Published online Sep 24, 2022. doi: 10.5306/wjco.v13.i9.738
Peer-review started: May 18, 2022
First decision: July 14, 2022
Revised: July 22, 2022
Accepted: August 17, 2022
Article in press: August 17, 2022
Published online: September 24, 2022
Processing time: 126 Days and 12.8 Hours
Many authorities advocate for Whipple’s procedures to be performed in high-volume centers, but many patients in poor developing nations cannot access these centers. We sought to determine whether clinical outcomes were acceptable when Whipple’s procedures were performed in a low-volume, resource-poor setting in the West Indies.
To study outcomes of Whipple’s procedures in a pancreatic unit in the West Indies over an eight-year period from June 1, 2013 to June 30, 2021.
This was a retrospective study of all patients undergoing Whipple’s procedures in a pancreatic unit in the West Indies over an eight-year period from June 1, 2013 to June 30, 2021.
This center performed an average of 11.25 procedures per annum. There were 72 patients in the final study population at a mean age of 60.2 years, with 52.7% having American Society of Anesthesiologists scores ≥ III and 54.1% with Eastern Cooperative Oncology Group scores ≥ 2. Open Whipple’s procedures were performed in 70 patients and laparoscopic assisted procedures in 2. Portal vein resection/reconstruction was performed in 19 (26.4%) patients. In patients undergoing open procedures there was 367 ± 54.1 min mean operating time, 1394 ± 656.8 mL mean blood loss, 5.24 ± 7.22 d mean intensive care unit stay and 15.1 ± 9.53 d hospitalization. Six (8.3%) patients experienced minor morbidity, 10 (14%) major morbidity and there were 4 (5.5%) deaths.
This paper adds to the growing body of evidence that volume alone should not be used as a marker of quality for patients requiring Whipple’s procedures. Low volume centers in resource poor nations can achieve good short-term outcomes. This is largely due to the process of continuous, adaptive learning by the entire hospital.
Core Tip: Although conventional recommendations suggest that Whipple’s procedures should only be performed in high-volume centers, this is not practical in many nations. This paper adds to the growing body of evidence that volume alone should not be used as a marker of quality for patients requiring Whipple’s procedures. Low volume centers in resource poor nations can achieve good short-term outcomes. This is largely due to the process of continuous, adaptive learning by the entire hospital.
- Citation: Cawich SO, Thomas DA, Pearce NW, Naraynsingh V. Whipple’s pancreaticoduodenectomy at a resource-poor, low-volume center in Trinidad and Tobago. World J Clin Oncol 2022; 13(9): 738-747
- URL: https://www.wjgnet.com/2218-4333/full/v13/i9/738.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i9.738
Whipple’s procedure is a major operation designed to treat malignant peri-ampullary lesions[1,2]. Many Whipple’s procedures tend to be concentrated in high-volume hospitals, usually found in high income nations[3,4,5].
Pancreatic surgeons in the West Indies work in limited-resource environments and perform small numbers of resections annually[5]. There are no centers that qualify as high-volume centers in this setting. Traditional teaching suggests that this scenario is not ideal. In this paper, we examine outcomes of Whipple’s procedures at a low volume/resource environment in a West Indian nation.
The country of Trinidad and Tobago is comprised of two small islands with a cumulative population of 1.35 million persons. A pancreatic surgery unit was established in the main referral hospital in 2013. This unit was led by a single fellowship-trained pancreatic surgeon, one dedicated senior resident and two junior residents. We received permission from the institutional review board to collect and examine data from all consecutive patients who underwent Whipple’s procedures in this setting over an eight-year period from June 1, 2013 to June 30, 2021.
We identified patients by reviewing the hospital records and operating room log books. The hospital records for all patients who underwent Whipple’s operations were retrieved for detailed review. The data extracted included diagnoses, performance scores, estimated operative blood loss, duration of operation (from incision to closure), therapeutic outcomes, post-operative morbidity and mortality. Complications were classified according to the modified Clavien-Dindo system[6]. Pancreatic leak was categorized according to the International Study Group on Pancreatic Fistula criteria. Cardiopulmonary complications included myocardial infarction, arrhythmia, congestive heart failure, pneumonia, pulmonary embolus, and respiratory failure. Statistical analyses were performed using SPSS ver 16.0.
There were 90 patients with operable peri-ampullary neoplasms who had Whipple’s procedures attempted (mean annual case volume of 11.25). The detailed paper-based hospital record could not be retrieved in 14 cases. A search of the intensive care unit (ICU) and hospital registers indicated that these 14 patients were discharged from hospital alive, but they were excluded from the final analysis since their clinical details were not available. We also excluded 4 patients who were deemed irresectable at the time of operation and had palliative bypasses. The final study population included 72 patients who underwent Whipple’s procedures.
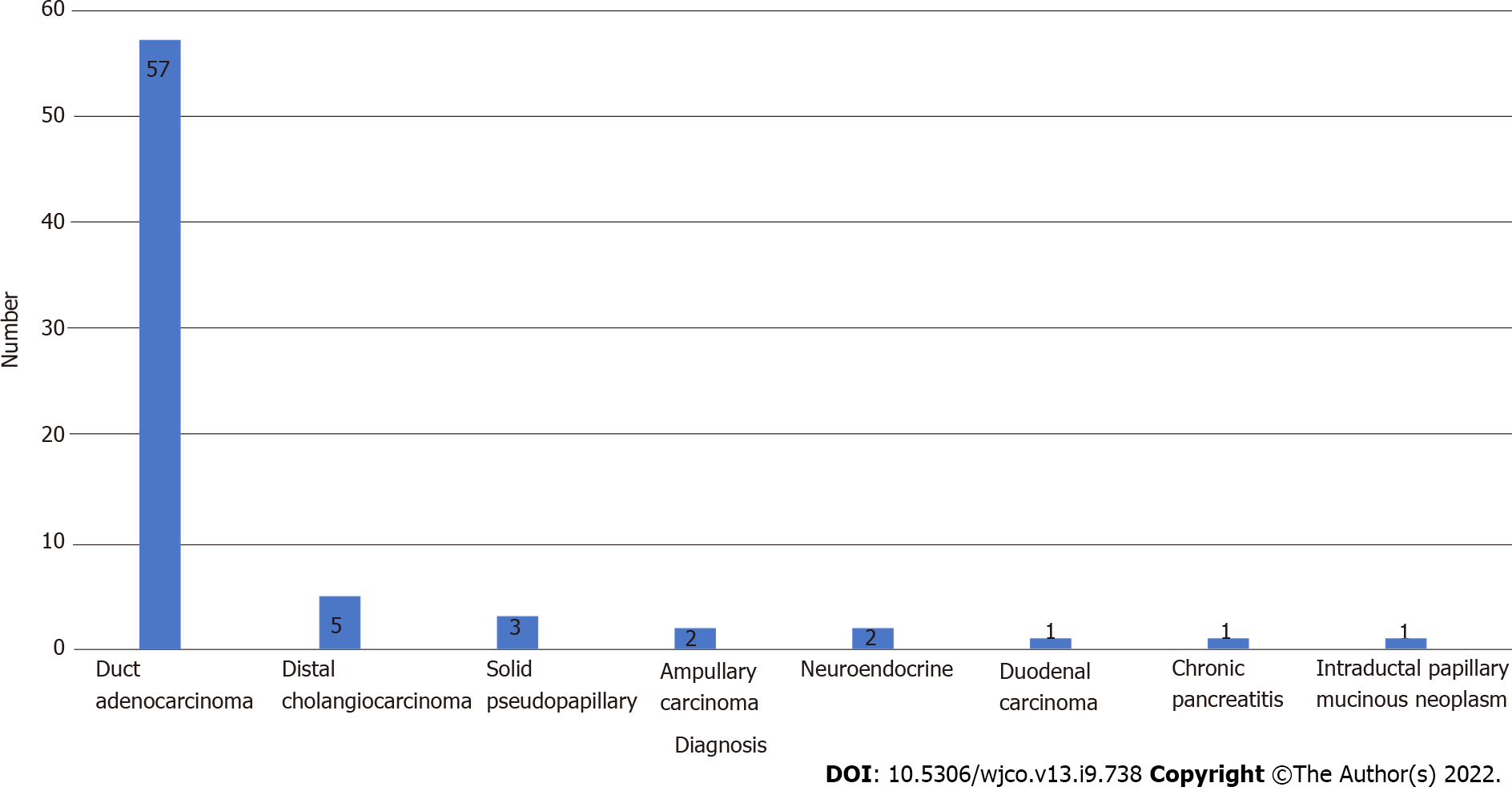
There were 32 men and 40 women at a median age of 61 years (range 46-77; mean 60.2; SD ± 9.28). There were 62 (86%) patients with > 1 comorbid condition, 38 (52.7%) with American Society of Anesthesiologists (ASA) scores > II and 39 (54.1%) with performance scores > 1 (Tables 1 and 2). Pancreatic ductal adenocarcinoma was the commonest pathology, as outlined in Figure 1.
| Score | American Society of Anesthesiologists Descriptor | No (%) |
| I | Completely healthy | 10 (13.9) |
| II | Mild systemic disease | 24 (33.3) |
| III | Severe systemic disease that is not incapacitating | 30 (41.7) |
| IV | Incapacitating disease that is a threat to life | 8 (11.1) |
| V | Moribund and not expected to survive > 24 h | 0 |
| Grade | Eastern Cooperative Oncology Group performance status | No (%) |
| 0 | Fully active, able to carry out all activities without restriction | 13 (18.1) |
| 1 | Restricted in physically strenuous activity, but ambulatory and able to carry out light work | 20 (27.8) |
| 2 | Ambulatory and capable of self care, but unable to carry out work activities. Up and about > 50% of waking hours | 34 (47.2) |
| 3 | Capable of limited self care and confined to bed or chair for more than 50% of waking hours | 4 (5.6) |
| 4 | Completely disabled and cannot carry on self care. Confined to bed or chair | 1 (1.4) |
| 5 | Dead | 0 |
The operation was anticipated to be technically difficult in 26 (36.1%) persons due to: vein involvement requiring resection and reconstruction (19 patients), prior open surgery for abdominal sepsis (5 patients) and planned laparoscopic approach (2 patients).
Four patients had palliative bypasses as they were deemed irresectable at the time of operation due to: invasion of common hepatic artery (1 patient), metastatic disease (2 patients) and portal vein encasement (1 patient). Data on these patients were excluded from further analysis.
In 70 cases, the operation was planned via the open approach using a modified Makuuchi incision, aided by an Omnitract® retractor (Integra Life Sciences, Princeton, NJ). This was our preferred incision as it afforded us good access to the pancreatico-duodenal complex in the retro-peritoneum.
Two patients underwent laparoscopic-assisted Whipple’s procedures. In these cases, kocherization of the duodenum, dissection of the pancreatic neck tunnel, dissection of the gallbladder and structures in the hepatoduodenal ligament, transection of the stomach and full mobilization of the duodenum were completed laparoscopically. A 7 cm midline incision was used to create a pancreatico-gastrostomy, hepatico-jejunostomy and for specimen removal. Both of these patients had ampullary lesions and none required vein resection or reconstruction.
The median operating time for open Whipple’s procedures was 350 min (range 260-485; SD ± 54.1; mean 367). The median blood loss was 1.2 L (range 0.6-4.0; SD ± 0.7; mean 1.4) and 2 packed red cell units was the median transfusion rate (range 0-5; SD ± 1.4; mean 1.88).
Nineteen (26.4%) patients underwent planned vein resections and reconstruction. Reconstruction was performed with primary anastomoses in 13 cases, vein patches in 4 cases and interposition grafts in 2 cases.
In the patients with technically difficult operations, the duration of operation was 374 ± 57.34 minutes (mean ± standard deviation), estimated blood loss was 1494 ± 815 mL (mean ± standard deviation) and 2 ± 1.6 packed red cell units (mean ± standard deviation) were transfused per patient.
We insisted on a policy of mandatory admission to intensive care (ICU) after Whipple’s resection since institutional limitations prevented the expected level of supportive care to be delivered in other areas. Our patients stayed in the ICU for 3 ± 7.22 d (mean ± standard deviation), with 29 (40.3%) needing extended stay > 72 h for ventilator and/or inotropic support. The median hospital stay for all patients was 12 ± 9.6 d (mean ± standard deviation).
There were no complications in 56 patients within this series. Patients without complications remained in ICU for 3.5 ± 1.5 (mean ± standard deviation) d and remained hospitalized for 14 ± 7.9 (mean ± standard deviation) d. Patients who experienced a complication remained in ICU for 9.8 ± 11.3 (mean ± standard deviation) d and remained hospitalized for 15.3 ± 8.4 (mean ± standard deviation) d.
There were 16 (22.2%) patients with overall morbidity - 6 (8.3%) minor and 10 (14.0%) major (Table 3). There were 4 (5.5%) in-patient deaths: (1) A man at 53 years of age with pancreatic head adenocarcinoma who developed massive bleeding from a pseudoaneurysm that could not be controlled at re-operation; (2) A 59-year-old man who did not receive pre-operative stenting and had frank pus in the biliary system at operation. He developed bacteremia and septic shock; (3) A 48-year-old man who developed a leak from the jejuno-jejunal anastomosis, leading to intra-abdominal sepsis and multiple organ failure; and (4) A 70-year-old man with no prior cardiac history who succumbed to a massive myocardial infarction on day 5 post-operation.
| Morbidity | Description | No | % |
| Overall | Number of patients with any complication | 16 | 22.2 |
| Minor | Clavien-Dindo I or II | 6 | 8.3 |
| Pneumonia | 2 | ||
| Deep vein thrombosis | 1 | ||
| Delayed gastric emptying | 1 | ||
| Gastrointestinal bleeding | 2 | ||
| Major | Clavien-Dindo III or IV | 10 | 13.9 |
| Anastomotic dehiscence | 1 | ||
| Massive upper gastrointestinal bleeding | 1 | ||
| Myocardial infarction | 3 | ||
| Pseudoaneurysm | 2 | ||
| Biliary sepsis as a source of septicemia | 2 | ||
| Post-operative pancreatic fistula/intra-abdominal collection | 1 | ||
| Mortality | 30-d mortality: All causes: (1) Massive bleeding from a pseudoaneurysm; (2) Generalized sepsis secondary to cholangitis; (3) Jejuno-jejunal anastomotic leak leading to multiple organ failure; and (4) Myocardial infarction | 4 | 5.6 |
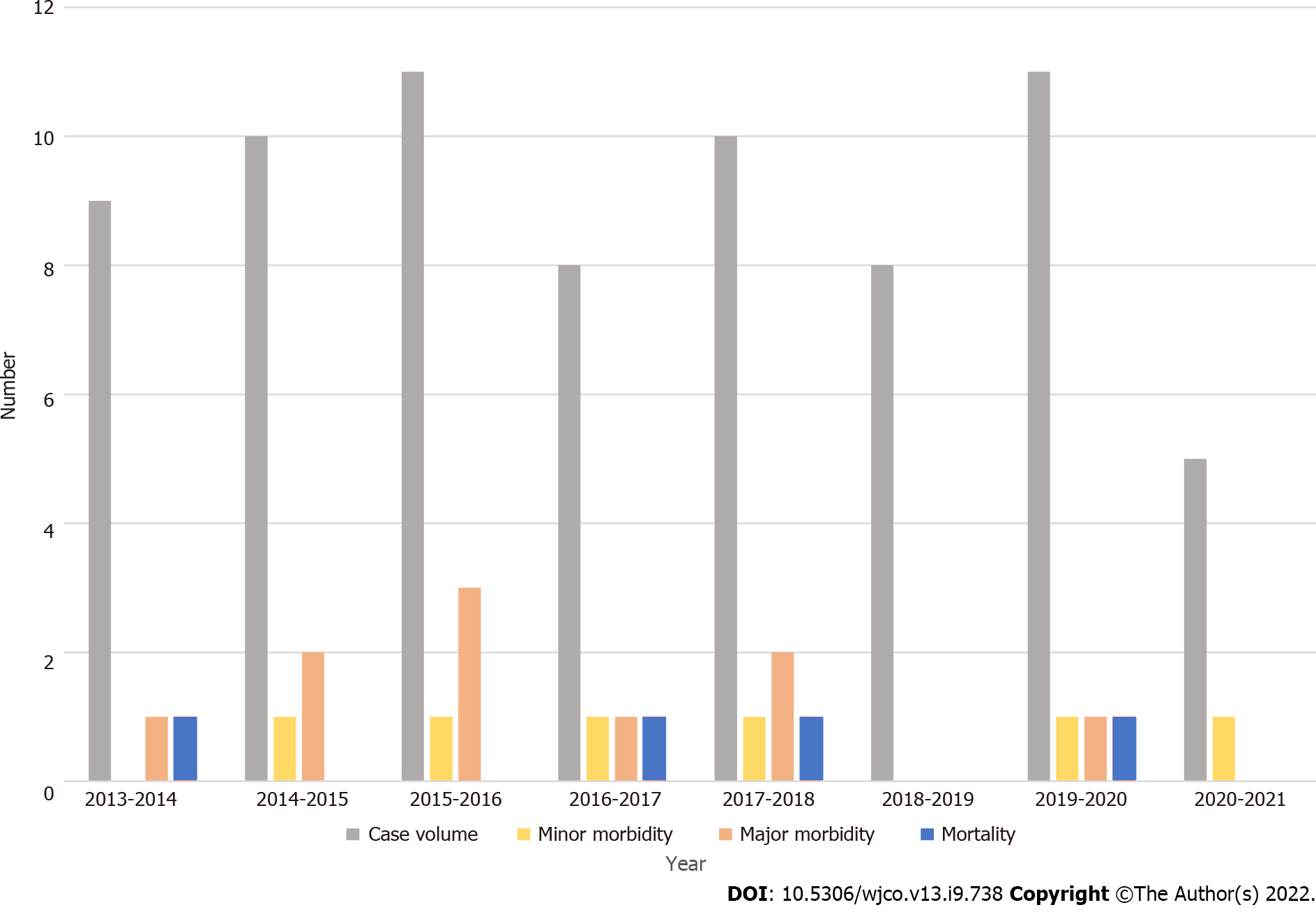
In an attempt to analyze the outcomes chronologically, we tabulated the case volume by year (Table 4). The case volume remained relatively stable with time, although there was a notable reduction in the case volume for the last period (June 2020 to June 2020) due to effects of the coronavirus disease 2019 pandemic. We also analyzed complications and mortality chronologically as outlined in Figure 2. Although minor morbidity reduced over time, there was no statistically significant change in outcomes over time.
| Year | Case volume | Minor morbidity | Major morbidity | Mortality |
| 2013-2014 | 9 | 0 | 1 | 1 |
| 2014-2015 | 10 | 1 | 2 | 0 |
| 2015-2016 | 11 | 1 | 3 | 0 |
| 2016-2017 | 8 | 1 | 1 | 1 |
| 2017-2018 | 10 | 1 | 2 | 1 |
| 2018-2019 | 8 | 0 | 0 | 0 |
| 2019-2020 | 11 | 1 | 1 | 1 |
| 2020-2021 | 5 | 1 | 0 | 0 |
| Total | 72 | 6 (8.3%) | 10 (13.9) | 4 (5.6%) |
We also analyzed clinical outcomes according to patient risk as stratified by their performance scores and ASA scores (Table 5). Although there were trends toward greater morbidity and mortality in high-risk patients, none of these parameters attained statistical significance.
| American Society of Anesthesiologists scores | |||
| Parameter | ASA I-II (34) | ASA III-IV (38) | P |
| Overall morbidity | 7 (20.6%) | 9 (23.7%) | 0.7843 |
| Overall mortality | 2 (5.9%) | 2 (5.3%) | 1.000 |
| Eastern Cooperative Oncology Group performance status | |||
| Parameter | ECOG 0-2 (67) | ECOG 3-4 (5) | P |
| Overall morbidity | 14 (20.9%) | 2 (40%) | 0.307 |
| Overall mortality | 3 (4.5%) | 1 (20%) | 0.255 |
Although it is a high-risk operation, Whipple’s procedure is the only existing treatment with the potential to cure peri-ampullary malignancies[7,8]. Early series in the late 1960s reported 60% post-operative morbidity and mortality rates approaching 25%[9-11], but with better surgical equipment and supportive care, the safety profile has improved. Modern reports document 30-d mortality rates between 4%-6%[7,8,12-14].
Much of the recent progress in surgical treatment has been aimed at minimizing peri-operative morbidity with a multidisciplinary approach to care[1,2], advanced cross-sectional imaging[1,15], specialized surgical equipment[5], appropriate support services[16,17], full-time intensive care, interventional radiology and gastroenterology services[1,14-17] and quaternary surgical training. In our center, we have been able to achieve many of these goals.
Another change was the centralization concept, that was popularized in the early 21st century[3,4,11-13]. Published data showed reductions in morbidity[1,2,18], cost[19], mortality[1,2,18,10,20-22] and hospitalization[1,18] in high-volume hospitals. However, the high-volume definition remained elusive. Some have designated centers performing as few as 3 Whipple’s procedures per annum as high-volume centers[12,14,19], while others reserve this designation for facilities performing ≥ 30 per annum[18,23,24]. Most researchers quote numbers ≥ 18 Whipple’s procedures per annum as high volume[2,7,11,18,22-26]. Using this definition, our facility did not qualify as high-volume. Although our center has documented the largest volume of Whipple’s procedures per annum in the region[5], we still only performed an average 11.25 cases per annum.
Centralization remains controversial. Even in developed countries, most Whipple’s procedures are still performed in low-volume centers[7,20,27]. In Texas, Riall et al[27] reported that ≥ 25% of Whipple’s procedures were performed in hospitals doing < 5 cases per annum and 35% were done in hospitals performing < 10 cases per annum. Similarly, McPhee et al[22] reported that 61% of Whipple’s procedures across the United States in the year 2007 were done outside of high-volume centers. Furthermore, it has been documented that centralization contributes to health care inequity, with significantly fewer females[27], non-caucasians[18,27-31], persons from low socio-economic brackets[32], persons from low-income zip codes and persons without private health insurers[18,27-30] being able to access care in these centers. We do not believe that the traditional centralization concept is practical for the West Indies due to travel restrictions, low health insurance rates, financial limitations and absent social support pathways.
Despite the fact that these surgeons performed small numbers of operations in a setting with scarce blood products, limited operating time and restricted intensive care support, short-term outcomes were still reasonable. The 30-d mortality in high-volume centers ranged widely, but most high-volume centers maintained 30-d mortality rates between 4%-6%[2-4,7,11-14,18,22,26,27]. At 5.5%, our 30-d mortality compared favorably. Similarly, our major morbidity rates compared favorably with high-volume centers reporting figures that ranged from 16%[33] to 26%[1]. Several authors have advocated documentation of procedure-related complications that include pancreatic fistula, delayed gastric emptying, intra-abdominal sepsis and intra-abdominal haemorrhage[34]. In our series the incidence of these procedure-specific complications was acceptable (1.4%, 1.4%, 2.8% and 2.8% of cases respectively).
It has been demonstrated in the medical literature that procedure-related complications are similar between low- and high-volume hospitals, but there is a significant difference in medical complications such as aspirations, pneumonia, pulmonary failure, renal failure and septicemia[33,34]. This reinforces the thinking that, while surgical expertise is necessary, it alone is not sufficient to guarantee good post-operative outcomes[2,33,34]. Medical complications occurred in 8.3% of our cases, suggesting that there may still be room for us to optimize support care/medical services.
Schmidt et al[2] introduced the concept of the “experienced surgeon” as being distinct from a “high-volume surgeon.” They defined the “experienced surgeon” as one who performed ≥ 50 Whipple’s procedures in their career, regardless of the interval[2]. They also made the point that experienced surgeons may not be high volume surgeons (which was time dependent) and demonstrated that experienced surgeons with low annual volumes had equivalent outcomes to high-volume surgeons[2]. The pancreatic surgeon in this setting was experienced, having performed ≥ 100 Whipple’s procedures. We believe that this contributed to the outcomes reported in this paper, and gives support to Schmidt’s concept of the experienced surgeon.
In their paper, Schmidt et al[2] counted the number of procedures in which a vein resection was performed as a surrogate marker of technical complexity and surgeon experience. In our series, 26.4% of patients had portal vein resection and reconstruction. It should be noted, however, that while these were experienced surgeons, they would have accrued much of their experience in high volume centers in developed countries during fellowship training. These facilities operate under different circumstances. Upon repatriation to the West Indies, these surgeons would have to adapt to challenging, new working environments. These surgeons adapted their practices to the new environment, focusing on peri-operative management and inter-disciplinary cooperation that evolved with time and were hospital-specific. This interaction and continuous institutional learning are difficult to measure and would evolve specific to each surgeon’s health care environment. Several authors have alluded to the concept of continuous, adaptive learning by the institution[1,2,7,18,35-37]. This is not limited to the surgeons alone, but includes pre-operative evaluation, multidisciplinary team interaction, intra-operative anaesthesia care, surgeon training, post-operative care pathways, post-procedure nursing care, ICU care, availability of emergency medical doctors and experienced subspeciality supportive care[1,2,7,18,35-37].
We agree that Whipple’s procedure is a complex operation that depends heavily on surgeon experience. At the same time, we believe that there is more to experience than technical facility. For example, the experienced surgeon would know how to resect and reconstruct the portal vein when required to achieve negative margins[2], when not to operate on patients[2], to recognize aberrant anatomy[2], how to get out of trouble when complications occur intra-operatively[7]. These can only be learned with experience and proper mentorship[2].
Recently, there has been focus on learning curves as a part of the concept of surgeon volume and surgeon experience. Tseng et al[38] suggested that after 60 Whipple’s procedures, surgeons improved on peri-operative outcomes such as blood loss, operation time, hospital stay and margin status. However, the most senior author in our paper performed over 300 Whipple’s and felt that he was still improving well beyond 200 cases, although the steepest part of the curve was the first 50. Similarly, the first author who performed all Whipple’s procedures in this series felt that he continued to improve during this series. It seems reasonable to conclude that the learning curve lies somewhere between 50 and 70 cases.
We believe that multiple factors contributed to the outcomes in our setting: (1) Population-based data[5]; (2) Training of unit staff; (3) Developing an intimate knowledge of the hospital; (4) Fostering teamwork; (5) Diligent administration of care; and (6) Regular audits. We also advocate two experienced surgeons operating to maximize experience. Also, if one surgeon is more experienced it speeds up the learning curve for the second surgeon. The key is overall team experience because, in addition to reducing intraoperative complications, effectively managing post-operative complications is important.
It is tempting to think that the outcomes reported here may be biased due to case selection. However, we do not believe that this was the case in our setting because this was a government funded hospital and we were required to provide care for all patients who presented to this unit. In addition, many of our patients were physiologically challenging, with 52.7% having ASA scores ≥ III and 54.1% with ECOG scores ≥ 2.
The retrospective study design did limit our ability to collect detailed clinical information, such as accurate blood loss, adherence to post-care pathways and, as previously noted, we were unable to locate paper-based records for 14 patients who underwent Whipple’s procedures.
This paper adds to the growing body of evidence that volume alone should not be used as a marker of quality for patients requiring Whipple’s procedures. Low volume centers in resource poor nations can achieve good short-term outcomes. This is largely due to the process of continuous, adaptive learning by the entire hospital and includes: Population-based data, good teamwork, effective staff training, regular audit and due diligence in care administration.
Whipple's operations are high-risk operations that should be done in high-volume centers for optimal outcomes. This is supported by data from several high-volume hospitals.
High-volume centers are usually in developed nations. There are no high-volume centers in the West Indies. In this setting, pancreatic surgeons have to perform Whipple's operations in resource-poor, low-volume settings. This scenario is not ideal, but it is the reality on the ground.
We sought to document the clinical outcomes when Whipple's operations were performed in resource-poor, low-volume centers in the West Indies. If the outcomes are poor, this would be impetus not to perform these operations in this setting or to develop service centralization with high-volume centers.
A retrospective audit of all Whipple's operation performed at a referral center over an eight-year period was performed. Data collected from hospital records included: diagnoses, performance scores, estimated operative blood loss, duration of operation, therapeutic outcomes, post-operative morbidity and mortality. Statistical analyses were performed using SPSS version 16.0.
This facility performed 11.25 Whipples procedures per annum. There were 72 patients in the final study population at a mean age of 60.2 years. Open Whipple’s procedures were performed in 70 patients and laparoscopic assisted procedures in 2. Portal vein resection/reconstruction was performed in 19 (26.4%) patients. In patients undergoing open procedures there was 367 ± 54.1 min mean operating time, 1394 ± 656.8 mL mean blood loss, 5.24 ± 7.22 d mean intensive care unit stay and 15.1 ± 9.53 d hospitalization. Six (8.3%) patients experienced minor morbidity, 10 (14%) major morbidity and there were 4 (5.5%) deaths.
Low volume centers in resource poor nations can achieve good short-term outcomes once they pay attention to continuous, adaptive learning. Volume alone should not be used as a marker of quality for patients requiring Whipple’s procedures.
The direction of future research is to identify specific hospital-based pathways and/or team-focused processes that improve clinical outcomes in low-volume facilities.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Trinidad and Tobago
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mijwil MM, Iraq; Pan Y, China S-Editor: Wang DM L-Editor: A P-Editor: Wang DM
| 1. | Søreide JA, Sandvik OM, Søreide K. Improving pancreas surgery over time: Performance factors related to transition of care and patient volume. Int J Surg. 2016;32:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Schmidt CM, Turrini O, Parikh P, House MG, Zyromski NJ, Nakeeb A, Howard TJ, Pitt HA, Lillemoe KD. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg. 2010;145:634-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 345] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 3. | Fong Y, Gonen M, Rubin D, Radzyner M, Brennan MF. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg. 2005;242:540-4; discussion 544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 290] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Neoptolemos JP, Russell RC, Bramhall S, Theis B. Low mortality following resection for pancreatic and periampullary tumours in 1026 patients: UK survey of specialist pancreatic units. UK Pancreatic Cancer Group. Br J Surg. 1997;84:1370-1376. [PubMed] |
| 5. | Cawich SO, Kluger MD, Francis W, Deshpande RR, Mohammed F, Bonadie KO, Thomas DA, Pearce NW, Schrope BA. Review of minimally invasive pancreas surgery and opinion on its incorporation into low volume and resource poor centres. World J Gastrointest Surg. 2021;13:1122-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 6. | Téoule P, Bartel F, Birgin E, Rückert F, Wilhelm TJ. The Clavien-Dindo Classification in Pancreatic Surgery: A Clinical and Economic Validation. J Invest Surg. 2019;32:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | van Heek NT, Kuhlmann KF, Scholten RJ, de Castro SM, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781-788, discussion 788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 289] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 8. | Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Gilsdorf RB, Spanos P. Factors influencing morbidity and mortality in pancreaticoduodenectomy. Ann Surg. 1973;177:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 72] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Lansing PB, Blalock JB, Oschner JL. Pancreaticoduodenectomy: a retrospective review, 1949-1969. Am Surg. 1972;38:79-86. [DOI] [Full Text] |
| 11. | Sosa JA, Bowman HM, Gordon TA, Bass EB, Yeo CJ, Lillemoe KD, Pitt HA, Tielsch JM, Cameron JL. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998;228:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 377] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Kotwall CA, Maxwell JG, Brinker CC, Koch GG, Covington DL. National estimates of mortality rates for radical pancreaticoduodenectomy in 25,000 patients. Ann Surg Oncol. 2002;9:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Ho V, Heslin MJ. Effect of hospital volume and experience on in-hospital mortality for pancreaticoduodenectomy. Ann Surg. 2003;237:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Bilimoria KY, Bentrem DJ, Feinglass JM, Stewart AK, Winchester DP, Talamonti MS, Ko CY. Directing surgical quality improvement initiatives: comparison of perioperative mortality and long-term survival for cancer surgery. J Clin Oncol. 2008;26:4626-4633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Mayo SC, Gilson MM, Herman JM, Cameron JL, Nathan H, Edil BH, Choti MA, Schulick RD, Wolfgang CL, Pawlik TM. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg. 2012;214:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Simianu VV, Zyromski NJ, Nakeeb A, Lillemoe KD. Pancreatic cancer: progress made. Acta Oncol. 2010;49:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Lassen K, Ljungqvist O, Dejong CH, Demartines N, Parks RW, Lobo DN, Coolsen MM, Fearon KC. Pancreaticoduodenectomy: ERAS recommendations. Clin Nutr. 2013;32:870-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Bliss LA, Yang CJ, Chau Z, Ng SC, McFadden DW, Kent TS, Moser AJ, Callery MP, Tseng JF. Patient selection and the volume effect in pancreatic surgery: unequal benefits? HPB (Oxford). 2014;16:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Gordon TA, Bowman HM, Tielsch JM, Bass EB, Burleyson GP, Cameron JL. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg. 1998;228:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Derogar M, Blomberg J, Sadr-Azodi O. Hospital teaching status and volume related to mortality after pancreatic cancer surgery in a national cohort. Br J Surg. 2015;102:548-57; discussion 557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Birkmeyer JD, Warshaw AL, Finlayson SR, Grove MR, Tosteson AN. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126:178-183. [PubMed] |
| 22. | McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, Anderson FA, Tseng JF. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 376] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 23. | Alsfasser G, Kittner J, Eisold S, Klar E. Volume-outcome relationship in pancreatic surgery: the situation in Germany. Surgery. 2012;152:S50-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Glasgow RE, Mulvihill SJ. Hospital volume influences outcome in patients undergoing pancreatic resection for cancer. West J Med. 1996;165:294-300. [PubMed] |
| 25. | Meguid RA, Ahuja N, Chang DC. What constitutes a "high-volume" hospital for pancreatic resection? J Am Coll Surg. 2008;206:622.e1-622.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Briceno P, Hutson J, Shridhar R, Meredit K. Pancreatic Resection at High Volume Centers Improves Survival. HPB. 2017;S171:131. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Riall TS, Eschbach KA, Townsend CM Jr, Nealon WH, Freeman JL, Goodwin JS. Trends and disparities in regionalization of pancreatic resection. J Gastrointest Surg. 2007;11:1242-51; discussion 1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Al-Refaie WB, Muluneh B, Zhong W, Parsons HM, Tuttle TM, Vickers SM, Habermann EB. Who receives their complex cancer surgery at low-volume hospitals? J Am Coll Surg. 2012;214:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, Brook RH, Ko CY. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA. 2006;296:1973-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 425] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 30. | Chang DC, Zhang Y, Mukherjee D, Wolfgang CL, Schulick RD, Cameron JL, Ahuja N. Variations in referral patterns to high-volume centers for pancreatic cancer. J Am Coll Surg. 2009;209:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Epstein AJ, Gray BH, Schlesinger M. Racial and ethnic differences in the use of high-volume hospitals and surgeons. Arch Surg. 2010;145:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Eppsteiner RW, Csikesz NG, McPhee JT, Tseng JF, Shah SA. Surgeon volume impacts hospital mortality for pancreatic resection. Ann Surg. 2009;249:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Halloran CM, Ghaneh P, Bosonnet L, Hartley MN, Sutton R, Neoptolemos JP. Complications of pancreatic cancer resection. Dig Surg. 2002;19:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Ho CK, Kleef J, Friess H, Buchler MW. Complications of Pancreatic Surgery. HPB. 2005;7:99-108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Gasper WJ, Glidden DV, Jin C, Way LW, Patti MG. Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference? Ann Surg. 2009;250:472-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Hashimoto DA, Bababekov YJ, Mehtsun WT, Stapleton SM, Warshaw AL, Lillemoe KD, Chang DC, Vagefi PA. Is Annual Volume Enough? Ann Surg. 2017;266:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Ihse I. The volume-outcome relationship in cancer surgery: a hard sell. Ann Surg. 2003;238:777-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Tseng JF, Pisters PW, Lee JE, Wang H, Gomez HF, Sun CC, Evans DB. The learning curve in pancreatic surgery. Surgery. 2007;141:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |