of “warming Yang and dredging intestines method” prescription is also assessed.
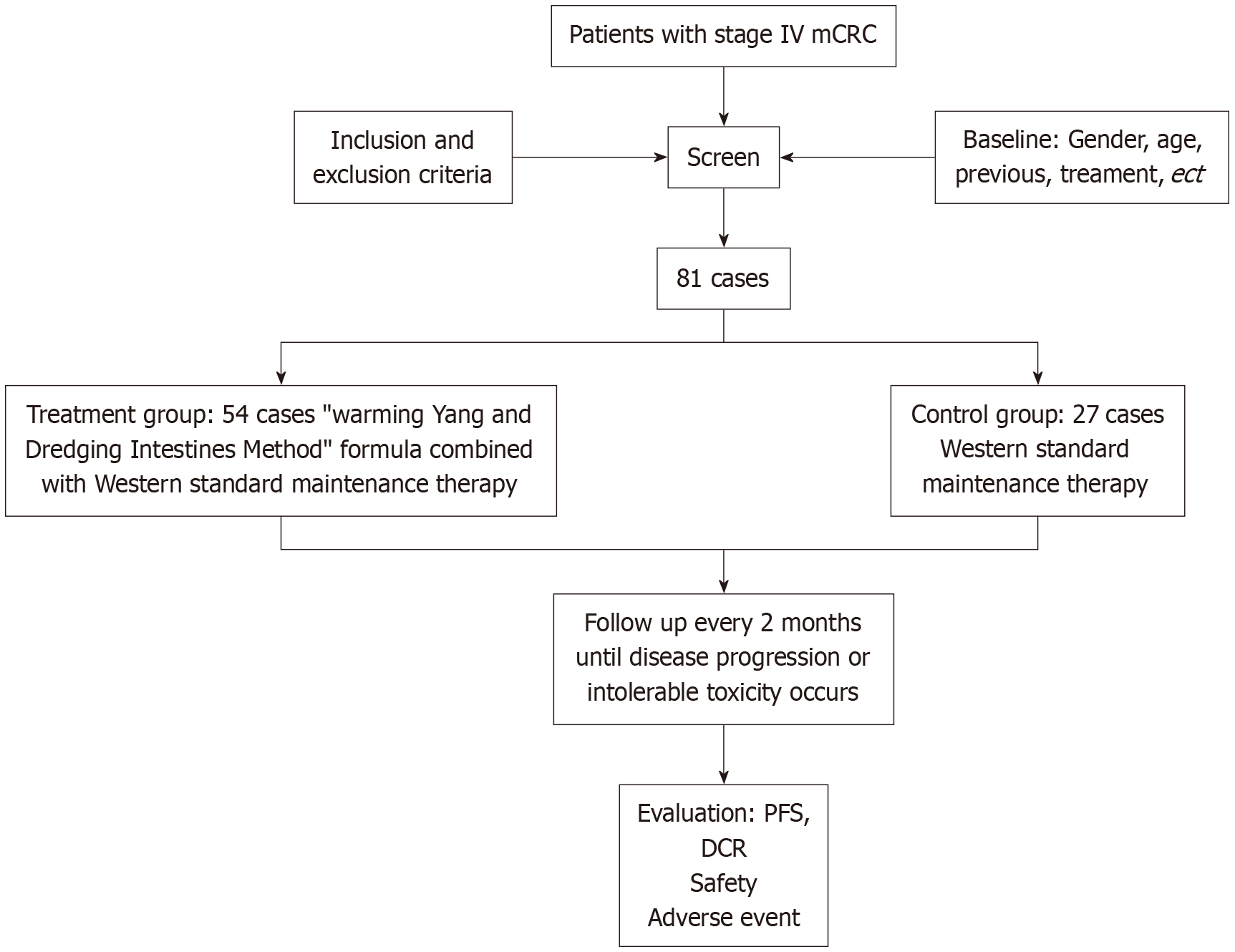
The study has a prospective, open-label, randomized, controlled study design. Patients have been recruited beginning November 2023 from Xiyuan Hospital of China Academy of Chinese Medical Sciences, Guang’anmen Hospital of China Academy of Chinese Medical Sciences, Dongfang Hospital of Beijing University of Chinese Medicine, and Xinjiang Uygur Autonomous Region Hospital of TCM. The study period is from March 2024 to March 2026. After screening in outpatient clinics or wards, subjects who met the inclusion criteria are randomized into the treatment or control group in a 2:1 ratio. The treatment group receives the “warming Yang and dredging intestines method” formula combined with Western standard maintenance therapy. The control group receives Western standard maintenance therapy formulated by the investigators based on the CSCO guidelines for CRC. The study protocol mandates therapy administration until disease progression events, fatal outcomes, or severe adverse events (AE) arise, followed by surveillance until patient decease. An independent panel of chief physicians with extensive clinical experience evaluates the progression of the disease. The subject flow chart is presented in Figure 1. The screening, intervention, and evaluation schedule is shown in Table 1.
| Item | Before treatment (within 2 weeks) | Each treatment cycle | End of treatment or withdrawal |
| Collection of basic information | |||
| Admission screening form | × | - | - |
| Signing the informed consent form | × | - | - |
| Baseline information | × | - | - |
| Effectiveness index | |||
| CT/MRI1 | × | Every 2 months or at a time point deemed appropriate by the investigator | × |
| Tumor marker2 | × | × | × |
| Safety indicator | |||
| Blood pressure | × | × | - |
| Blood routine | × | × | - |
| Blood coagulation function | × | × | - |
| Thyroid gland function | × | Every 2 months or at a time point deemed appropriate by the investigator | - |
| 24-hour urinary protein quantification | × | Every 2 months or at a time point deemed appropriate by the investigator | - |
| Liver and kidney function | × | × | - |
| ECG | × | × | - |
| Echocardiogram | × | × | - |
| Urinalysis | × | × | - |
| Stool routine + occult blood | × | × | - |
| Recording adverse events | - | × | × |
The following eligibility requirements are used: (1) Aged 18-80 years, gender not limited; (2) Pathologic diagnosis of clinical stage IV CRC; (3) Intent to receive maintenance therapy after criteria according to National Comprehensive Cancer Network or CSCO colorectal cancer guidelines; (4) Eastern Cooperative Oncology Group score 0-2; (5) Expected survival ≥ 3 months; (6) Measurable lesions according to Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 criteria; (7) No severe cardiac, pulmonary, hepatic, or renal function abnormalities; (8) Normal coagulation function, no active bleeding or thrombotic disorders; (9) Voluntary participation with signed informed consent; and (10) Expected good compliance, able to follow-up concerning efficacy and adverse reactions as required by the protocol.
The following exclusion requirements are used: (1) Clinical symptoms of brain metastases; (2) Other active malignancies without prior treatment; (3) Positive immunohistochemistry for deficient mismatch repair microsatellite instability-high for molecular diagnostics; (4) Refractory hypertension; (5) Refractory cardiac conditions with uncontrolled clinical manifestations; (6) Conditions impairing oral medication absorption, including dysphagia and intestinal obstruction; (7) Bleeding risk factors, such as active peptic ulcers, melena or hematemesis within the past 3 months, and hemoptysis within the previous month before study participation; (8) Major surgery, open biopsy, or significant trauma within 28 days before enrollment; current or planned; (9) Those who will be, or are, participation in other clinical trials; and (10) Other conditions, such as pregnancy, considered by the researchers to be unsuitable for inclusion.
Participation can be ended by the researchers for: (1) Patients who should not have been included because of serious violations of the inclusion or exclusion criteria; (2) Non-compliance with the research protocol after enrollment; and (3) Cases judged by other researchers as not suitable to continue the study.
Participation can be ended by patients who: (1) For whatever reason, are unwilling or unable to continue the clinical study and requesting withdrawal from the study from the supervising physician; and (2) Do not explicitly withdraw from the study, but no longer accept medication and testing and are lost to follow-up.
Regulations for dropout cases: (1) Dropout cases are study subjects who have signed the informed consent and are screened and qualified to enter the observation, regardless of when and why they withdrew, as long as they have not completed the observation cycle stipulated in the protocol; and (2) When a study subject drops out of the study, the investigator should make every effort to contact the subject, ask for the reason for dropout and record the time of the last medication administration, and complete the assessment items that can be accomplished.
Termination criteria: Researchers reserve the right to terminate the study at any time. When terminating the study, researchers will ensure the protection of the subjects’ interests. Criteria for terminating the study are: (1) Death; (2) Protocol deviation; (3) Withdraw of informed consent by the subject; (4) Loss to follow-up; and (5) Enrollment in a clinical trial that does not allow simultaneous participation in the registered study.
Participant recruitment and selection: Subjects are recruited through the public WeChat website of each hospital, recruitment advertisements posted in outpatient clinics, hospital electronic bulletin boards, and recommendations from frontline clinicians. The screening process for study participants is conducted by the oncology department’s attending physicians and deputy chief physicians at each hospital. All candidates must provide prior pathology reports to confirm their diagnosis and detailed clinical examination records to determine their treatment progression stage. All subjects should receive an informed consent form to be signed and should be informed of all precautions to be taken. In addition, blood tests, computed tomography scans, or magnetic resonance imaging are performed on subjects to assess their eligibility against the inclusion criteria.
Study protocol: Eligible subjects are randomly assigned to treatment and control groups. Efficacy is evaluated every 2 months.
Control group: Researchers in each center choose maintenance therapy options according to the 2023 National Comprehensive Cancer Network and CSCO guidelines for CRC. The available treatment regimens include, but are not limited to, the following regimens: (1) Capecitabine tablets, 1000 mg/m2, twice daily, taken orally 30 minutes after breakfast and dinner, from days 1-14 repeated every 21 days, or from days 1-7 repeated every 14 days; (2) Cetuximab injection, 500 mg/m2, by intravenous infusion every 14 days, or 250 mg/m2 by intravenous infusion every 7 days; and (3) Bevacizumab injection, 7.5 mg/kg, by intravenous infusion every 21 days, or 5 mg/kg by intravenous infusion every 14 days.
Bevacizumab injection can be combined with capecitabine tablets. Cetuximab can be combined with capecitabine tablets or used as a single agent. Other optional drugs, such as raltitrexed, irinotecan, and trifluridine trifluridine-tepidopyrimidine tablets, can be selected by the investigator according to the guidelines and clinical practice.
Treatment group: The treatment group used the formula of “warming Yang and dredging intestines method” combination with the standard Western medicine maintenance therapy for intervention. “Warming Yang and dredging intestines method” formula produced by Xiyuan Hospital Pharmaceutical Factory. The effects included warming Yang and dredging intestines, dispersing knots, and removing toxins. The ingredients included Epimedium, turmeric, cinnamon, rhubarb in wine, etc. Take 1 sachet twice daily. Treatment until disease progression or intolerable toxicity occurs.
Dose adjustment: According to CTCAE (5.0), for grade 1-2 adverse reactions, which can be routine, the combination of drugs used is recorded. In the event of an adverse reaction of grade 3, which does not recover after symptomatic treatment, a first dose adjustment is made with a reduction to 80 per cent of the full dose. The second dose adjustment was reduced to 50 per cent of the full dose.
Regimen adjustment: The third occurrence of a grade 3 adverse reaction should prompt a change in the treatment regimen. PFS is not determined at this stage for individuals who adjust their protocol secondary to adverse tolerance and RECIST 1.1 based assessment of tumor progression. Instead, their PFS is calculated according to the time of evaluation of disease progression according to RECIST 1.1. If PD is evaluated, the researcher will decide to change the treatment regimen.
Tailoring cancer therapy: If the altered regimen (allowing multiple times) remains intolerable, standard conventional therapy is discontinued. The researcher may further individualize the therapeutic approach according to the patient’s clinical situation and autonomous decisions.
Post-disease progression management is decided by the attending physician, with the therapeutic strategy remaining unrestricted.
This protocol has undergone rigorous peer review and refinement by interdisciplinary experts in oncology, clinical methodology, and biostatistics. Prior to trial initiation, all researchers must complete mandatory training in standardized operating procedures covering enrollment criteria, intervention protocols, CRF completion protocols, RECIST criteria application, and data governance frameworks. Professionals are hired to enter, manage, and monitor data for the study process.
“Warming Yang and dredging intestines method” formula are developed by the Pharmaceutical Factory of Xiyuan Hospital, Chinese Academy of Medical Sciences.
This study has been approved by the ethics committee of Xiyuan Hospital, Chinese Academy of Chinese Medical Sciences, approval 2024XLA071-4.
The main observational index of this study is the difference in PFS between the two groups of maintenance therapy. The sample size is chosen based on the formula:
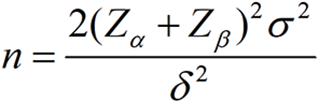
The means of the two samples is compared using test level α = 0.05 and β = 0.8, according to the pre-experimental data δ = 11.7-8 = 3, σ2 = 18.85, substituting into the parameter calculations to obtain a sample size of 23 cases in each group. Accounting for an expected failure rate of 20%, the sample size is 27 for each group. Finally, based on a 2:1 ratio of the treatment and control groups, a total of 81 patients is required.
Maintenance PFS, the primary outcome indicator, is defined as the time from the start of the subject’s signing of the informed consent form until the first tumor progression or the patient’s death.
The secondary outcome include of disease control rate (DCR) includes number of cases that achieve remission (including partial remission + complete remission) and stabilization of lesions (stable disease) after treatment. DCR represents the proportion of assessable cases, quantified as a percentage: DCR = (complete remission + partial remission + stable disease/sample size of the group) × 100.
AE are evaluated using NCI CTCAE version 5.0 before the start of each treatment cycle. All AE occurring from the start of treatment to 1 month after the end of treatment are recorded and evaluated. Blood, urine, stool, biochemistry, coa
Statistical analyses will be performed using SAS JMP Pro (14.0). Measurement information conforming to normal distribution are expressed as indicated, non-normally distribution is expressed as median and interquartile range. Baseline analysis is performed using the χ2 test to detect whether there is a balance between the two groups, including age, gender, tumor primary site, gene type, and treatment regimen. The Fisher exact test is applied when the sample size fails to meet the χ2 test requirements. For the main index median PFS, the Kaplan-Meier method of survival analysis is used to determine the survival curve, and the Log-rank test is used for comparison between groups. The test level is α = 0.05. Subgroups include age, gender, primary tumor site, treatment plan, gene type, and liver metastasis.
This study aims to clarify whether the combination of warming Yang and dredging intestines method formula with standard Western medicine regimens can prolong PFS during maintenance therapy for mCRC and whether the treatment has a favorable safety profile.
This study aims to clarify whether the combination of warming Yang and dredging intestines method formula with standard Western medicine regimens can prolong PFS during maintenance therapy for mCRC and whether the treatment has a favorable safety profile. The goal is to provide a combined Chinese and Western medicine treatment option for clinical physicians and mCRC patients. Notably, with the actual sample size, this study has an 80% probability of detecting a significant difference if a true difference exists. Small sample sizes may lead to increased instability of the results of subgroup analyses, and may also result in findings that are only applicable to patients with characteristics highly similar to those of the present study population (e.g., specific genotypes, therapeutic backgrounds, etc.), making it difficult to generalize to the broader mCRC population. In the future, it may be possible to expand the sample size based on this study to further validate the efficacy and safety of combining Chinese and Western medicine in the treatment of mCRC. Basic research on the therapeutic combination of warming Yang and dredging intestines method formula and standard Western regimen will be performed in parallel.
This study aims to clarify whether the combination of warming Yang and dredging intestines method formula with standard Western medicine regimens can prolong PFS during maintenance therapy for mCRC and whether the treatment has a favorable safety profile.
| 1. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3024] [Article Influence: 504.0] [Reference Citation Analysis (3)] |
| 2. | Wu CC, Wang JH, Lin PC, Liang CA, Huang CY, Lien HC, Chen CY, Chou KJ, Su YC. Tumor sidedness and efficacy of first-line therapy in patients with RAS/BRAF wild-type metastatic colorectal cancer: A network meta-analysis. Crit Rev Oncol Hematol. 2020;145:102823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Modest DP, Pant S, Sartore-Bianchi A. Treatment sequencing in metastatic colorectal cancer. Eur J Cancer. 2019;109:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 4. | Ciliberto D, Staropoli N, Caglioti F, Chiellino S, Ierardi A, Ingargiola R, Botta C, Arbitrio M, Correale P, Tassone P, Tagliaferri P. The best strategy for RAS wild-type metastatic colorectal cancer patients in first-line treatment: A classic and Bayesian meta-analysis. Crit Rev Oncol Hematol. 2018;125:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Castleberry AW, Güller U, Tarantino I, Berry MF, Brügger L, Warschkow R, Cerny T, Mantyh CR, Candinas D, Worni M. Discrete improvement in racial disparity in survival among patients with stage IV colorectal cancer: a 21-year population-based analysis. J Gastrointest Surg. 2014;18:1194-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Dienstmann R, Salazar R, Tabernero J. Molecular Subtypes and the Evolution of Treatment Decisions in Metastatic Colorectal Cancer. Am Soc Clin Oncol Educ Book. 2018;38:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Huang HY, Shi JF, Guo LW, Bai YN, Liao XZ, Liu GX, Mao AY, Ren JS, Sun XJ, Zhu XY, Wang L, Song BB, Du LB, Zhu L, Gong JY, Zhou Q, Liu YQ, Cao R, Mai L, Lan L, Sun XH, Ren Y, Zhou JY, Wang YZ, Qi X, Lou PA, Shi D, Li N, Zhang K, He J, Dai M. Expenditure and financial burden for the diagnosis and treatment of colorectal cancer in China: a hospital-based, multicenter, cross-sectional survey. Chin J Cancer. 2017;36:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Shao C, Zuo Q, Lin J, Yu RJ, Fu Y, Xiao M, Sun LL, Lin L. Effect of Chinese Herbal Medicine on the Survival of Colorectal Cancer Patients With Liver-Limited Metastases: A Retrospective Cohort Study, 2008 to 2017. Integr Cancer Ther. 2019;18:1534735419883687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Zhang T, Yang YF, He B, Yi DH, Hao J, Zhang D. Efficacy and Safety of Quxie Capsule () in Metastatic Colorectal Cancer: A Double-Blind Randomized Placebo Controlled Trial. Chin J Integr Med. 2018;24:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (35)] |