Published online May 24, 2025. doi: 10.5306/wjco.v16.i5.101378
Revised: February 19, 2025
Accepted: March 31, 2025
Published online: May 24, 2025
Processing time: 249 Days and 18.2 Hours
Radiation therapy is an important treatment for esophageal tumors. However, there is still controversy regarding the total dose and fraction dose. The optimal dose and fractionation schedule have not yet been clearly established. Hypofractionated radiotherapy is becoming more popular, but it is unknown whether this is the optimal choice for esophageal tumors. In addition, the appropriate dose per fraction is uncertain. We performed a retrospective study to address these issues.
To report the cumulative survival and toxicity associated with the delivered dose escalation and hypofractionation schedule of radiation therapy for esophageal squamous cell carcinoma.
Forty-seven patients treated for inoperable locally advanced thoracic esophageal squamous cell carcinoma with helical tomotherapy using different total doses and doses per fraction were enrolled. Toxicity and adverse events were evaluated in all patients to determine the acute and long-term effects according to the Toxicity Criteria of The Radiation Therapy Oncology Group. Overall survival was cal
Six patients died of bleeding related to aorto-esophageal fistulization. Four patients died of tracheo-esophageal fistulas, and 7 patients died of local recu
Esophageal toxicity can be tolerated below a prescribed radiation dose of 60 Gy and less than 2.3 Gy per fraction.
Core Tip: Hypofractionated radiotherapy is becoming more popular. However, it is unknown whether it is an optimal choice for esophageal tumors. In addition, the appropriate dose per fraction is unclear. At present, there is no systematic report on these issues. In this study, 47 patients with thoracic esophageal carcinoma treated with radiotherapy at different prescribed doses and doses per fraction were enrolled retrospectively. Toxicity and adverse events were evaluated to determine the acute and long-term treatment effects. It was shown that esophageal toxicity can be tolerated below a prescribed dose of 60 Gy of radiation and less than 2.3 Gy per fraction.
- Citation: Zhang SM, Sun J, Pan XO, Zhu WC, Zhou YK. Toxicity of dose-escalated, hypofractionated helical tomotherapy for inoperable thoracic esophageal squamous cell carcinoma. World J Clin Oncol 2025; 16(5): 101378
- URL: https://www.wjgnet.com/2218-4333/full/v16/i5/101378.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i5.101378
External beam radiation therapy is an integral part of treatment and has played an important role in the management of unresectable esophageal carcinoma[1]. Additionally, local recurrence is an important cause of treatment failure in esophageal squamous cell carcinoma (ESCC). The dose-response effect for local control has been confirmed, but the optimal dose and fractionation schedule in esophageal carcinoma have not yet been clearly established. The Radiation Therapy Oncology Group (RTOG) 85-01 trial and RTOG 94-05 trial concluded that 50.4 Gy is the standard dose used in the United States due to a lack of improved locoregional control and increased mortality in the high-dose radiotherapy group in these randomized trials[2,3]. However, in China, the treatment regimen of definitive radiotherapy was intensified using late-course accelerated hypofractionated radiotherapy with a dose of 68-71 Gy, which showed the potential to improve the survival of patients and to achieve better local control compared to conventional fractionated radiotherapy[4]. In addition, preoperative chemoradiotherapy prolonged local disease-free survival with a dose of 3.7 Gy per fraction of 37 Gy[5]. However, hypofractionated radiotherapy can result in less radiation time and a higher radiation dose for esophageal tumors. Hypofractionated radiotherapy represents an accelerated form of radiation delivery, and treatment once a day improves patient convenience and is now becoming more popular for many solid tumors such as lung cancer and liver cancer. Up to date, no systematic study on hypofractionated radiotherapy for ESCC has been reported. Here, we report, for the first time, the cumulative survival and toxicity associated with the delivered dose escalation and fractionation schedule in patients with ESCC.
Between June 2011 and December 2013, a total of 401patients with esophageal cancer were treated with radiotherapy at the Zhongshan Hospital, Fudan University (Shanghai, China). We retrospectively reviewed the records of 47 patients treated with helical tomotherapy for inoperable locally advanced thoracic ESCC. All patients were initially diagnosed and did not have a history of surgery or chemotherapy. Demographic information included a complete medical history, physical examination, complete blood cell count, adequate liver and renal function, esophageal barium contrast examination, esophagogastroduodenoscopy, contrast-enhanced computed tomography (CT), positron emission tomography/CT (if necessary), etc. Histological diagnoses in all patients were verified by esophagogastroduodenoscopy biopsy examinations. Clinical staging was based on the tumor-node-metastasis classification for esophageal cancer of the Union for International Cancer Control criteria (7th edition, 2009), after comprehensive assessment of the findings from the patient’s physical and imaging examinations. The T stage was defined by the maximum transverse diameter of the esophageal tumor: Less than 1 cm (T1), between 1 and 3 cm (T2), and larger than 3 cm (T3). Tumors with invasion of any neighboring structures were classified as T4. T4a were defined as a resectable tumor that invaded the pleura, pericardium or diaphragm. T4b was defined as an unresectable tumor that invaded other adjacent structures, such as the aorta, vertebral body, and trachea. The lymph nodes were classified as N1 (invaded) if the maximum transverse diameter of these nodes was larger than 1.5 cm; otherwise, they were classified as N0. T1 and T2 were excluded. T3 tumors with lymph node metastasis and T4 tumors did not undergo surgery and were analyzed in this study. This retrospective study was approved by the Ethical Committee of Zhongshan Hospital, Fudan University. The nature of the illness, treatment and possible complications were explained to the patient, and written consent was obtained.
Helical tomotherapy was limited to the primary site and clinically proven or suspicious nodal regions on CT imaging. A custom-made double vacuum-based immobilization system was utilized. Planned imaging included thin slice treatment planning CT. Gross tumor volume (GTV) of the primary tumor was determined, and metastatic lymph nodes were judged to be positive clinically. CT and/or positron emission tomography imaging were utilized to define the extent of the GTV. The Helical TomoTherapy Treatment Planning system (TomoTherapy, Inc., Middleton, WI, United States) was used for treatment planning. Patients received sequential chemotherapy after completion of radiation therapy.
According to the Toxicity Criteria of The RTOG, toxicity and adverse events were evaluated in all patients to determine the acute and long-term effects attributable to the treatment regimen[6]. Esophageal radiation toxicities were scored as follows: Grade 1 esophageal toxicity requiring topical anesthetic non-narcotic analgesics for mild dysphagia or odynophagia; grade 2 esophageal toxicity consisted of moderate dysphagia or odynophagia, usually requiring narcotic analgesics; grade 3 esophageal toxicity consisted of severe dysphagia or odynophagia with > 15% weight loss; and grade 4 esophageal toxicity consisted of complete obstruction, ulceration, fistula, or perforation. Acute toxicity was monitored at least once weekly during radiation treatment. When excessive morbidity was confirmed, the patients were hospitalized and received treatment. During the follow-up period, patients were seen every month during the first year, bimonthly in the second year and then every 3 to 6 months thereafter.
Patients were followed weekly by a multidisciplinary team while receiving treatment. Then, they were followed up monthly for the first year, bimonthly during the second year, and every 3 to 6 months thereafter until death. Follow-up evaluation included an interval history, physical examination, CT of the chest and abdomen, serum chemistry, blood tumor markers etc. Swallowing evaluations including barium fluoroscopy were completed prior to therapy and serially during follow-up. Response Evaluation Criteria in Solid Tumors were used to evaluate treatment response based on CT studies[7].
Overall survival was calculated using the Kaplan-Meier method. The survival time was defined as the time from initial diagnosis to death. Locoregional control was defined as the time from the end of radiation therapy to local recurrence. Logistic analysis was used to identify the correlation between dose to primary tumor and the degree of toxicity. In the multivariate analysis, all variables were entered in a single step using the method of backward stepwise regression. P < 0.05 was considered statistically significant. Analyses were performed using the SPSS18.0 software (SPSS Inc., Chicago, IL, United States) for Windows.
Forty-seven patients with inoperable locally advanced thoracic ESCC treated between June 2011 and December 2013 were enrolled in this study. Their baseline characteristics are summarized in Table 1. The median age of these patients was 61years (range 36-86). Most of the patients were male and had lymph node involvement and T3 or T4 disease. As shown in Table 1, 38%, 15% and 47% of patients had T3, T4a and T4b disease, respectively. Additionally, 11% were stage IIIA, 28% were stage IIIB, and 62% were stage IIIC. However, almost all patients had lymph node metastases, and only 4% of patients did not. The median follow-up for the entire study population was 11.3months. Median follow-up in surviving patients (n = 10) was 28.4 months (range 14.1 to 38.9 months). None of the patients were lost to follow-up with respect to survival and tumor control information. All the patients received helical tomotherapy. In one patient, tracheo-esophageal fistulas were confirmed at the 4th radiation treatment, and radiation was administered at his request after stent implantation. The dose and the fractions are detailed in Table 2. Thirty-eight percent of patients (18/47) were treated with radiation therapy without chemotherapy, and only 27.7% of patients received full chemotherapy after radiation therapy (Table 1). The regimen of fixed-dose chemotherapy included docetaxel and cisplatin, based on the National Comprehensive Cancer Network guideline.
| Characteristics | Median (range) | n | % |
| Sex | |||
| Man | 41 | 87.2 | |
| Woman | 6 | 12.8 | |
| Age, years, | 61 (36-86) | ||
| Location of tumor | |||
| Upper | 11 | 23.4 | |
| Middle | 17 | 36.2 | |
| Lower | 19 | 40.4 | |
| Tumor classification | |||
| T3 | 18 | 38.3 | |
| T4A | 7 | 14.9 | |
| T4B | 22 | 46.8 | |
| Lymph node status | |||
| N0 | 2 | 4.3 | |
| N1 | 24 | 51.1 | |
| N2 | 21 | 44.7 | |
| Disease stage | |||
| IIIa | 5 | 10.6 | |
| IIIb | 13 | 27.7 | |
| IIIc | 29 | 61.7 | |
| TP chemotherapy | |||
| 0 | 18 | 38.3 | |
| 1 | 4 | 8.5 | |
| 2 | 12 | 25.5 | |
| 4 | 13 | 27.7 |
| Dose level (Gy per fraction) | Number of fractions | BED (α/β), 10 | EQD2 (α/β), 10 | EQD2 (α/β), 15 | EQD2 (α/β), 3 | Number of patients | Toxicity IV grade | Time, months |
| 3.6 | 15 | 73.44 | 61.20 | 59.08 | 71.28 | 1.00 | ||
| 3.5 | 20 | 94.50 | 78.75 | 76.18 | 91.00 | 1.00 | ||
| 3.3 | 20 | 87.78 | 73.15 | 71.05 | 83.16 | 1.00 | ||
| 3.2 | 20 | 84.48 | 70.40 | 68.52 | 79.36 | 3.00 | B | 5 |
| 3 | 25 | 97.50 | 81.25 | 79.41 | 90.00 | 1.00 | B | 6 |
| 3 | 23 | 89.70 | 74.75 | 73.06 | 82.80 | 1.00 | ||
| 3 | 22 | 85.80 | 71.50 | 69.88 | 79.20 | 1.00 | ||
| 3 | 20 | 78.00 | 65.00 | 63.53 | 72.00 | 2.00 | A | 6 |
| 2.8 | 27 | 96.77 | 80.64 | 79.16 | 87.70 | 1.00 | A | 5 |
| 2.8 | 20 | 71.68 | 59.73 | 58.64 | 64.96 | 1.00 | ||
| 2.7 | 27 | 92.58 | 77.15 | 75.90 | 83.11 | 1.00 | B | 3 |
| 2.7 | 25 | 85.73 | 71.44 | 70.28 | 76.95 | 1.00 | ||
| 2.5 | 27 | 84.38 | 70.31 | 69.49 | 74.25 | 2.00 | a: A; b: A | a: at 4th RT; b: 10 |
| 2.5 | 25 | 78.13 | 65.10 | 64.34 | 68.75 | 2.00 | ||
| 2.5 | 20 | 62.50 | 52.08 | 51.47 | 55.00 | 1.00 | ||
| 2.5 | 18 | 56.25 | 46.88 | 46.32 | 49.50 | 1.00 | ||
| 2.4 | 28 | 83.33 | 69.44 | 68.78 | 72.58 | 1.00 | ||
| 2.4 | 26 | 77.38 | 64.48 | 63.87 | 67.39 | 1.00 | ||
| 2.4 | 25 | 74.40 | 62.00 | 61.41 | 64.80 | 3.00 | ||
| 2.4 | 20 | 59.52 | 49.60 | 49.13 | 51.84 | 1.00 | B | 2 |
| 2.3 | 28 | 79.21 | 66.01 | 65.54 | 68.26 | 1.00 | ||
| 2.3 | 27 | 76.38 | 63.65 | 63.20 | 65.83 | 1.00 | ||
| 2.3 | 26 | 73.55 | 61.30 | 60.86 | 63.39 | 2.00 | ||
| 2.3 | 25 | 70.73 | 58.94 | 58.51 | 60.95 | 2.00 | B | 7 |
| 2.3 | 23 | 65.07 | 54.22 | 53.83 | 56.07 | 1.00 | ||
| 2.2 | 28 | 75.15 | 62.63 | 62.32 | 64.06 | 2.00 | ||
| 2.2 | 25 | 67.10 | 55.92 | 55.65 | 57.20 | 2.00 | ||
| 2 | 30 | 72.00 | 60.00 | 60.00 | 60.00 | 2.00 | ||
| 2 | 27 | 64.80 | 54.00 | 54.00 | 54.00 | 2.00 | B | 7 |
| 2 | 25 | 60.00 | 50.00 | 50.00 | 50.00 | 5.00 |
During the study, dose prescription to the median dose point of the entire primary tumor and lymph node metastases started from a dose of 2 Gy per fraction to 50 Gy, extending up to a maximum dose of 3 Gy per fraction to 81.25 Gy. This corresponded to the total equivalent dose at 2 Gy per fraction, considering an α/β ratio of 10 Gy and the same biologic effective dose of 97.5 Gy delivered in 2 Gy per fraction. The details are shown in Table 2.
The cumulative incidence of acute grade 1, 2, 3 and 4 esophageal toxicity was 46.8% (22 patients), 23.4% (11 patients), 14.9% (7 patients) and 14.9% (7 patients), respectively. Three patients had late-onset toxicity at 7, 7 and 10 months after radiation treatment. The incidence of serious life-threatening esophageal toxicity is summarized in Table 2. Grade 4 esophageal toxicity required hospitalization and resulted in death. Acute grade 2, 3 and 4 pulmonary toxicity was not observed, and none of the patients was treated for pulmonary toxicity induced by radiation therapy. Grade 1 and 2 hematological toxicity was observed in 35 patients during the radiation therapy, while other patients with grade ≥ 3 hematological toxicity received granulocyte colony-stimulating factor treatment. Radiation was not split due to hematological toxicity.
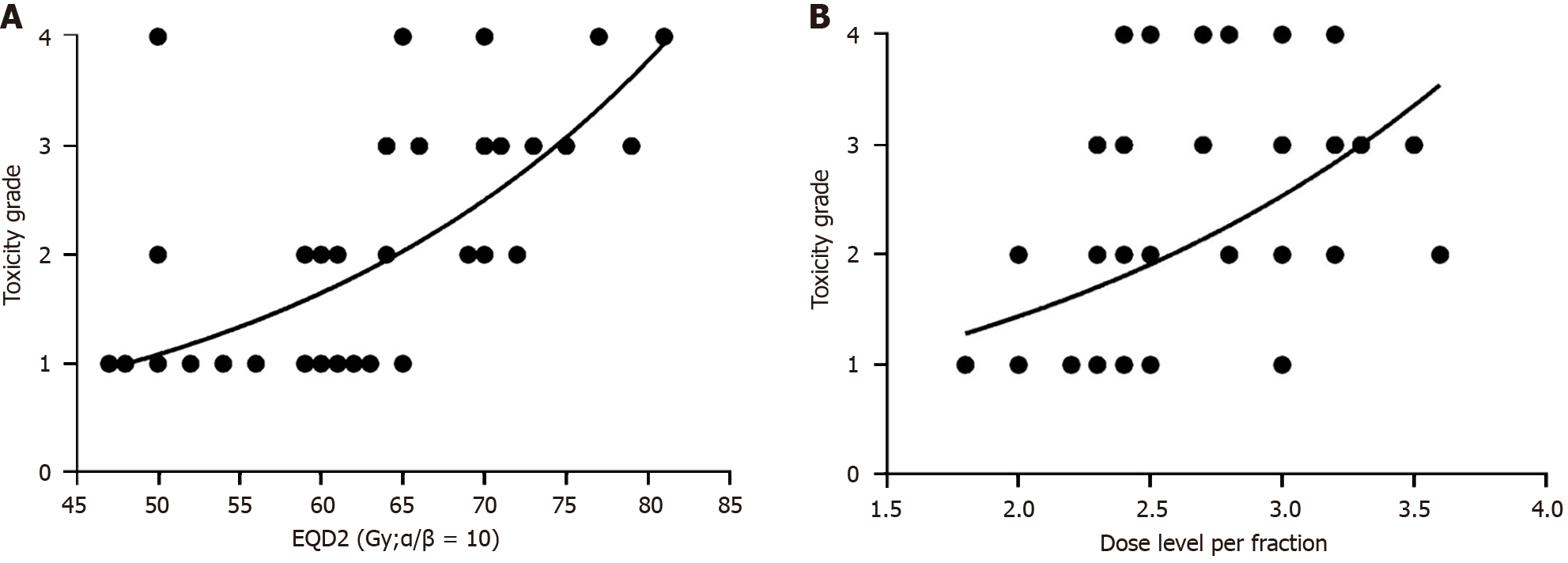
In this study, we aimed to analyze the dose prescription and fraction dose of radiation associated with esophageal toxicity. Nonlinear regression of the dose and acute esophageal toxicity are shown in Figure 1A. It can be seen that the dose of radiation is positively correlated with esophageal toxicity. The esophageal toxicity becomes much higher with dose escalation and can be tolerated below a radiation dose of 60 Gy. If the radiation dose is much greater than 60 Gy, esophageal toxicity can be life-threatening. Thus, a dose of 60 Gy is suggested. Figure 1B shows the nonlinear regression of the dose level per fraction and acute esophageal toxicity. It can be seen that the dose level is positively correlated with esophageal toxicity. Esophageal toxicity is much greater when the dose level was escalated. If the level is greater than 2.3 Gy per fraction, esophageal toxicity can be greater and life-threatening. If the dose level is less than 2.3 Gy per fraction, esophageal toxicity may be tolerated. Thus, we suggest a dose of 60 Gy and a dose per fraction less than 2.3 Gy. Multivariate analysis was also performed to identify significant factors using the ordinal logistic regression of esophageal toxicity. The details are shown in Table 3. The prescription dose was a significant factor in esophageal toxicity. However, the dose level per fraction was not the main factor. Further studies should be carried out to confirm this finding.
| Estimate | SE | Wald | P value | 95% confidence interval | |
| Dose level (Gy per fraction) | -0.96 | 1.14 | 0.70 | 0.40 | -3.20, 1.28 |
| EQD2 (α/β = 10) | 0.17 | 0.06 | 6.99 | 0.01 | 0.04, 0.29 |
| Tumor classification | -0.12 | 0.37 | 0.11 | 0.74 | -0.86, 0.61 |
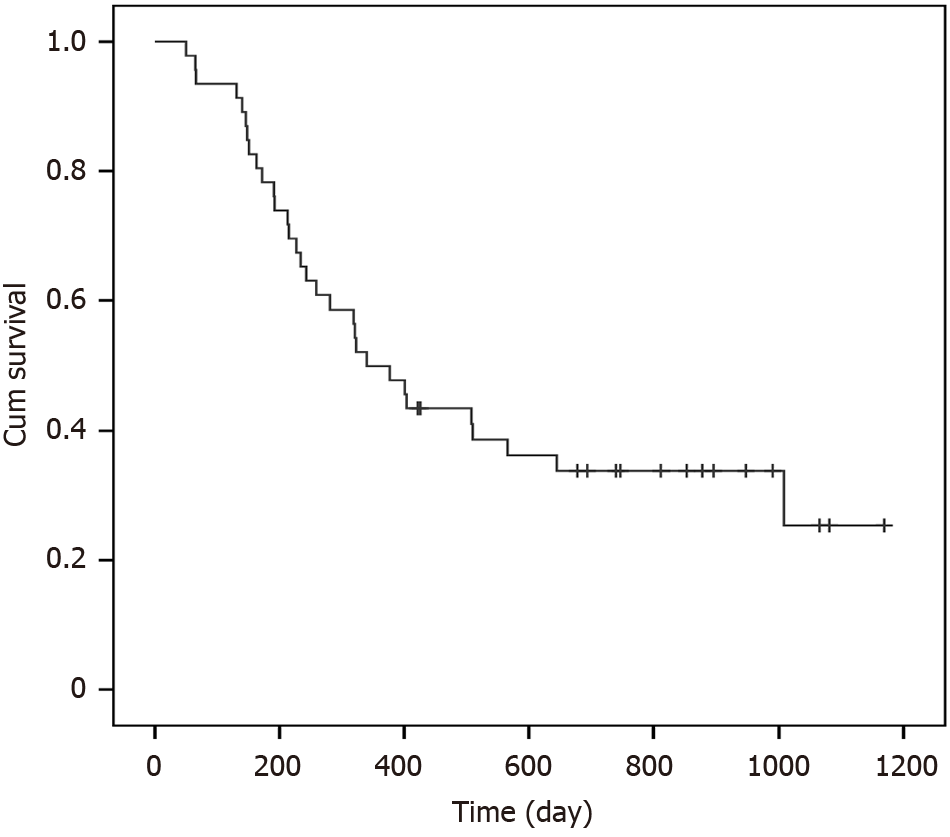
At the time of analysis, 10 patients were alive, and 37 had died. Six patients died of bleeding related to aorto-esophageal fistulization. Four patients died of tracheo-esophageal fistulas, and 7 patients died of local recurrence. The remaining 20 patients died of metastases and multi-organ failure due to organ metastases. The median overall survival of the entire study population was 348 days (Figure 2). The 1- and 2-year cumulative survival rates were 47.8% and 33%, respectively. The incidence of local failure was 21.9% (7/32). The esophageal tumor progressed and led to death in 36% of patients (17/47). The majority of local recurrences occurred around one year of treatment.
Definitive treatment for unresectable, locally advanced esophageal carcinoma remains a challenge, although there is useful palliative treatment for dysphagia and sustained remission[1,8]. The survival rate at 3 and 5 years is very poor. In a randomized trial, the 2-year survival was 31% to 40%[3]. None of the patients in the radiation therapy group survived more than 5 years[2]. The National Comprehensive Cancer Network panel recommends that the standard dose range should be 50 to 50.4 Gy. However, the incidence of locoregional failure and persistence of locoregional disease was 50% to 55%[3]. More than 75% of patients failed in terms of the GTV, and 85% failed in terms of the planning target volume[9]. The low local control rate is still a challenge for modern radiotherapy, especially for locally advanced esophageal carcinoma.
In radiation biology, the dose-response effect for local control has been confirmed. Many factors have contributed to improvements in the local control rate, including an increase in the nominal dose, an increase in the biologic effective dose, the use of technical innovation in radiation delivery, and the addition of concurrent chemotherapy[10]. In one trial[5], preoperative chemoradiotherapy prolonged local disease-free survival with a dose of 37 Gy and 3.7 Gy per fraction. This indicated that prolongation of overall treatment time allows accelerated repopulation of tumors during radiotherapy. Hypofractionated radiotherapy represents an accelerated form of radiation delivery, and treatment once a day improves patient convenience.
To our knowledge, the current study is the first to report the use of helical tomotherapy with hypofractionated radiotherapy and dose escalation for inoperable, locally advanced thoracic ESCC. Helical tomotherapy allows delivery of image-guided radiation therapy to permit higher conformal delivery to diseased tissue while minimizing the dose delivered to normal tissue. These plan optimization possibilities are more flexible and slightly better due to the rotational nature, resulting in a specific type of dose delivery[10]. Thus, in our study, acute grade 2, 3 and 4 pulmonary toxicity was not observed, and none of the patients died of radiation-induced pulmonary failure. However, severe life-threating toxicity related to the esophageal tumor was observed which included tracheo-esophageal fistulas and aorto-esophageal fistulization. The dose level was less than 2.3 Gy per fraction, and the prescribed dose gradually declined. The incidence of severe life-threating toxicity related to the esophageal tumor was much higher than that previously reported (12.8% vs 1%)[3]. We found that the primary tumor was unresectable and locally advanced in almost all patients, thus resulting in a greater chance of morbidity even without treatment.
Nevertheless, the incidence of local recurrence was 21.9% (7/32), which is much less than that observed in a previous report[9]. This also coincided with the results of using preoperative chemoradiotherapy with hypofractionation of 3.7Gy per fraction[5]. We analyzed the prescribed total dose and fraction dose of radiation associated with esophageal toxicity. We found that the dose of radiation and the dose level per fraction were positively correlated with esophageal toxicity (Figure 1). In addition, esophageal toxicity was much greater with escalation of total dose or dose per fraction. Esophageal toxicity could be tolerated below a radiation dose of 60 Gy at less than 2.3 Gy per fraction. Multivariate analysis was performed to identify significant factors associated with esophageal toxicity using ordinal logistic regression (Table 3). The prescribed dose was a significant factor in esophageal toxicity. However, the dose level per fraction was not the main factor. Further studies should be conducted to confirm these findings, and patients with different tumor classifications and stages should been enrolled to determine the best protocol.
We advocate a radiation dose of 60 Gy and less than 2.3 Gy per fraction for unresectable, locally advanced esophageal carcinoma.
The authors are profoundly grateful to all who participated in the study.
| 1. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1959] [Article Influence: 163.3] [Reference Citation Analysis (5)] |
| 2. | Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, Asbell SO, Graham MV, Leichman LL. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1261] [Cited by in RCA: 1370] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 3. | Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 874] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 4. | Zhang YW, Chen L, Bai Y, Zheng X. Long-term outcomes of late course accelerated hyper-fractionated radiotherapy for localized esophageal carcinoma in Mainland China: a meta-analysis. Dis Esophagus. 2011;24:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, Lozach P, Ollier JC, Pavy JJ, Mercier M, Sahmoud T. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 968] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 6. | Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3186] [Cited by in RCA: 3503] [Article Influence: 116.8] [Reference Citation Analysis (0)] |
| 7. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13074] [Article Influence: 523.0] [Reference Citation Analysis (0)] |
| 8. | Pöttgen C, Stuschke M. Radiotherapy versus surgery within multimodality protocols for esophageal cancer--a meta-analysis of the randomized trials. Cancer Treat Rev. 2012;38:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Settle SH, Bucci MK, Palmer MB, Liu H, Liengsawangwong R, Guerrero TM, Mcaleer MF, Cox JD, Komaki R, Liao Z. PET/CT Fusion with Treatment Planning CT (TP CT) Shows Predominant Pattern of Locoregional Failure in Esophageal Patients Treated with Chemoradiation (CRT) is in GTV. Int J Radiat Oncol. 2008;72:S72-S73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Bral S, Duchateau M, Versmessen H, Verdries D, Engels B, De Ridder M, Tournel K, Collen C, Everaert H, Schallier D, De Greve J, Storme G. Toxicity report of a phase 1/2 dose-escalation study in patients with inoperable, locally advanced nonsmall cell lung cancer with helical tomotherapy and concurrent chemotherapy. Cancer. 2010;116:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |